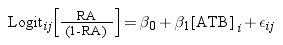A bioassay containing Kluyveromyces marxianus in microtiter plates was used to determine the inhibitory action of 28 antibiotics (aminoglycosides, beta-lactams, macrolides, quinolones, tetracyclines and sulfonamides) against this yeast in whey. For this purpose, the dose–response curve for each antibiotic was constructed using 16 replicates of 12 different concentrations of the antibiotic. The plates were incubated at 40°C until the negative samples exhibited their indicator (5–7h). Subsequently, the absorbances of the yeast cells in each plate were measured by the turbidimetric method (λ=600nm) and the logistic regression model was applied. The concentrations causing 10% (IC10) and 50% (IC50) of growth inhibition of the yeast were calculated. The results allowed to conclude that whey contaminated with cephalosporins, quinolones and tetracyclines at levels close to the Maximum Residue Limits inhibits the growth of K. marxianus. Therefore, previous inactivation treatments should be implemented in order to re-use this contaminated whey by fermentation with K. marxianus.
Se utilizó un bioensayo en placas de microtitulación que contenían Kluyveromyces marxianus para determinar la acción inhibitoria en suero de 28 antibióticos (incluyendo aminoglucósidos, betalactámicos, macrólidos, quinolonas, tetraciclinas y sulfonamidas) contra esta levadura. Para ello, se construyeron curvas dosis/respuesta utilizando 16 réplicas de 12 concentraciones diferentes de cada antibiótico. Las placas se incubaron a 40°C hasta el viraje del indicador de las muestras negativas (5-7h). Posteriormente, se midieron las absorbancias de las células de levadura en cada placa por el método turbidimétrico (λ=600nm), y se aplicó el modelo de regresión logística. Se calcularon las concentraciones que causan el 10% (IC10) y el 50% (IC50) de inhibición del crecimiento de la levadura. Los resultados permitieron concluir que el suero contaminado con cefalosporinas, quinolonas y tetraciclinas en niveles cercanos a los límites máximos de residuos inhiben el crecimiento de K. marxianus. Por lo tanto, deberían implementarse tratamientos previos de inactivación para reutilizar sueros contaminados por fermentación con K. marxianus.
Antibiotics are widely used to treat different diseases of dairy cattle45. Consequently, some antibiotic molecules can be eliminated in feces, urine and milk17.
Whey, the by-product of the dairy industry obtained during cheese making, may contain residues of antibiotics such as penicillin22, amoxicillin and ampicillin19, erythromycin and oxytetracyclines6,22,45, cefquinome and ceftiofur19, ciprofloxacin31 and tetracyclines1.
Among the various industrial uses of whey, it is important to highlight the production of different products24, such as biofuels7,15, bioethanol3,12,35,37,39–41, enzymes5,32, higher alcohols with antibacterial–antifungal properties2,10,11,33, beverages18,20,26,38 and biomass9,30,50,51, among others.
It should be noted that many of these industrial whey applications use yeasts, preferably Kluyveromyces marxianus, due to their ability to ferment lactose4,14,21,28,34,35,40,48. However, residues of antibiotics in whey at concentrations close to the Maximum Residue Limits (MRLs) established by the legislation8,16 could inhibit the fermentative processes of K. marxianus.
Therefore, the objective of the present work was to analyze the inhibitory effect of antibiotics on the growth of K. marxianus through a simple bioassay in microtiter plates.
Materials and methodsA yeast inhibition bioassay in microtiter plates was developed according to the following stages:
- 1.
Yeast: K. marxianus (ATCC 8554) was obtained from the strain collection of the Departamento de Microbiología of the Facultad de Ingeniería Química of the Universidad Nacional del Litoral, Santa Fe, Argentina.
Culture medium consisting of 5g/l yeast extract (Merck Millipore, USA), 30g/l casein peptone (Biokar Diagnostics, France) and 40g/l lactose (Merck Millipore, USA) was adjusted to pH=7.0 and sterilized at 121°C for 15min. Then, the medium was inoculated with K. marxianus and incubated at 40°C for 24h, in order to obtain cells in exponential phase (OD=0.5, λ=600nm).
- 2.
Culture medium with yeast: A semisynthetic matrix made up of antibiotic-free whey deproteinized by heat treatment at 120°C for 20min was used. Then, the culture medium was fortified under sterile conditions with 0.5% yeast extract (Merck Millipore, USA), 3% casein peptone (Biokar Diagnostics, France) and lactose (Merck Millipore, USA) in order to obtain a matrix containing 0.9% protein and 5.0% lactose. Furthermore, the pH was adjusted to 7.0 with NaOH 0.1M. Subsequently, 20% suspension of K. marxianus (ATCC 8554) in exponential phase and 50mg/l of bromothymol blue were added. It should be noted that, the acid–base indicator is used to visualize the endpoint of the incubation of K. marxianus.
- 3.
Antibiotic-fortified solutions in culture medium: Aqueous solutions (1000mg/l) were prepared for the following 27 antibiotics (Sigma Chemical Co., St. Louis, MO, USA): 3 aminoglycosides, 10 beta-lactams, 3 macrolides, 4 quinolones, 3 tetracyclines and 4 sulfonamides. For each antibiotic, 12 concentrations were prepared (Table 1S online Supplementary File) using the culture medium described in “Materials and methods” section.
- 4.
Yeast inhibition bioassay: Two microtiter plates were used for each antibiotic and 16 replicates of the 12 solutions in whey according to “Materials and methods” section were tested. In each well of the microtiter plates, 200μl of antibiotic-fortified medium solution was added using an electronic dispenser (Eppendorf Research® Pro, Hamburg, Germany). Subsequently, the plates were incubated at 40°C until the negative controls turned from blue to yellow. Previous studies revealed that an adequate incubation time is between 5 and 7h, since prolonging this time (e.g. 9h) produces no modifications in the bioassay response.
Subsequently, the microtiter plates were measured at 600nm using a Biotek EL800 reader (BioTek Instruments Inc., Winooski, Vermont, USA) and the Relative Absorbances (RA) in each bioassay were calculated.
The RA were analyzed using the Maximum Likelihood estimation method contained in the Logistic Regression procedure46. The following logistic regression model (logit) was used:
where β0 and β1 are the parameters calculated with the logistic model; [ATB]i is the antibiotic concentration; and ɛij is the model error.For each antibiotic, IC10 and IC50 were calculated as the antibiotic concentrations that reduce 10% and 50% of RA44.
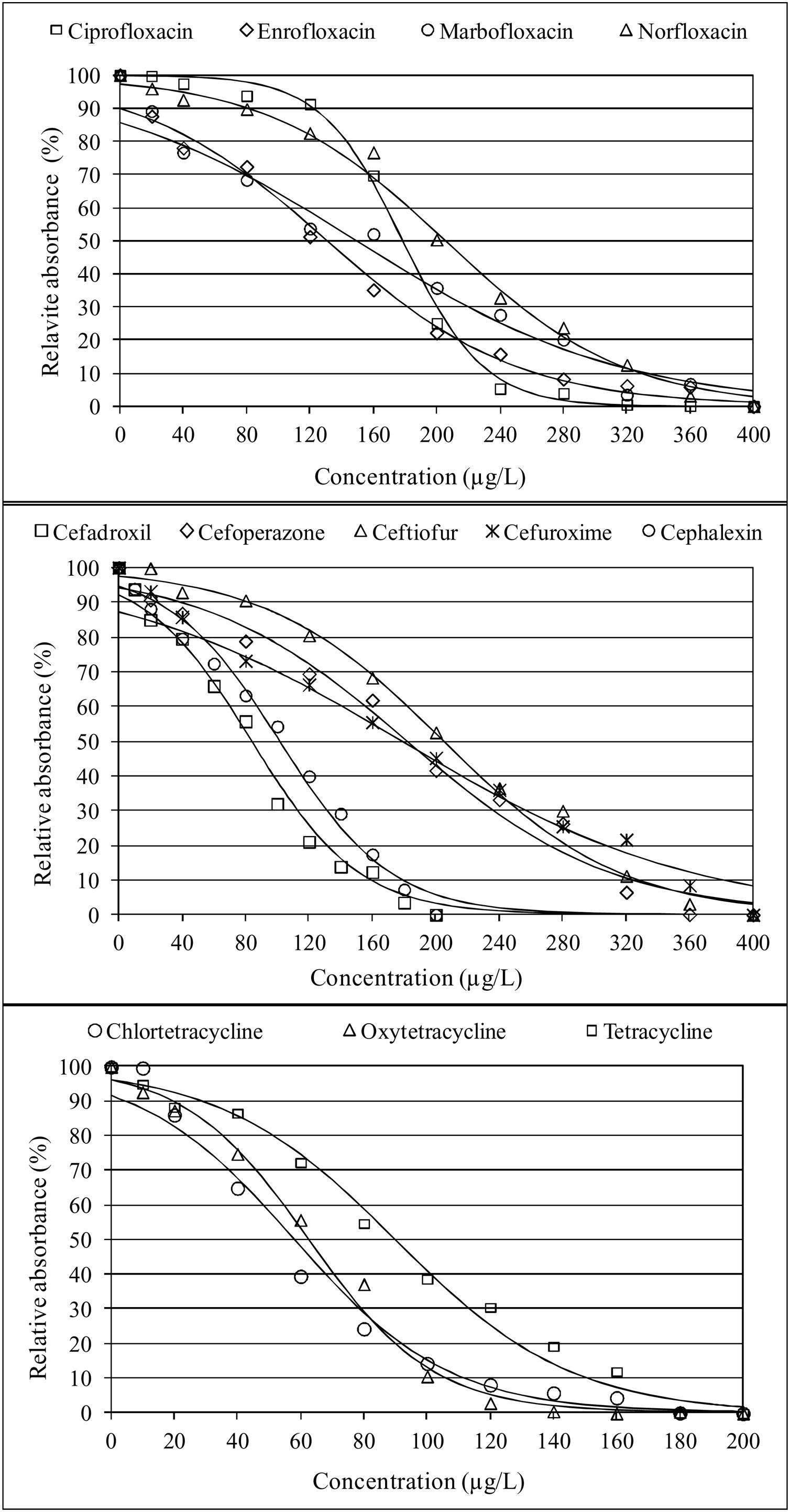
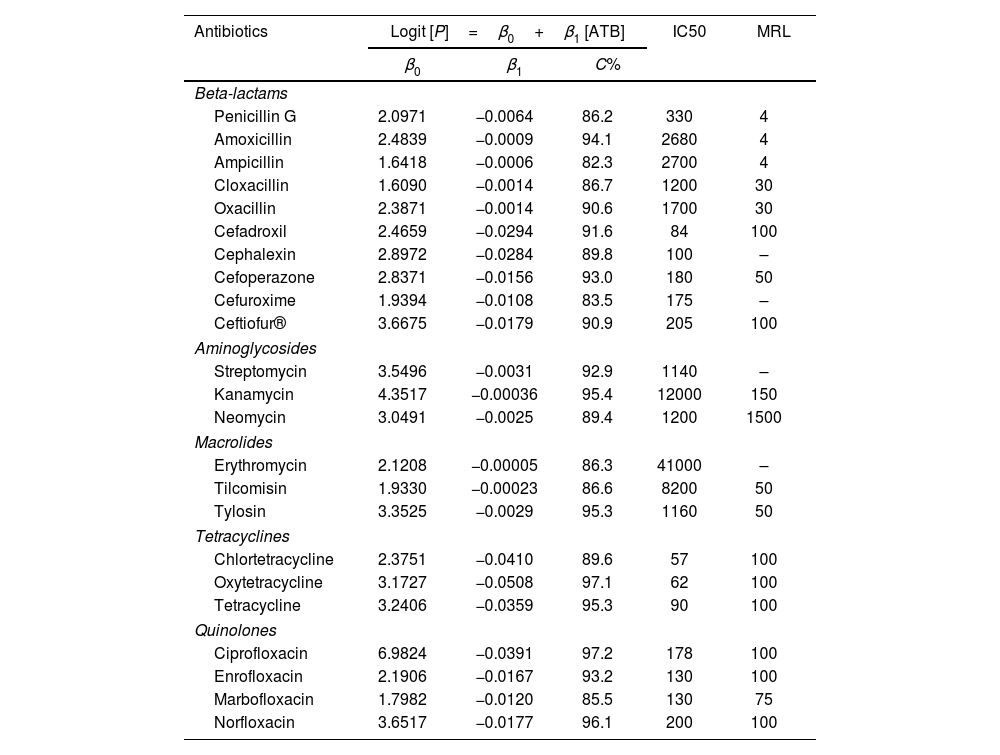
ResultsThe application of the logistic regression model was adequate because the high concordance coefficients were between 82.3% for ampicillin and 97.3% for ciprofloxacin, as observed in Table 1. The values of coefficient β1 represent the decrease in growth of K. marxianus due to the increase in antibiotic concentration. Thus, higher values of this parameter indicate greater inhibition by the antibiotic. The β1 parameters for cephalosporins (from β1, cefadroxil=−0.0294 to β1, cefuroxime=−0.0108), quinolones (from β1, ciprofloxacin=−0.0391 to β1, marbofloxacin=−0.0120) and tetracyclines (from β1, oxytetracyclin=−0.0508 to β1, tetracyclin=−0.0359) were higher than those for the other antibiotics studied (Table 1). To visualize the growth inhibition of K. marxianus due to the concentrations of cephalosporins, tetracyclines and quinolones, dose–response curves were constructed (Fig. 1).
Logistic equations that represent the effect of antibiotic concentrations on the growth inhibition of Kluyveromyces marxianus.
| Antibiotics | Logit [P]=β0+β1 [ATB] | IC50 | MRL | ||
|---|---|---|---|---|---|
| β0 | β1 | C% | |||
| Beta-lactams | |||||
| Penicillin G | 2.0971 | −0.0064 | 86.2 | 330 | 4 |
| Amoxicillin | 2.4839 | −0.0009 | 94.1 | 2680 | 4 |
| Ampicillin | 1.6418 | −0.0006 | 82.3 | 2700 | 4 |
| Cloxacillin | 1.6090 | −0.0014 | 86.7 | 1200 | 30 |
| Oxacillin | 2.3871 | −0.0014 | 90.6 | 1700 | 30 |
| Cefadroxil | 2.4659 | −0.0294 | 91.6 | 84 | 100 |
| Cephalexin | 2.8972 | −0.0284 | 89.8 | 100 | – |
| Cefoperazone | 2.8371 | −0.0156 | 93.0 | 180 | 50 |
| Cefuroxime | 1.9394 | −0.0108 | 83.5 | 175 | – |
| Ceftiofur® | 3.6675 | −0.0179 | 90.9 | 205 | 100 |
| Aminoglycosides | |||||
| Streptomycin | 3.5496 | −0.0031 | 92.9 | 1140 | – |
| Kanamycin | 4.3517 | −0.00036 | 95.4 | 12000 | 150 |
| Neomycin | 3.0491 | −0.0025 | 89.4 | 1200 | 1500 |
| Macrolides | |||||
| Erythromycin | 2.1208 | −0.00005 | 86.3 | 41000 | – |
| Tilcomisin | 1.9330 | −0.00023 | 86.6 | 8200 | 50 |
| Tylosin | 3.3525 | −0.0029 | 95.3 | 1160 | 50 |
| Tetracyclines | |||||
| Chlortetracycline | 2.3751 | −0.0410 | 89.6 | 57 | 100 |
| Oxytetracycline | 3.1727 | −0.0508 | 97.1 | 62 | 100 |
| Tetracycline | 3.2406 | −0.0359 | 95.3 | 90 | 100 |
| Quinolones | |||||
| Ciprofloxacin | 6.9824 | −0.0391 | 97.2 | 178 | 100 |
| Enrofloxacin | 2.1906 | −0.0167 | 93.2 | 130 | 100 |
| Marbofloxacin | 1.7982 | −0.0120 | 85.5 | 130 | 75 |
| Norfloxacin | 3.6517 | −0.0177 | 96.1 | 200 | 100 |
Logit [P]: logistic model; β0, β1: parameters estimated by the model; ATB: antibiotic; C%: concordance percentage; IC50: concentrations producing 50% of inhibition of yeast growth (μg/l); MRL: Maximum Residue Limits (μg/l).
IC50 (μg/l) values in whey were close to those of MRLs (μg/l) in milk for cephalexin (100 vs 100), cefoperazone (180 vs 50), ceftiofur (205 vs 100), chlortetracycline (57 vs 100) oxytetracycline (62 vs 100), tetracycline (90 vs 100), ciprofloxacin (178 vs 100), enrofloxacin (130 vs 100), marbofloxacin (130 vs 75) and norfloxacin (130 vs 100). In contrast, IC50 values of penicillins, aminoglycosides, macrolides and sulfonamides, except neomycin (Table 1), were very high compared to their MRLs (>30 MRLs).
DiscussionWith respect to cephalosporins, Hamilton-Miller23 observed growth inhibition of some pathogenic yeasts and filamentous fungi when analyzing semisynthetic cephalosporins that possess an N-benzyldithiocarbamate side group. Furthermore, an in vitro study to determine the inhibition of yeasts reveals the use of high concentrations (16000μg/ml) of a combination of cefoperazone sodium, sulbactam sodium, and cefradine in proportions of 2:2:329.
In the case of fungal species, Sanyal et al.42 reported that the degradation products of cephalosporins inhibit the growth of Trichophyton mentagrophytes (dermatophytes) and Macrophomina phaseolina (a plant pathogen), but are not effective against Candida albicans (an opportunistic yeast) or Aspergillus niger (a saprophyte).
Therefore, the differences in sensitivity observed between cephalosporins and penicillins (Table 1) in this study could be attributed to the production of penicillinase by K. marxianus, which reduces the effectiveness of penicillins, thus enabling normal yeast fermentation.
With regard to quinolones, growth inhibition of K. marxianus by these antibiotics (Fig. 1) can be attributed to their effect on the topoisomerase enzyme that participates in the relaxation of the DNA double helix. Zhang et al.52 described genotoxic effects against non-target species due to the binding of quinolones to topoisomerase. Similarly, Stergiopoulou et al.47 reported growth inhibition of Candida spp. by fluoroquinolones.
Moreover, in an in vivo study involving fluoroquinolones, Dalhoff13 emphasizes the effectiveness of topically administered moxifloxacin and gatifloxacin in treating infections caused by Candida spp. Additionally, in an in vitro study using a paper disk diffusion test, the author observes that gatifloxacin and sparfloxacin inhibit Trichophyton rubrum, Fusarium solani and C. albicans.
The in vitro study involving Fusarium spp. isolates, conducted by Kawakami et al.27, reported inhibitory effects at high concentrations of fluoroquinolones: 750mg/l of gatifloxacin, 312.5mg/l of levofloxacin, and 1250mg/l of moxifloxacin.
Finally, regarding tetracyclines, their effect on K. marxianus growth (Fig. 1) can be attributed to the inhibition of yeast protein synthesis, since these substances interfere with the binding of aa-tRNA to the 30S ribosomal subunit49. In this regard, Oriel and Waterworth36 highlighted that minocycline can inhibit the growth of the yeast C. albicans, while Schwartz et al.43. suggested that the combined effect of polymyxin B and tetracycline can inhibit the growth of C. albicans and Saccharomyces cerevisiae. Similarly, Hooper et al.25. observed synergism in the inhibition of Candida spp. when using combinations of doxycycline with fluconazole and tigecycline with fluconazole.
ConclusionIn summary, a simple, fast (6h) and low-cost bioassay was developed to analyze the inhibitory effect of antibiotics on the growth of K. marxianus. Results showed that whey contaminated with cephalosporin, quinolone or tetracycline residues at levels close to the MRLs in milk inhibits K. marxianus growth.
Therefore, whey contaminated with antibiotics at levels accepted by the legislation must be treated before fermentation with K. marxianus. Additionally, bioassays utilizing this methodology could be developed to determine the in vitro inhibition of pathogenic yeasts and fungi, aiming to reduce response times (5–7h) compared to the current radial diffusion methods in Petri dishes. These future bioassays in microtiter plates could provide a rapid response (to antibiotics and/or antifungals, including their interactions) for patients with infections caused by fungi and/or yeast.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This research work was carried out as part of the projects PICT 455-2012 and PICT 2017-2841 of the Agencia Nacional de Promoción Científica y Tecnológica of Argentina (ANPCyT). We thank María Victoria Gonzalez Eusevi for revising the English of the manuscript.