Equid alphaherpesvirus 1 (EHV-1) infection causes abortion, respiratory disease, perinatal deaths and neurological disorders in horses. The natural infection and available vaccines provide only partial and short-lived protection against reinfections. In the present study, we analyzed the ability of purified baculovirus-expressed glycoprotein D (gD) administered by different routes to induce protective immunity in BALB/c mice after challenge with the EHV-1 AR8 strain. Clinical signs varied among the different groups of mice immunized by parenteral routes, and, although gD induced a specific serum IgG response, it did not prevent the virus from reaching the lungs. Intranasally immunized mice showed no clinical signs, and virus isolation from lungs, histological lesions and antigen detection by immunohistochemistry were negative. In addition, by this route, gD did not stimulate the production of serum IgG and IgA. However, a specific IgA response in the respiratory tract was confirmed in intranasally immunized mice. Thus, we conclude that the mucosal immune response could reduce the initial viral attachment and prevent the virus from reaching the lungs. Our findings provide additional data to further study new immunization strategies in the natural host.
La infección con alfaherpesvirus equino 1 (EHV-1) causa abortos, enfermedad respiratoria, muertes perinatales y desórdenes neurológicos en equinos. La infección natural y las vacunas disponibles solo proporcionan protección parcial y de corta duración contra las reinfecciones. En el presente estudio se analizó la inducción de inmunidad protectiva de la glicoproteína D (gD) expresada en baculovirus y purificada al ser administrada por diferentes rutas en ratones BALB/c desafiados con la cepa AR8 de EHV-1. Los signos clínicos fueron variables entre los grupos de ratones inmunizados por rutas parenterales y, aunque la gD indujo respuesta específica de IgG en suero, no logró prevenir la llegada del virus al pulmón. En los ratones inmunizados intranasalmente no se observaron signos clínicos ni lesiones histopatológicas, y el aislamiento viral y la detección de antígenos por inmunohistoquímica en pulmón fueron negativos. Además, por esta ruta la gD no estimuló la producción de IgG y de IgA en suero. Sin embargo se confirmó la respuesta de IgA específica en el tracto respiratorio de ratones inmunizados intranasalmente. Esta respuesta inmune mucosal podría haber reducido la unión inicial del virus a la célula huésped y, de este modo, prevenir la llegada del virus al pulmón. Nuestros hallazgos proporcionan un aporte para continuar estudiando nuevas estrategias de inmunización en el huésped natural.
Equid alphaherpesvirus 1 (EHV-1) induces respiratory infection, neurological disorders and abortions in horses throughout the world, thus causing a significant negative economic impact on equine production. Since the natural respiratory infection does not result in long-lived protection against subsequent infections, animals remain susceptible to new infections1. The immunological protection of horses depends on the cooperation of virus-specific humoral and cellular immune responses35, in which antibodies with virus-neutralizing activity are required to block free infectious viral particles and cytotoxic T lymphocytes are required to lyse infected cells. EHV-1 infection elicits a local immune response at the primary site of replication and a systemic humoral and cellular immune response; however, protective immunity against reinfection lasts 3 to 6 months19. The specific local mucosal immunity induced by EHV-1 is important because it might prevent both the virus entry through the basement membrane barrier and the induction of mononuclear leukocyte-associated viremia19,32. Breathnach et al.4 showed that virus-specific antibodies are secreted following an experimental infection and that Immunoglobulin (Ig) A is the main isotype and IgGa the most prominent sub-isotype of IgG. These authors also showed that the duration of secretion is short; however, this period increases upon repeated stimulation. In addition, antiviral cytotoxic activity has been observed in lymphoid tissue within the mucosa of the nasopharynx, which is the main site for primary replication of infectious EHV-1 particles2,5. This cytotoxic activity may help clear the virus and prevent its arrival at downstream locations by the bloodstream5.
Effective control of EHV-1 infection relies on management practices and vaccination. Modified live virus and inactivated vaccines are currently available for protection against EHV-1. Although the modified vaccines have the risk of reversion to virulence, they have an excellent safety record and can protect horses against clinical disease. However, their efficacy in preventing viremia, abortion, and neurological disease is not yet clear19. The inactivated vaccines, which are produced exclusively with inactivated viruses and adjuvants, fail to prevent challenge infection but induce the production of serum virus-neutralizing antibodies and reduce both the amount of infectious viral particles and the duration of viral shedding from the nasopharynx19,21,22. In addition, adverse effects on antibody and cellular immunity are observed when the EHV vaccine is given in short intervals. Therefore, the vaccination protocols should be optimized35.
Envelope glycoproteins of herpesviruses are strong candidates as immunogens for the development of effective subunit vaccines, since they are essential for the infectious process and the induction of cellular and humoral immune responses2,6. Different glycoproteins of EHV-1 have been produced and their ability to induce humoral and cellular responses have been evaluated in murine models25,29,33,39,42 and the natural host10,11,36. In addition, experimental trials using individual or combined baculovirus-expressed glycoproteins B, C, D and H in mice have shown that these glycoproteins accelerate viral clearance from mouse lungs after challenge with EHV-124,25. Glycoprotein D (gD) is a component of the viral envelope, which is found on the surface of EHV-1-infected cells and is involved in viral entry into the host cells6. The strong protective response elicited by this glycoprotein may be due to the complement-independent neutralizing epitope (s) of gD9. Most of the studies on vaccine development against EHV-1 with gD have been performed in mice by using systemic routes, and, although these vaccines stimulate the specific immune response, they do not completely prevent infection25,29,30,37. In horses, few studies on EHV-1 gD immunogenicity have been reported10,23,28,39, and all of them have shown specific serum antibody response. Recently, Liu et al.20 have evaluated the use of the live-attenuated Herpes Simplex Virus 1 vaccine strain VC2 expressing EHV-1 gD and have shown that it can efficiently infect equine cells and generate strong and protective anti-EHV-1 immune responses in mice. In addition, Allen et al.2 suggested that the stimulation of the mucosal immunity of the respiratory tract is the best approach for immunoprophylaxis against EHV-1 and EHV-4 respiratory disease. Thus, the development of a successful vaccine against EHV-1 with recombinant gD administered by the intranasal route may provide appropriate immunoprotection.
The aims of this work were to study the specific immune response induced in mice immunized with previously obtained complete gD expressed by a recombinant baculovirus12, using different inoculation routes with or without adjuvants, and to evaluate its effectiveness to prevent the virus entry into the lungs after challenge with an EHV-1 strain.
Materials and methodsViral strain, cell cultures and recombinant glycoprotein DThe Argentinean EHV-1 AR8 strain was used in all the experiments. This strain was isolated in 1996 from an aborted equine fetus after a storm of abortions occurring in a farm in 1995 (data not published). Rabbit kidney cells (RK13) (Argentine Cell Bank Association, Argentina, ABAC) were used to prepare a viral stock for virus neutralization and virus isolation. An antigen for indirect enzyme-linked immunosorbent assay (ELISA) was prepared on Madin–Darby bovine kidney (MDBK) cells (ABAC). Both cell lines were grown in minimum essential medium (MEM) (Gibco, Invitrogen, USA) supplemented with 2mM glutamine (Gibco), 100IU/ml penicillin, 100μg/ml streptomycin (Ritchet, Buenos Aires, Argentina), and 100IU/ml nystatin (Parafarm, Buenos Aires, Argentina). For cell growth, 10% of fetal calf serum was added (growth medium) and then reduced to 2% for cells maintenance (M-MEM). Confluent monolayers of RK13 cells grown with growth medium were infected with AR8 strain and incubated with M-MEM at 37°C in a 5% CO2 atmosphere until an extensive cytopathic effect was observed. After three cycles of freezing and thawing, the cells and infectious supernatant were centrifuged at 8000g for 20min to remove cell debris. The final infectious supernatant was fractionated in small volumes and this viral stock was stored at−70°C until use. Virus titer was determined by the Reed and Muench method27 (107.6 TCID50/ml).
For recombinant gD production, total DNA of the EHV-1 AR8 strain was used to generate by PCR a 1373 bp fragment encompassing the EHV-1 gD open reading frame (ORF) flanked by restriction sites, according to the methodology described by Fuentealba et al.12 Then, it was cloned into the pCR2.1-TOPO vector by using the TOPO TA Cloning Kit (Life Technologies Corporation, Carlsbad, CA, USA) and digested with restriction endonucleases corresponding to restriction sites added to the primers. The obtained fragment corresponding to the gD gene was inserted into the transfer plasmid pFastBacHTB and transformed into DH10Bac cells (Invitrogen, Carlsbad, CA, USA). The recombinant baculovirus was obtained in Spodoptera frugiperda (Sf21) cells (Gibco, Grand Island, NY, USA) transfected with recombinant bacmid DNA. For glycoprotein expression, Trichoplusia ni cells (High Five™ cells, Invitrogen) were infected with 1 PFU of recombinant baculovirus/cell. Then, the glycoprotein was purified by immobilized metal ion affinity chromatography (IMAC), using Ni-NTA agarose (Qiagen, Maryland, USA)12.
Animal handling and immunization strategiesSpecific pathogen-free five-week-old BALB/c mice were provided by the Laboratory of Experimental Animals (Faculty of Veterinary Sciences, National University of La Plata, Buenos Aires, Argentina) and kept in conventional animal rooms. Animal handling and all experimental procedures were carried out in compliance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Research Council17 and supervised by the Institutional Committee for Care and Use of Laboratory Animals (Faculty of Veterinary Sciences, National University of La Plata, protocol 600-004104/08-001. Res. 129/09).
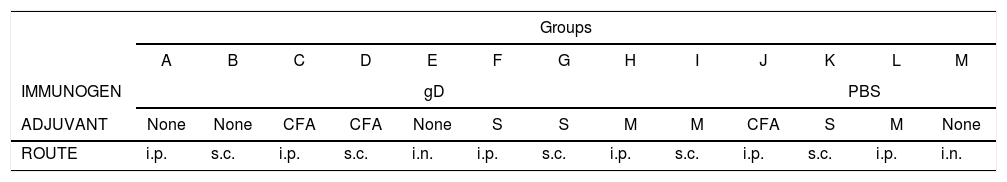
Mice were divided into 13 groups (groups A–M; 10 animals each). Groups A–I were immunized with gD under light anesthesia with isoflurane (Baxter Co., Deerfield, IL, USA) by using different routes (i.p.: intraperitoneal; s.c.: subcutaneous; i.n.: intranasal). For the i.p. and s.c. routes, the following adjuvants were used: Complete Freund's Adjuvant (CFA), specol, and Montanide (Table 1). Each mouse received a first dose of 50μl (15μg of gD in phosphate buffered saline, PBS) and a second dose 20 days later. Control groups (groups J–M) received either PBS or PBS with adjuvants. Pooled blood samples of each group were taken from the maxillary vein 10 days after each immunization (days 10 and 30) and serum samples were collected and processed for ELISA, virus neutralization and immunoblotting assays. Twenty days after the second immunization, mice were anesthetized as previously described, and challenged with ∼106.3 TCID50/50μl of EHV-1 AR8 using the i.n. route.
Immunization protocol with purified recombinant Equid alphaherpesvirus 1 gD, different adjuvants and inoculation routes.
| Groups | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | |
| IMMUNOGEN | gD | PBS | |||||||||||
| ADJUVANT | None | None | CFA | CFA | None | S | S | M | M | CFA | S | M | None |
| ROUTE | i.p. | s.c. | i.p. | s.c. | i.n. | i.p. | s.c. | i.p. | s.c. | i.p. | s.c. | i.p. | i.n. |
CFA, complete Freund's adjuvant; S, specol; M, Montanide ISA 206; i.p., intraperitoneal; s.c., subcutaneous; i.n., intranasal.
Mice were examined daily to record clinical signs and body weight. At 1, 2, 3 and 4 days post-challenge (pc), two mice of each group were anesthetized, killed by exsanguination, and heparinized blood (20IU/ml) was collected for virus isolation15 and DNA detection by polymerase chain reaction (PCR)14. The right lung of each mouse was processed for virus infectivity titration, whereas the left lung was selected for histological studies. In addition, on day 4, serum samples were collected for antibody detection by ELISA, virus neutralization and immunoblotting. The remaining mice in each group were observed to record body weight and were bled on days 60 and 90 after the first immunization.
Antibody detection in seraIndirect ELISABlood samples from each group were taken from the maxillary vein 10 days after each immunization and on day 4pc. Serum samples were obtained from each group and analyzed by ELISA for IgG and IgA detection, using a soluble antigen produced with AR8-infected Madin Darby bovine kidney (MDBK) cells. Control antigen was prepared following the same procedure with uninfected MDBK cells7. An optimum dilution of 1 in 40 was determined for the serum samples. Anti-mouse IgG peroxidase (Sigma-Aldrich, St Louis, MO, USA) and Goat Anti-mouse IgA with peroxidase (Southern Biotech, Birmingham, AL, USA) were used as secondary antibody, and an ABTS (2,20-azino-di-[3-ethyl-benzothiazoline-6-sulphonic acid]) solution containing H2O2 was used as a substrate indicator. The optical density (OD) was determined by the use of an ELISA reader (Thermo Scientific Multiskan FC, Vantaa, Finland) at 405nm. The cut-off value (0.3) was calculated as two average OD values of pooled serum samples of the control groups taken before challenge34.
Virus neutralization testSerial two-fold dilutions (1:2 to 1:512) of each heat-inactivated serum samples were incubated for 1h with ∼100 TCID50 of the EHV-1 AR8 strain, and then 100μl of RK-13 (3×105 cells/ml) was added and incubated at 37°C in a 5% CO2 atmosphere. Each sample was evaluated in quadruplicate. The titers were expressed as the reciprocal of the highest serum dilution that prevented EHV-1 cytopathic effects in 50% of the wells.
Immunoblot AnalysisThe soluble antigen used for ELISA was separated by electrophoresis in 10% SDS–PAGE, transferred to a nitrocellulose membrane and incubated with the 1:100 dilution of each serum sample. Then, the membrane was incubated with an Anti-Mouse IgG Peroxidase diluted 1:800 and revealed with chromogen 3,3-diaminobenzidine tetrahydrochloride (DAB) (Sigma-Aldrich, St Louis, MO, USA).
Infectivity titration and PCRThe right lung of each mouse was processed by preparing a 10% (w/v) homogenized suspension in M-MEM and clarified by centrifugation at 6000 g for 20min. Ten-fold serial dilutions of lung homogenates in M-MEM were inoculated onto preformed cell monolayers and checked for 1 week, and samples without cytopathic effect were re-passaged twice before being considered negative. The viral titers in positive lungs (log TCID50/ml) were calculated by the Reed and Muench method27 at 96h. Positive and negative samples by viral isolation were also processed, using a commercial kit (Wizard Genomic DNA Purification Kit, Promega, Madison, WI, USA) for DNA extraction and subsequent PCR detection of gC14.
Histology and immunohistochemistrySamples of left lungs of all groups were fixed in 10% neutral buffered formalin and processed using standard techniques for histological studies. Five sections of 3-μm of the lungs of each animal in each group were stained with hematoxylin and eosin. Some sections were used for immunohistochemistry (IHC) to detect EHV-1 antigens or IgA, following protocols previously described40. Briefly, EHV-1 antigens were detected using a primary rabbit polyclonal anti-EHV-1 rabbit serum produced in our laboratory, diluted 1/1600 in PBS with BSA 0.1%, followed by the secondary anti-rabbit EnVision® detection system + HRP (DakoCytomation, Carpinteria, CA, USA). To detect IgA, we used direct IHC using a primary Goat Anti-Mouse IgA antibody labeled with peroxidase (Southern Biotech, Birmingham, AL, USA). The reaction was revealed with DAB (Sigma-Aldrich, St Louis, MO, USA) and 10 fields of each section were analyzed for each cut.
Mucosal immune responseThe specificity of mucosal immune response was analyzed in a new experiment of mice immunized with the same protocol used for group E (Table 1). Six mice were immunized with two doses of 15μg of gD in 50μl of PBS by the i.n. route. In addition, a control group (six mice) received 50μl of PBS intranasally. Bronchoalveolar lavages (BAL) were collected at 7 and 14 days (n=3 each time) after the last immunization. Mice were killed by exsanguination, the trachea was exposed and BAL was performed with a catheter by injecting 0.5ml of PBS. The fluids recovered were clarified immediately by centrifugation at 8000 g for 10min and the supernatants were stored at −70°C. The mucosal immune response specific to gD of EHV-1 was measured by the described indirect ELISA. Undiluted BAL samples were used, Anti-mouse IgA peroxidase (Sigma-Aldrich, St Louis, MO, USA) was used as secondary antibody and an ABTS (2,20-azino-di-[3-ethyl-benzothiazoline-6-sulphonic acid]) solution containing H2O2 was used as a substrate indicator. The cut-off value for the optical density (OD) was determined as two average OD values of BAL sample of control group.
Statistical analysisBody weights and virus titers in the lungs of mice were evaluated to verify their normal distribution by the Shapiro–Wilk test. Then, body weights were analyzed with one-way ANOVA, with repeated measures for each mouse nested in days, followed by Bonferroni's post hoc test of pairwise comparisons of marginal linear predictions. Results of the virus neutralization test were analyzed with a nested two-way ANOVA followed by the pairwise comparisons of marginal linear predictions (Bonferroni's post hoc test). Virus titers in lungs were analyzed with a two-way ANOVA in a nested design model, using the STATA 11IC software. A p-value of 0.05 was considered significant.
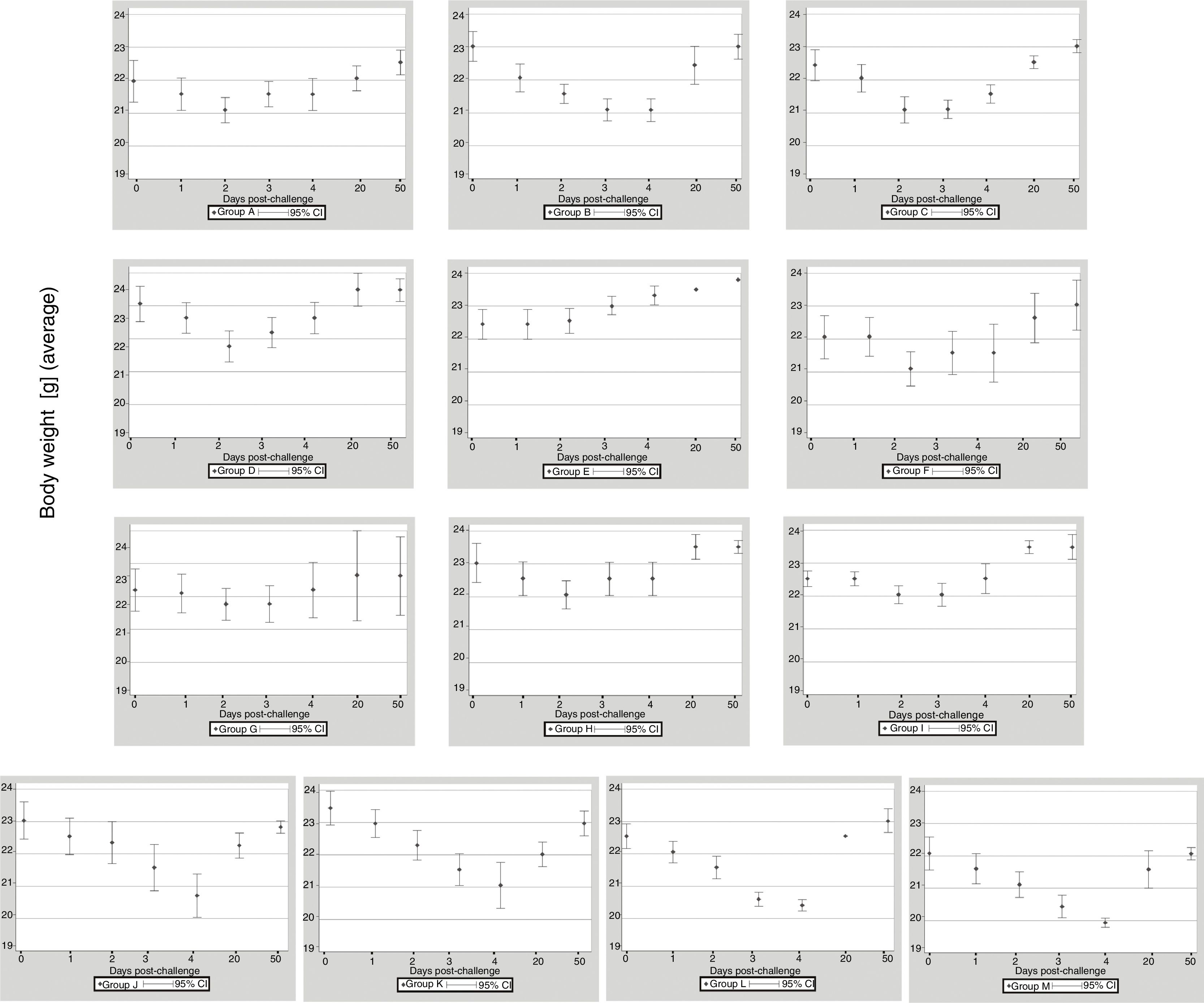
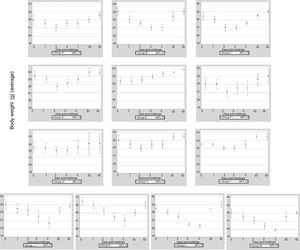
ResultsClinical assessmentThe 13 groups of mice immunized with EHV-1 recombinant gD using different routes and adjuvants were challenged with the EHV-1 AR8 strain. After challenge, all mice in group B (gD, s.c. route) and the control groups (J, K, L and M) showed some clinical signs of infection such as ruffled fur, hunched posture, crouching in corners and dyspnea. Body weights before challenge were normally distributed and showed no differences among the groups (one-way ANOVA, p>0.05). All mice in group B showed a decrease in body weight at 2 days pc, and only began to recover their weight as from day 5 pc. Mice in group E (gD, i.n. route) showed no clinical signs or body weight loss. In the remaining immunized groups (groups A, B, D–I) some mice in each group showed slight clinical signs and body weight loss at 1–2 days pc and began to recover their body weight as from day 3 pc (Fig. 1). Body weight loss was significantly lower (p<0.05) by ANOVA on days 3 and 4 pc in the groups immunized with Freund's adjuvant compared to the groups immunized with the other adjuvants. Bonferroni's post hoc analysis showed significant differences between immunized groups and controls (p<0.05).
Weight of BALB/c (n=10 each group) mice immunized with EHV-1 gD and challenged with ∼106.3 TCID50/50μl of EHV-1 AR8. Each point represents the average of n=10 at day 0 and 1 post-challenge (days 40–41 post-immunization), n-2 at days 2–4 post-challenge (days 42–44 post-immunization) and n=2 at days 20 and 50 post-challenge (days 60 and 90 post-immunization).
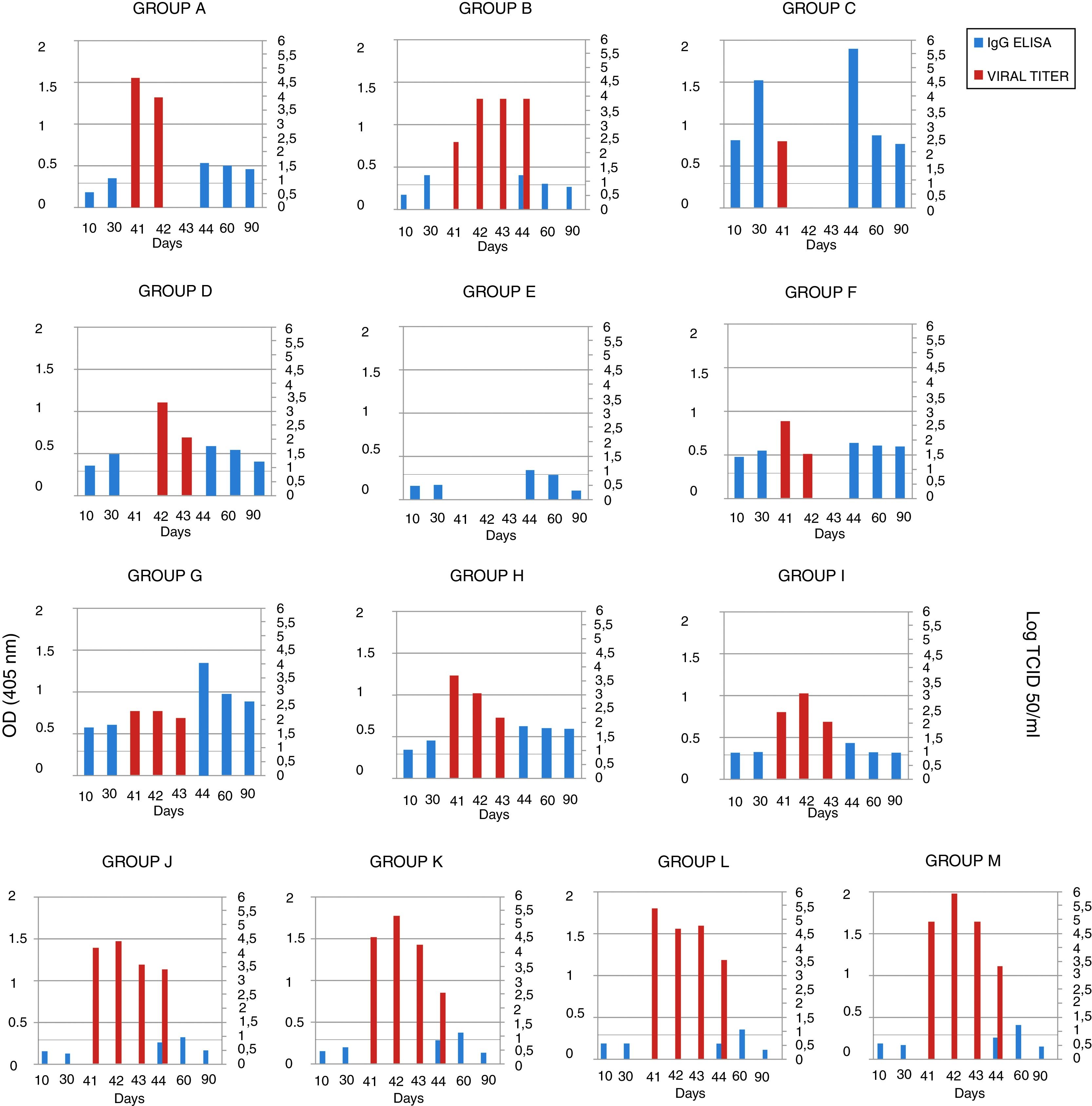
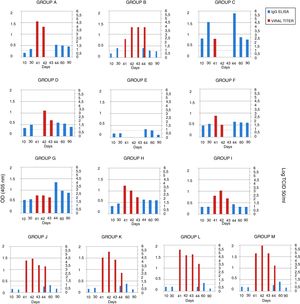
The serum samples were analyzed by ELISA, and IgG was not detected in the mice in groups A, B and E (groups immunized with gD without adjuvant) 10 days after the first immunization. After the second immunization, antibodies were detected in the mice of group A (gD, i.p. route) and B (gD, s.c. route), and were undetectable in sera from the mice of group E (gD, i.n. route). Only at 4 days pc serum IgG was detected in group E. In the remaining immunized groups, antibodies were detected after the first immunization and remained detectable until the end of the experiment. IgG detection in sera from mice in the control groups (groups J–M) was negative 10 days after each immunization and positive 20 days pc (Fig. 2). Serum IgA was not detected in any group.
EHV-1 gD specific ELISA antibody response and viral titers from BALB/c mice immunized and challenged. The mice were immunized twice, 20 days apart, with different formulations of purified recombinant EHV-1 gD. Pooled blood serum samples of each group were taken 10 and 30 days later, and, 40 days after immunization mice were challenged with ∼106.3 TCID50/50μl of EHV-1 AR8. Lung samples of each mouse were collected 41, 42, 43 and 44 days after the first immunization (days 1, 2, 3 and 4 post-challenge), and serum sample at 44, 60 and 90 days post-immunization. Serum samples were analyzed by ELISA. Viral titers from lungs were calculated by the Reed and Muench method and expressed as log10 TCID50/ml. Data represent the average titer obtained from two lungs individually processed. Horizontal lines of each graphic indicate the cut-off value of the assay. CFA, complete Freund's adjuvant; S, specol; M, Montanide ISA 206; i.p., intraperitoneal; s.c., subcutaneous; i.n., intranasal. ELISA absorbance values at 405nm and viral titers are plotted on the primary y-axis and secondary y-axis, respectively.
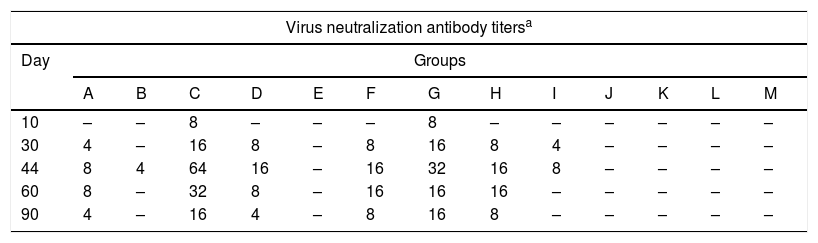
The antibody titers obtained by the virus neutralization test for each group at each time point are shown in Table 2. In groups C (gD+CFA, i.p. route) and G (gD+specol, s.c. route) neutralizing antibodies were detected after the first immunization, and the titers showed an increase at 4 days pc, which remained detectable after the first immunization until day 90, and higher than in the remaining groups.
Virus neutralizing antibodies detected in mice immunized with purified recombinant Equid alphaherpesvirus 1 gD, different adjuvants and inoculation routes.
| Virus neutralization antibody titersa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Groups | ||||||||||||
| A | B | C | D | E | F | G | H | I | J | K | L | M | |
| 10 | – | – | 8 | – | – | – | 8 | – | – | – | – | – | – |
| 30 | 4 | – | 16 | 8 | – | 8 | 16 | 8 | 4 | – | – | – | – |
| 44 | 8 | 4 | 64 | 16 | – | 16 | 32 | 16 | 8 | – | – | – | – |
| 60 | 8 | – | 32 | 8 | – | 16 | 16 | 16 | – | – | – | – | – |
| 90 | 4 | – | 16 | 4 | – | 8 | 16 | 8 | – | – | – | – | – |
Virus neutralizing antibodies detected in groups of mice at different times (10, 30, 44, 60 and 90 days) post-immunization with purified recombinant Equid alphaherpesvirus 1 gD, different adjuvants and inoculation routes.
The immunoblot analysis of the serum samples recovered after the second immunization and pc from mice immunized with gD+adjuvants showed a single band at 65KDa in EHV-1 polypeptide preparations (Supplemental data).
Viral isolation, infectivity titration and PCRThe lung samples and heparinized blood were processed for virus isolation and DNA extraction for detection by PCR. Virus isolation from the lungs and DNA detection in plasma rich in leukocytes were positive on day 1 pc in all groups, except in group D (gD+CFA, s.c. route), in which virus isolation was positive on day 2 pc, and in group E (gD, i.n. route), which was negative on all days pc. Virus isolation from the lungs of all the mice in group B (gD, s.c. route) was positive up to day 4 pc, and the titers were significantly lower than those in the control groups (groups J–M) (two-way ANOVA). Viral clearance from the lungs of mice in the group C (gD+CFA, i.p. route) was observed at 2 days pc, whereas viral clearance in groups A (gD, i.p. route) and F (gD+specol, i.p. route) occurred at 3 days pc; however, the virus titer was lower in group F than in group A. The viral titers of the remaining groups varied according to the immunization route and the use or not of adjuvants; however, they were lower than those in the control groups. Results of virus isolation were confirmed by PCR. All data are shown in Figure 2.
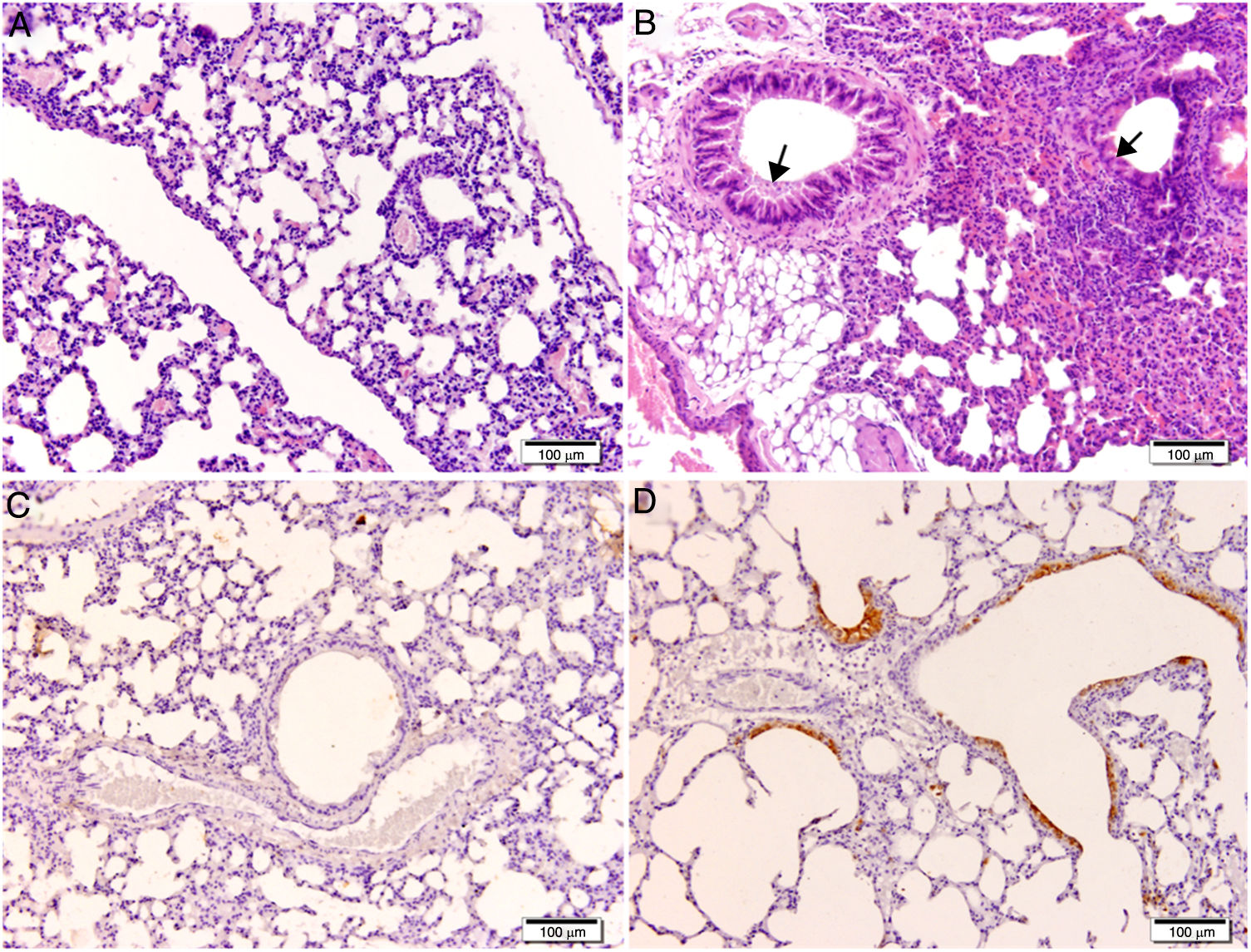
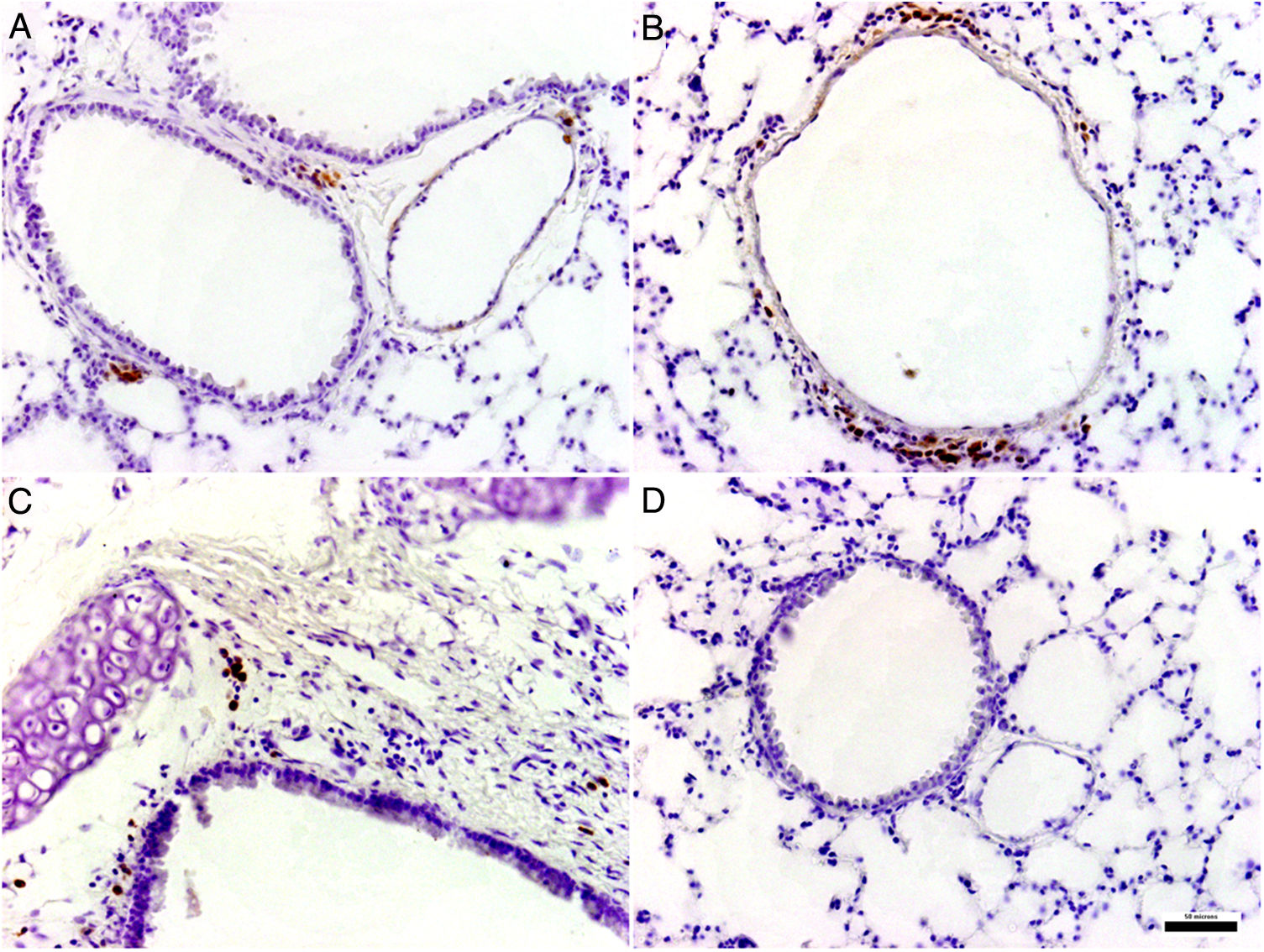
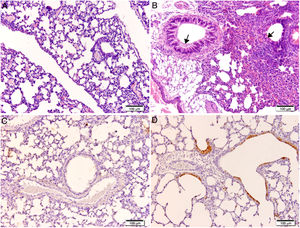
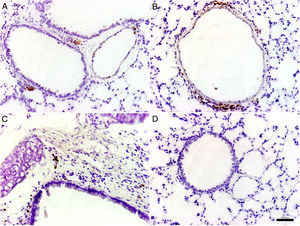
Histological and immunohistochemical studiesHistological studies of the left lungs of the control mice (groups J–M) challenged with the EHV-1 AR8 strain showed infection foci consisting of inflammatory infiltrate, loss of normal alveolar architecture, and desquamation and necrosis of bronchial, bronchiolar and alveolar epithelia (Fig. 3B). Positive IgA cells were not detected by IHC (Fig. 4D), whereas viral antigens were found in bronchial and bronchiolar epithelia (Fig. 3D). Lungs of mice in group E (gD, i.n. route) showed no lesions (Fig. 3A), the IHC for viral antigens was negative (Fig. 3C), and positive IgA cells were observed at 2 and 3 days pc, mainly in peribronchial and perivascular tissues (Fig. 4A–C). In the remaining groups, immunized syncytia and eosinophilic inclusion bodies were observed in the bronchiolar epithelium, with neutrophilic and mononuclear infiltrate around blood vessels and the respiratory tract. Moreover, viral antigens were detected in bronchial and bronchiolar epithelia, but no positive IgA cells were found.
Histopathology and immunohistochemistry. Hematoxylin and eosin staining. (A) Lung without lesions of intranasally immunized mice challenged with the EHV-1 AR8 strain (group E, gD, i.n. route); (B) Representative lesions observed in lungs of non-immunized mice challenged with the EHV-1 AR8 strain (groups J, K, L and M) and mice immunized by systemic routes (groups A and B). Infection foci consisting of inflammatory infiltrates and desquamation of bronchial and bronchiolar epithelia are shown (arrows). Immunohistochemical detection of EHV-1 antigen (diaminobenzidine chromogen and hematoxylin counterstaining); (C) Negative immunostaining of EHV-1 antigens in the lungs of intranasally immunized mice challenged with the EHV-1 AR8 strain (group E: gD by i.n. route); (D) Positive immunostaining of EHV-1 antigens in the lungs of non-immunized and challenged mice (control groups J, K, L and M) and mice immunized by systemic routes (groups A and B) in bronchial and bronchiolar epithelia.
BAL fluid of BALB/c mice immunized with two doses of EHV-1 gD by i.n. route was analyzed by ELISA. The intranasal administration induced a specific immune response to gD of EHV-1 with presence of IgA antibodies in BAL at two evaluated times, whereas IgA production was not detected in the control groups (Supplemental data).
DiscussionEHV-1 infections cause significant negative economic impact on equine production and although commercial vaccines and new alternative vaccination strategies have been developed and evaluated, none of them are effective22. In this work, we studied the immune responses induced by purified recombinant EHV-1 gD and its protective effect on mice challenged with the EHV-1 AR8 strain. To this end, we evaluated different routes of immunization and adjuvants.
Studies evaluating different immunogens, such as infected cells with recombinant baculovirus25,33, DNA encoding gD with recombinant baculovirus-expressed protein30, or gD expressed in E. coli39,41,42, have shown high titers of ELISA and neutralizing antibodies when using systemic routes of immunization. Our results are in accordance with these studies, since we showed that the gD protein combined with adjuvants was able to induce a specific serum antibody response in mice immunized using systemic routes. Conventional adjuvants, such as Freund's Adjuvant, show general immunostimulatory capabilities that have not been surpassed by any other adjuvant; however, this property has been associated with local inflammatory reactions31. Our results confirm the immunogenic potential of this adjuvant and showed that gD-CFA formulations induce high neutralizing antibody titers. We evaluated two alternative commercial mineral oil adjuvants with fewer reported side effects: Montanide ISA 20616 and specol31. In contrast to other authors16, adjuvant Montanide ISA 206 did not induce significant differences in neutralizing antibody response when compared to the group immunized with gD alone. On the other hand, it has been reported that specol induces an antibody response against several antigens in rabbits and mice, comparable with Freund's Adjuvant31. In our study, specol showed high efficacy to induce neutralizing antibody response and persistence when the s.c. route was used. This variable response may be explained by differences in the effectiveness of the combination of adjuvants with certain antigens31.
To evaluate whether gD induces protective immunity against infection, immunized mice were challenged with EHV-1 AR8 and viral isolation from the lungs was performed. We observed faster viral clearance of the lungs in all the groups of mice immunized by systemic routes when compared to their controls, except in those mice immunized by the s.c. route without adjuvants (group B). Moreover, all these groups of mice showed clinical signs of disease. The results obtained here are consistent with previous data in mice immunized with different immunogens25,29,33. Virus was not detected in the lungs of the intranasally immunized mice (group E) in any sample taken after the challenge, while high levels of virus were detected in the lungs of the control mice. In addition, the mice in group E showed no lesions in their lungs, IgA was detected by IHC, and viral antigen detection by IHC was negative. Interestingly, total protection from the challenge was observed only in the intranasally immunized mice. The other routes showed partial protection. Although we detected a serum antibody response when gD was parenterally administered with adjuvants, this response did not prevent the virus entry into the lungs and the production of IgA was not detected in the samples analyzed by IHC. In the intranasally immunized mice, the absence of serum IgG and IgA may indicate that the protection was mainly provided by mucosal immunity. This result was confirmed with a new assay in which mice were intranasally immunized to detect IgA response specific for gD in BAL. Previously, we have also observed a protective response of i.n. recombinant gD immunization in pregnant mice challenged with three EHV-1 strains, although in that work we did not evaluate the specific response to IgA13.
Local virus-specific mucosal immune responses are believed to represent a first line of defense against EHV-1 infection, and may prevent EHV-1 leukocyte-associated viremia, which is a critical step in the pathogenesis of EHV-1 abortion2. It is known that secretory IgA blocks the interaction between cellular receptors and pathogens, and that i.n. immunization stimulates mucosal immunity. In this sense, Zhang et al.41,42 studied the immunogenic potential using the i.n. immunization route of a recombinant EHV-1 gD expressed as a glutathione –S-transferase (GST) fusion protein in E. coli, and found that the generation of an appropriate immune response at the potential site of infection may provide the basis for an effective subunit vaccine. Park and Chang26 showed that i.n. immunization of mice with a fusion protein against the Human respiratory syncytial virus and the influenza virus confers complete protection to both viral infections. A local secretory IgA response in the upper respiratory tract mucosa may prevent or reduce the initial attachment of the virus3, as has been shown after i.n. immunization against the influenza virus18,38. Ferrer et al.8 reported that a recombinant modified vaccinia virus Ankara (MVA) vector expressing the secreted version of gD of Bovine herpesvirus-1 is effective to induce IgA. In addition, in contrast to our results, these authors also reported the systemic production of IgG after i.n. immunization, but they used two doses of MVA-gD combined with cholera toxin. In our study, IgA production was detected by IHC in the lungs and by ELISA in BAL of intranasally immunized mice; however, negative results were obtained for serum IgG and IgA, probably because mucosal adjuvants were not used for the immunization. These results suggest that intranasal vaccination provides protection against EHV-1 infection through a secretory immune response in the respiratory tract, probably due to its ability to prevent viral penetration and cell-to-cell spread in the nasopharyngeal mucosa. EHV-specific cytolytic cell immune responses could have also contributed to the elimination of EHV-1 at a primary replication site5. However, as in this work gD was used without a mucosal adjuvant, we believe that this response was not stimulated, although this was not evaluated.
The present results provide additional information with respect to immunization routes, mainly the intranasal one. Further studies are needed to evaluate the immune response in the upper respiratory tract when immunizing with gD by the i.n. route with mucosal adjuvants. In addition, our results will be used to propose new strategies to test gD immunization in natural hosts, for example, the use of the intranasal immunization route in foals before weaning in order to improve immune protection, since at this stage, the decrease in colostral antibodies makes animals more susceptible to EHV-1 infection.
Conflict of InterestThe authors declare that they have no conflicts of interest.
We thank Professor Miguel Ayala (Laboratory of experimental animals, Faculty of Veterinary Sciences, UNLP) for his professional assistance. We also thank the technicians R. Mario, C. Leguizamón and A. Conde for their support. This study was supported by grants from ANPCyT (PICT 2011-1123), Department for Science and Technology of the UNLP, the National Academy of Agronomy and Veterinary and CIC-PBA.