Urinary tract infections (UTIs) are a common health concern. Urine culture is the “gold standard” for UTI diagnosis but takes 48h. Rapid methods like dipstick tests are used as point-of-care tests. However, their sensitivity and specificity are variable. In this work, a rapid immunochromatographic test (IT) for detecting Escherichia coli in urine was developed, and its performance was evaluated in urine samples from patients with suspected UTI. The “universal lateral flow assay kit” was employed using an E. coli capture antibody. One hundred and five (105) urine samples were analyzed using the IT, dipstick test, and urine culture. The sensitivity of the IT was 74.5%, specificity 88.9%, positive predictive value (PPV) 86.3%, and negative predictive value (NPV) 78.7%. The combination of the IT with the dipstick test increases sensitivity to 94.1%, specificity to 66.7%, PPV to 72.7%, and NPV to 92.3%. Using the IT for detecting E. coli in urine could be a valuable technique for UTI screening, showing better specificity and diagnostic precision but lower sensitivity than the dipstick test. Based on these results, we propose that the combined use of both screening techniques would allow a rapid and more precise diagnosis of UTI, rationalizing the indication for empirical antibiotics.
Las infecciones del tracto urinario (ITU) son un problema de salud frecuente. El urocultivo es el gold standard para el diagnóstico de ITU, pero demora unas 48horas. En muchos centros de atención se emplean los métodos rápidos, como la tira reactiva. Sin embargo, la sensibilidad y la especificidad de esas técnicas son variables. En el presente trabajo se desarrolló un test inmunocromatográfico (TI) rápido para la detección de Escherichia coli en orina y se evaluó su performance en muestras de orina de pacientes con sospecha de ITU. Para el desarrollo del test se utilizó el kit Universal Lateral Flow Assay, junto con un anticuerpo de captura para E. coli. Se analizaron 105 muestras de orina empleando el TI, la tira reactiva y el urocultivo. La sensibilidad del TI fue del 74,5%, su especificidad del 88,9%, el valor predictivo positivo (VPP) del 86,3% y el valor predictivo negativo (VPN) del 78,7%. La combinación del TI con la tira reactiva incrementa la sensibilidad al 94,1% y el VPN al 92,3%, aunque reduce la especificidad al 66,7% y el VPP al 72,7%. El test de TI para la detección de E. coli en orina puede ser sumamente útil como técnica de cribado de ITU, ya que posee mejor especificidad y precisión diagnóstica que la tira reactiva, pero una menor sensibilidad. De acuerdo con los resultados obtenidos, proponemos el uso de ambas técnicas de cribado en conjunto para una mayor precisión diagnóstica en menor tiempo, lo que, a su vez, disminuirá el uso de antibióticos empíricos.
Urinary tract infections (UTIs) are a common health concern that affects approximately 50% of all women and 12% of men and children6,9. About 20% of these women will have a recurrence in the next 6–12 months, and 30% will have more than one episode of UTI7. The costs of treating UTIs are high, about 3.5 billion dollars annually only in the United States. More than 11 million consultations for UTI were reported to the health system, 1.7 million in emergencies, and nearly half a million resulting in hospital admissions in that country in 20063,12.
Uropathogenic Escherichia coli (UPEC) is the most frequent etiological agent5. UPEC is detected in more than 80% of UTI cases, and its frequency is similar across different age groups and geographical areas5,19. The other most frequently isolated microorganisms are Klebsiella pneumoniae (6%), Staphylococcus saprophyticus (6%), and Proteus mirabilis (4%)20.
UTI diagnosis is confirmed by the presence of symptoms and signs such as painful urination with increased frequency, incontinence, fever, abdominal and lower back pain, and the detection of bacteria in urine in significant quantities16,18,22.
Urine culture is the “gold standard” technique for UTI diagnosis16,18,22. Significant bacteriuria is defined by the presence of 100000 or more CFU/ml of a unique microorganism when the urine specimen is collected by midstream. This technique requires at least 24h for colony growth and another 24–36h for bacteria identification and antibiotic susceptibility studies14,16. Meanwhile, the patient receives antibiotic treatment empirically without the confirmation of infection. Thus, contributing to the increasing antimicrobial resistance observed among isolated UTIs13. Another disadvantage of uroculture is the need for trained staff and a well-equipped microbiology laboratory.
Rapid methods for UTI screening, such as dipstick tests, are used as point-of-care tests8,11. The dipstick test presents the advantages of not requiring equipment or qualified staff; results are obtained in 2min and are not expensive. Nitrite, leukocyte esterase, specific gravity, pH, protein, glucose, ketones, bilirubin, urobilinogen, and blood are evaluated in the dipstick strip. For UTI diagnosis, nitrites and leukocyte esterase are the parameters that show greater accuracy24. However, the sensitivity and specificity of the dipstick test are very heterogeneous4.
In recent years, new technologies have been developed for the diagnosis of UTI, such as MALDI-TOF (matrix-assisted laser desorption ionization-time of flight mass spectrometry), exhibiting a sensitivity of 79.2% and specificity of 73.5%1. Furthermore, technologies such as flow cytometry (Sysmex UF1000), real-time PCR, and spectroscopy techniques are developed for UTI diagnosis, but the need for expensive equipment and qualified staff limits the applicability of these diagnostic methods2. On the other hand, immunochromatographic tests (IT) are practical options as point-of-care tests, being easy-to-use and interpret.
In this study, we developed a rapid IT for detecting E. coli in urine and evaluated its performance in urine samples from patients with suspected UTI. Its use in combination with a dipstick test would allow faster and more accurate diagnosis of UTI, improving diagnosis and rationalizing empirical misuse of antibiotics.
Materials and methodsBacterial strainsDifferent strains were used in order to develop the immunochromatographic test. E. coli ATCC 25922 strain was grown in Luria Bertani broth (LB) and kept at −80°C until use. UPEC strains were obtained from a collection of clinical isolates previously characterized by our work group19. P. mirabilis 292125, Klebsiella spp, Enterobacter spp, S. saprophyticus, and S. aureus were used for cross-reactivity evaluation and grown in LB.
Development of the immunochromatographic testThe IT development was divided into two phases: the selection of the best capture and detection antibodies (Abs) and IT setup.
Different commercial Abs against E. coli ATCC 25922 or lipopolysaccharide (LPS) were evaluated using ELISA. Briefly, high-affinity 96-well ELISA plates were sensitized with an E. coli suspension in PBS (105CFU/ml) and incubated at 4°C overnight. After three washes with 0.05% PBS-Tween 20, plates were blocked with 5% skim milk for 1h at 37°C. Following three washes with 0.05%, PBS-Tween 20, the Abs (all of them IgG) to be evaluated were added at 10μg/ml in 5% milk in 0.05% PBS-Tween 20 and incubated for 1h at 37°C. Then, three washes with 0.05% PBS-Tween 20 were performed, and secondary anti-IgG Abs conjugated with peroxidase were added and incubated for 30min at 37°C. Fifty microliters of TMB (3,3′,5,5′-tetramethylbenzidine) was added as a substrate and incubated for 15min at room temperature, and finally, the same volume of 1N HCl was added to stop the reaction. The optical density (OD) results were read at 450nm.
Applying the same protocol, different UPEC strains were used to sensitize the plates, and bacterial suspension in artificial urine was also performed to evaluate whether urine affects the recognition of the different capture and detection antibodies.
The selection criteria for the best Ab used in the IT were based on that with the highest average OD for the detection of E. coli strains in PBS and artificial urine, with less cross-reactivity. Artificial urine was prepared as previously described21.
Cross-reactivity of the selected Abs with other Enterobacteria and gram positive bacteria (Klebsiella spp, Enterobacter spp, P. mirabilis, S. saprophyticus, and S. aureus) was assessed using the same protocol, by sensitizing ELISA plates with different strain suspensions in PBS and artificial urine.
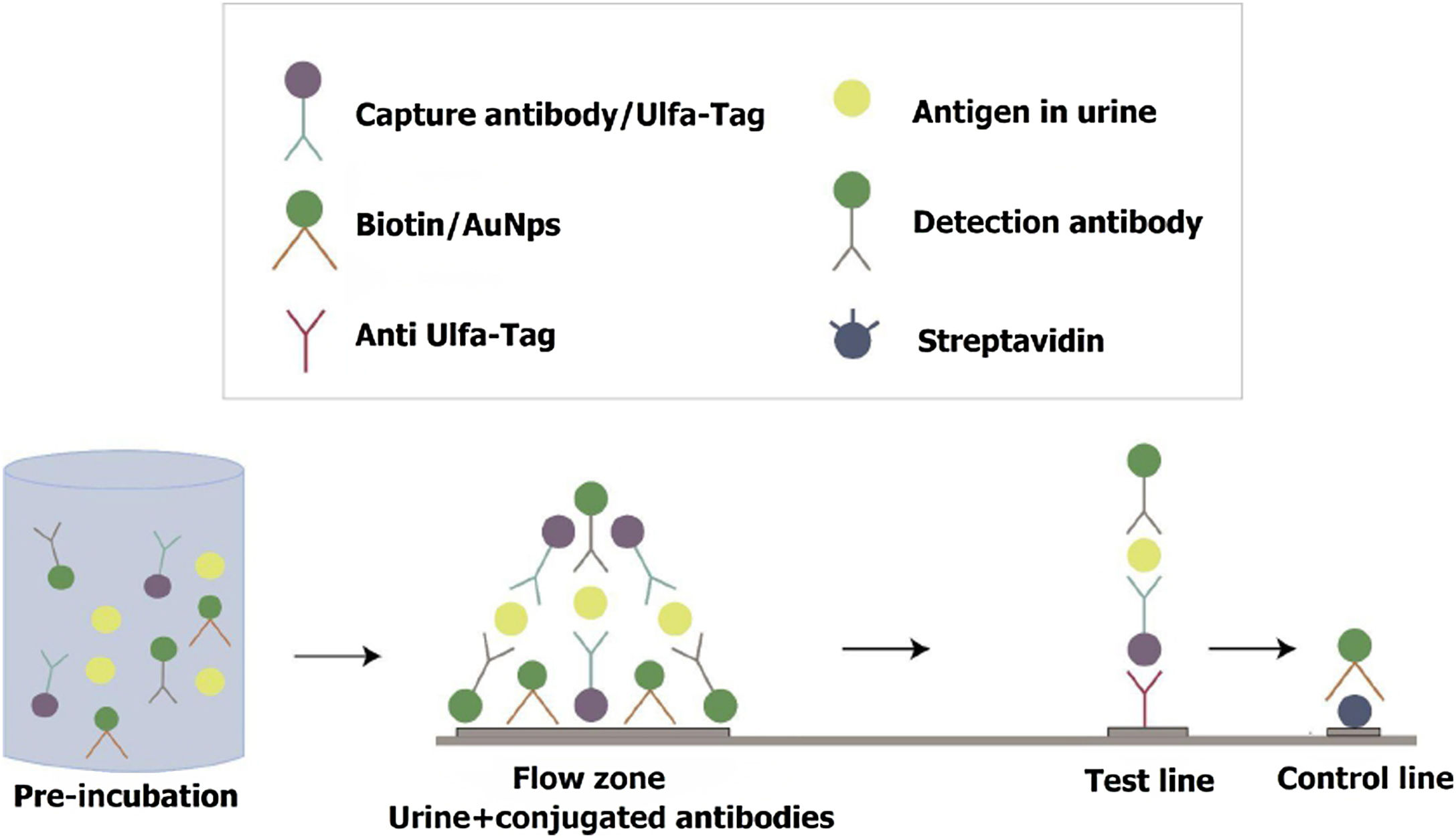
For the IT setup, the “universal lateral flow assay (LFA) kit” (Abcam 270537) was employed. A Universal LFA strip consists of a nitrocellulose membrane with a test line containing anti-Ulfa-Tag Ab and a control line containing streptavidin (IT functionality is explained in Fig. 1).
Immunochromatographic test functioning. Urine sample is preincubated with E. coli Ulfa-Tag-Ab conjugate, AuNps-LPS Ab and Biotin-AuNps conjugate for 5min. Then the mix is run on Universal LFA strip. This strip consists of a nitrocellulose membrane with a test line with anti-Ulfa-Tag Ab that binds the Ulfa-Tag-conjugated capture antibody which binds the analyte in complex with the Gold detection antibody. If the analyte is present a red line appears. The control line contains streptavidin which binds biotin, and a red line confirms that the test is valid.
The first step was to conjugate the selected E. coli capture Ab and LPS detection Ab using the Lightning-Link® Ulfa-Tag kit and the Gold Conjugation kit (40nm, 20 OD) respectively, following the manufacturer's instructions.
In order to conjugate the E. coli capture antibody, the Ulfa-Tag kit was used. First 1μl of LL-Modifier reagent was added for every 10μl of antibody to be labeled and mixed gently. After that, the antibody with the LL-Modifier was resuspended directly onto the vial of Ulfa-Tag mix. Then, the mixture was incubated overnight at room temperature. Finally, 1μl of LL quencher reagent was added for every 10μl of antibody used. The conjugate was ready to be used after 30min of incubation.
For the conjugation of the anti-LPS detection Ab with gold nanoparticles (AuNps-LPS), the anti-LPS Ab was diluted to a concentration of 0.1mg/ml in a final volume of 12μl and mixed with 42μl of reaction buffer. This mixture was added to a vial with gold nanoparticles, vigorously homogenized, and incubated for 15min at room temperature. Lastly, the quencher was added, and the AuNps-LPS was obtained.
For each strip, the test procedure was as follows: preparation of the reaction mix by adding 5μl of E. coli Ulfa-Tag-Ab conjugate (40μg/ml), 5μl of AuNps-LPS Ab 6 OD, 5μl of Biotin-AuNps conjugate 1 OD, and 75μl of the urine sample. The mix was incubated for 5min, and then 80μl were transferred to a 96-well plate, where the strip was placed into the well (with the sample pad dipped into the well) without touching the nitrocellulose. Incubation was allowed for 20min before the results were read. The presence of a line at the test site and another line at the control site is interpreted as a positive result, only one line at the control line is a negative test, and the absence of a control line means that the test is not valid and should be repeated.
In order to determine the limit of detection of the IT test, dilution series of E. coli ATCC 25922 from 106–102 CFU/ml were used.
Different E. coli strains and other gram negative and gram positive species in urine from patients with confirmed UTI by these agents were tested with the IT. In this first instance, the investigator knew the result of the urine culture. It was carried out to evaluate if the test worked correctly and determine cross-reactions, prior to validation with a larger number of clinical samples.
Validation of the IT testUrine samples from patients with clinically suspected UTIs were randomly selected. Both children and adults, as well as men and women, were included. Urine samples were analyzed by uroculture at the clinical microbiology laboratory of the Centro Hospitalario Pereira Rossell (a tertiary hospital) by independent investigators. The uroculture results were not reported to the investigators who performed the IT. The number of samples to be studied was predefined considering the resources available for the development of the IT. A 1:1 ratio was considered (urine culture positive samples:samples without urine culture development). The minimum number of samples to be processed was 100.
A urine aliquot was analyzed by the dipstick test (URIT 10V, URIT Medical Electronic Co, Guilin, China) according to the manufacturer's instructions, and by the IT at the Microbiology Laboratory of the Instituto de Higiene.
The dipstick test was considered positive for UTI when: leukocyte esterases were 2++ and/or nitrates positive or leukocyte esterases 1+ and blood or proteins in the urine.
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic precision, and positive and negative likelihood ratio (LR) were calculated using OpenEPi. IT and the dipstick tests were compared to the urine culture (gold standard assay).
Ethical approval of human research committeeThe study was approved by the Ethical Committee of the Faculty of Medicine (Exp. No. 070153-000360-19) and followed all the national regulations governing human research. Informed consent was obtained from participants before their involvement in the present study.
ResultsSelection of capture and detection Abs and IT developmentThe binding ability of four different Abs against whole E. coli or LPS was tested by ELISA. The average OD results of the evaluated Abs are shown in Table 1.
Evaluation of different antibodies for uropathogen detection by ELISA.
| Antibody | Immunogen | OD average (450nm) | Selected antibody for IT |
|---|---|---|---|
| Anti E. coli, monoclonal, mouse. LsBIO (LS-C744252/145842) | O from E. coli antigen | 0.035 (Ec) | |
| Anti E. coli, monoclonal, mouse. UsBiological (E3500-19A) | Complete E. coli | 0.004 (Ec) | |
| Anti E. coli, polyclonal, rabbit. Thermo Fisher (PA1-73029) | O and K antigens from E. coli | 1.5 (Ec) | Capture antibody |
| Anti-LPS, polyclonal, goat. UsBiological (L2525) | Lipid from E. coli. Reactivity anti Enterobacteriaceae and Morganellaceae | 0.7 (Kp)1.32 (Ec)1.29 (Pm) | Detection antibody |
OD: optical density; Ec: E. coli; Kp: Klebsiella pneumoniae; Pm: Proteus mirabilis; IT: immunochromatographic test.
Due to its high binding ability and specificity, the anti E. coli Ab (Thermo Fisher, PA1-73029) was selected as the capture Ab. This antibody did not recognize other Enterobacterales species (K. pneumoniae median OD 0.002, P. mirabilis median OD 0.006, Enterobacter cloacae OD 0.004) and S. saprophyticus (median OD 0.002). Anti-LPS (UsBiological, L2525) was selected as the detection Ab due to its high binding capacity.
The IT detection limit was evaluated using E. coli ATCC 25922 serial dilutions in LB and was ≥104CFU/ml.
Fifty-six samples were initially studied by IT and urine culture to evaluate the IT performance and cross-reactivity with other species in urine.
The IT was positive in 20 of 27 (74%) samples that developed E. coli in the urine culture and in one case in a polymicrobial urine culture (with a predominance of E. coli colonies). In those cases with urinary infection of non-E. coli etiology and samples without bacterial growth, the IT was negative (Table 2).
Immunochromatographic test results for E. coli detection and cross-reactivity with other species in urine samples.
| Urine culture (n) | IT positive |
|---|---|
| E. coli (27) | 20 |
| S. saprophyticus (1) | 0 |
| Klebsiella oxytoca (1) | 0 |
| Klebsiella pneumoniae (2) | 0 |
| Klebsiella aerogenes (1) | 0 |
| Polymicrobial (5) | 1 |
| No growth (19) | 0 |
One hundred and five urine samples from patients with clinical suspected UTI were studied by IT, dipstick test, and urine culture. In 51 samples, ≥100000 CFU/ml of E. coli were obtained. The remaining urine cultures showed no bacterial growth. Thirty-eight out of 51 E. coli positive urine cultures were positively detected by IT, while 13 urine cultures with E. coli growth tested negative for IT. Six of the 54 negative urine cultures were positive for IT (Table 3). The sensitivity of the IT was 74.5%, specificity 88.9%, PPV 86.3%, NPV 78.7%, diagnostic precision 81.9%, positive LR 6.7, and negative LR 0.28 compared with the uroculture, the gold standard technique (Table 3).
Performance of the immunochromatographic test compared with urine culture.
| IT | Urine culture (n) | Parameter | Result (%) | 95% CI | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | ||||
| Positive | 38 | 6 | 44 | Sensitivity | 74.5 | 61.1–84.4 |
| Negative | 13 | 48 | 61 | Specificity | 88.9 | 77.8–94.8 |
| Total | 51 | 54 | 105 | PPV | 86.3 | 73.2–93.6 |
| NPV | 78.7 | 66.9–87.1 | ||||
| Diagnostic accuracy | 81.9 | 73.4–88.1 | ||||
IT: immunochromatographic test; PPV: positive predictive value; NPV: negative predictive value.
When the dipstick comparison was assessed, 42 out of 51 positive urine cultures were positive by the dipstick test. Fifteen samples without bacterial growth by urine culture tested positive for the dipstick test (Table 4). Thus the sensitivity of the dipstick test was 82.3%, specificity 72.2%, PPV 73.6%, NPV 81.2%, diagnostic precision 77.1%, positive LR 2.9, and negative LR 0.2 (Table 4).
Dipstick test performance results compared with urine culture.
| Dipstick test | Urine culture (n) | Parameter | Result (%) | 95% CI | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | ||||
| Positive | 42 | 15 | 57 | Sensitivity | 82.3 | 69.7–90.4 |
| Negative | 9 | 39 | 48 | Specificity | 72.2 | 59.1–82.3 |
| Total | 51 | 54 | 105 | PPV | 73.6 | 61–83.3 |
| NPV | 81.2 | 68–89.8 | ||||
| Diagnostic accuracy | 77.1 | 68.2–84.1 | ||||
PPV: positive predictive value; NPV: negative predictive value.
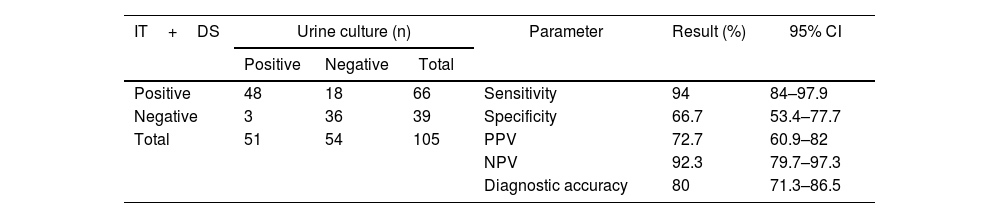
Finally, we analyzed the performance of using both tests (IT and dipstick test) in combination. Forty-eight out of 51 samples with E. coli bacterial growth in urine culture were positive by at least one of the tests (IT and/or dipstick), while 18 out of 54 samples without bacterial growth in the urine culture were positive by one of these tests (Table 5). Sensitivity was 94.1%, specificity 66.7%, PPV 72.7%, NPV 92.3%, diagnostic accuracy 80%, positive LR 2.8, and negative LR 0.08 (Table 5).
Performance results of the immunochromatographic test complemented with the dipstick test and compared with the urine culture.
| IT+DS | Urine culture (n) | Parameter | Result (%) | 95% CI | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | ||||
| Positive | 48 | 18 | 66 | Sensitivity | 94 | 84–97.9 |
| Negative | 3 | 36 | 39 | Specificity | 66.7 | 53.4–77.7 |
| Total | 51 | 54 | 105 | PPV | 72.7 | 60.9–82 |
| NPV | 92.3 | 79.7–97.3 | ||||
| Diagnostic accuracy | 80 | 71.3–86.5 | ||||
DS: dipstick test; IT: immunochromatographic test; PPV: positive predictive value; NPV: negative predictive value.
Antibiotic resistance is a growing concern worldwide. The irrational use of antimicrobials is one of the predisposing factors. About 15% of the antibiotics prescribed in the United States are for UTI treatment. A review of antibiotic prescriptions shows that the use of antibiotics in UTIs was appropriate in only 75% of cases24. One of the main approaches to reduce irrational antibiotic use is to develop a rapid and accurate method for UTI diagnosis. Despite being a frequent reason for consultation, adequate UTI diagnosis continues to be a complex issue. If only clinical symptoms and signs are considered, UTI diagnosis and, therefore, antibiotics would be unnecessary in 25% of cases24. These aspects reinforce the need for accurate, rapid, inexpensive, accessible, and easy-to-use diagnostic UTI tests.
In addition, a study carried out by members of our research group on UTI diagnosis in a pediatric population, found that only 29.6% of the children in whom UTI had been suspected due to clinical symptoms and/or the dipstick test results had microbiological confirmation in the urine culture. In this same population, 35% of children received antibiotics unnecessarily10.
Although the dipstick test is the point-of-care test most frequently used for UTI diagnosis, its accuracy is variable. One of the disadvantages of the dipstick strip is that not all bacteria can reduce urine nitrates to nitrites (only Enterobacterales can do so). Therefore, common uropathogens such as S. saprophyticus, Enterococcus, or Pseudomonas cannot be detected24. Another disadvantage is that leukocyte esterases indirectly detect the presence of pyocytes in urine so that it could be positive in situations other than UTIs, such as vulvovaginitis or urethritis. Different meta-analyses showed major heterogeneity in diagnostic accuracy across studies4. In the meta-analysis of Devillé et al., the sensitivity of the urine dipstick test for nitrites was low (45–60% in most situations) with higher levels of specificity (85–98%)4. The test for nitrites had the highest accuracy in pregnant women, urology patients, and older adults. The sensitivity and specificity for leukocyte esterase were very heterogeneous, 48–86% and 17–93%, respectively4. The presence of both parameters improves sensitivity and specificity (80%)24.
Immunochromatographic tests are a practical option for use as a point-of-care tests, as they are rapid, inexpensive, and easy-to-use and interpret. A kit based on lateral flow immunoassay for the rapid detection of gram positive and gram negative bacteria in urine, “RapidBac”, is available on the market for veterinary use only (https://rapidbacvet.com/pages/how-to). Stapleton et al. evaluated this test in human urine samples, showing a sensitivity of 86% and specificity of 94% for the detection of bacterial counts greater than or equal to 104 CFU23. Its disadvantage is that it does not identify the etiological agent and does not discriminate between pure or contaminated samples. To the best of our knowledge, this is the first report of a lateral flow test for E. coli detection in urine.
In the present study, we were able to set up a lateral flow IT for the detection of E. coli in urine, with better specificity (88.9%), PPV (86.3%), and diagnostic precision (81.9%) than the dipstick test that is commonly used in clinical settings as a screening test. The IT was positive in 6 out of 54 (11%) samples that showed no bacterial growth in the urine culture. These positive tests were not related to any specific characteristic of the urine (nitrites, leukocytes, Vogel, blood, ketones, bilirubin, glucose). In the last decade, many articles described the presence of a urinary microbiota and the performance of an expanded urine culture with greater capacity to detect microorganisms that are not detected by the standard urine culture15,17. In the standard urine culture, 10μl of urine is spread quantitatively onto 5% sheep blood agar plates and MacConkey agars and incubated aerobically at 35°C for 24h. In the expanded urine culture, the urine volume streaked onto agar plates increases to 100μl, and the culture media and atmospheric conditions differ. One explanation for these cases of positive IT and urine culture without bacterial growth could be the presence of E. coli in low quantities, not detected by conventional urine culture since 7.5 times more urine volume is used for IT.
The advantages of the IT developed in the present study are: (1) the ability to rapidly detect bacteria directly from the urine sample, (2) identifying E. coli, the main etiological agent present in more than 80% of UTI cases, (3) its straightforward interpretation (positive/negative), (4) no need for particular infrastructure or trained staff, and (5) implementation at the site where the patient is being assisted. Another advantage of directly detecting E. coli in the IT is that the selection of antibiotics will be directed to this microorganism, taking into account its antibiotic susceptibility profiles at each site of care.
Our results suggest that the best strategy for UTI screening would be the combined use of IT and the dipstick test, exhibiting excellent sensitivity (94%) and diagnostic precision (80%), compared to the dipstick test alone, whose sensitivity is 82.3%, and diagnostic precision 77.1%. Both tests are quick and can be performed as point-of-care tests without the need for a microbiology laboratory. However, this strategy should be contextualized depending on the clinical situation. In clinically stable patients, who do not urgently require the initiation of antibiotic treatment, the best strategy would be to use IT. If positive, empirical antibiotics would be indicated, due to their high specificity and diagnostic accuracy. If negative, it is advisable to wait for the urine culture results to decide on antibiotic therapy. This strategy would be the most appropriate to avoid the unnecessary use of antibiotics.
This study is a first approach to the development and performance evaluation of an immunochromatographic test for the diagnosis of E. coli in urine. A limitation of the test for its large-scale development is that it requires the use of commercial antibodies and a universal lateral flow kit, which increases production costs.
Further studies are required for its future application, including the use of the IT in different clinical scenarios, with a larger number of patients, and performed by health care staff (physicians, nurses and laboratory personnel) to evaluate its performance on a larger scale.
This technology could also be developed to detect other uropathogens and antibiotic resistance mechanisms. The development of a single IT to detect the most frequent uropathogens as well as mechanisms of resistance would be of great value in clinical practice.
Conflict of interestThe authors declare no commercial or financial conflict of interest.
Thanks to Dr. Ines Mota, Dr. Adriana Varela and Dr. Evangelina Costa for the collaboration in the Microbiology laboratory of the CHPR.
This work was supported by Agencia Nacional de Investigación e Innovación, ANII. FMV_3_2018_1_148363.