Routine microbiological monitoring of rodent colonies in animal facilities is essential to evaluate the health status of the animals used in research studies. In the present study, animals were examined for the presence of selected microbial infections. In order to determine the contamination rates of mice and rats in Argentina, animals from 102 conventional facilities were monitored from 2012 to 2016. The most frequent bacteria isolated were Pseudomonas aeruginosa and Proteus spp. The common parasites identified were Syphacia spp. and Tritrichomonas spp. Serological assays demonstrated the highest prevalence for Mouse hepatitis virus in mice and Sialodacryoadenitis virus in rats. The results indicate that there is a high incidence of infections, so it is suggested that an efficient management system and effective sanitary barriers should be implemented in conventional facilities in Argentina in order to improve sanitary standards.
Los controles microbiológicos de rutina en colonias de roedores en bioterios son esenciales para evaluar el estado de salud de los animales que se utilizan en las investigaciones. En el presente estudio se examinaron animales de bioterios de Argentina con el objeto de detectar la presencia de infecciones microbianas seleccionadas. Con el fin de determinar los porcentajes de contaminaciones en estos individuos, se controlaron animales de 102 bioterios convencionales entre 2012 y 2016. Las bacterias más frecuentes aisladas fueron Pseudomonas aeruginosa y Proteus spp. Los parásitos comunes identificados fueron Syphacia spp. y Tritrichomonas spp. Los ensayos serológicos demostraron la mayor prevalencia del virus de hepatitis del ratón en ratones y del virus de la Syalodacryoadenitis en ratas. Los resultados indican que hay una alta incidencia de infecciones, por lo que se sugiere que se debe implementar un sistema de gestión eficiente y barreras sanitarias eficaces en instalaciones convencionales en Argentina con el objeto de mejorar los estándares sanitarios.
Microbiological monitoring is the practice of scheduled and repetitive testing of an animal colony for evidence of selected microbial infections3,4,8,9,11,12. For many years, experiments failed because of the presence of diseases that killed animals during the assay or produced interferences. Therefore, it is important to determine the prevalence of infections in mice and rats colonies since this data will allow to know their sanitary condition6,10. In the case of Argentina, there is a lack of information and no data about this issue. International guidelines6 recommend to perform the experiments with microbiological and genetically defined animals since Infections are considered a complicating factor in biomedical research. Establishing laboratory rat and mice health status is an important issue to be achieved worldwide since it is a requirement to comply with international standards regarding the animal quality15. The Experimental Animal Laboratory (LAE) at the Faculty of Veterinary Sciences-National University of La Plata (FCV-UNLP) is the former facility that has formally performed since 1996 microbiological monitoring for health surveillance of small rodents in Argentina. In the present study the microbiological status of 2972 mice and rats colonies from conventional facilities in Argentina was studied.
All the protocols used in this study have been approved by the Institutional Animal Care and Use Committee (IACUC) at the FCV-UNLP.
The animals were randomly taken according to age and sex as follows, 2 male and 2 females over 24 weeks old, 2 males and 2 females from 6 to 12 weeks old and 1 male and 1 female 3 weeks old. In the case of rats the samples were obtained from WKAH/Hok, WKY, LEW and F344 strains and SD stock and in mice BALB/c, C57BL/6 strains and Crl:CD1, CF1, NLAE: NIH (S)-Fox1nu and NLAE: NIH (Swiss) stocks. The animals characteristics could be obtained in Charles River Laboratories (https://www.criver.com/), The Jackson Laboratory (https://www.jax.org/) or Taconic (https://www.taconic.com/)
The animals were euthanized by CO2 gas in order to performed microbiological monitoring to survey the presence of the listed pathogens. It was considered the recommendation proposed by Federation of European Laboratory Animal Science Associations (FELASA) for the selection of the microorganisms to be monitored10.
Blood collection was made by cardiac puncture. Blood was stored overnight at 4°C in order to get the sera. Serum specimens were inactivated at 56°C for 30min and diluted in phosphate-buffered saline (PBS) (1:5) and stored at −20°C until use1.
The serological tests were carried out by using Indirect Immunofluorescence Assay (IFA)5 for: Mouse hepatitis virus (MHV), Sendai virus (HVJ), Minute virus of mice (MVM), Theiler's mice encephalomyelitis virus (TMEV in rats, TMEV-GDVII in mice), Sialodacryoadenitis virus (SDAV), Kilham rat virus, Mycoplasma spp., Cilia-Associated Respiratory Bacillus (CAR Bacillus) and Clostridium piliforme. The IFA tests were performed by the method of Cherry et al., 19612. Microagglutination test (MAT) was used to detect antibodies against Corynebacterium kutscheri and Bordetella bronchiseptica. It was carried out as described by Suzuki et al., 198613.
Samples for respiratory tract bacteriological monitoring were taken from the trachea by using a swab and from cecal content for digestive system bacteria. Samples were seeded on blood agara, McConkey agara, cetrimide agara and FNC agara and cultured as required. Culture tests were performed to isolate B. bronchiseptica, Citrobacter rodentium, Corynebacterium kutscheri, Klebsiella spp., Mycoplasma spp., Pasteurella spp., Proteus spp., Pseudomonas aeruginosa, Salmonella spp., Staphylococcus aureus and Streptococcuspneumoniae5,10,14 according with microbiological culture methods.
Sources and manufacturers:
a: Britania S.A.
Simple biochemical tests were performed for the identification of the above bacteria according with Bergey's Manual of Systematic Bacteriology8 and The Handbook of Laboratory Animal Bacteriology 20007. In order to isolate Mycoplasma spp., the samples were cultured in PPLO brotha, afterwards in PPLO agara and observed 5–7 days after incubation to identify typical colonies.
Cellophane tape test, direct smears from duodenal and cecal contents and flotation method were performed in order to detect ectoparasites and endoparasites respectively4.
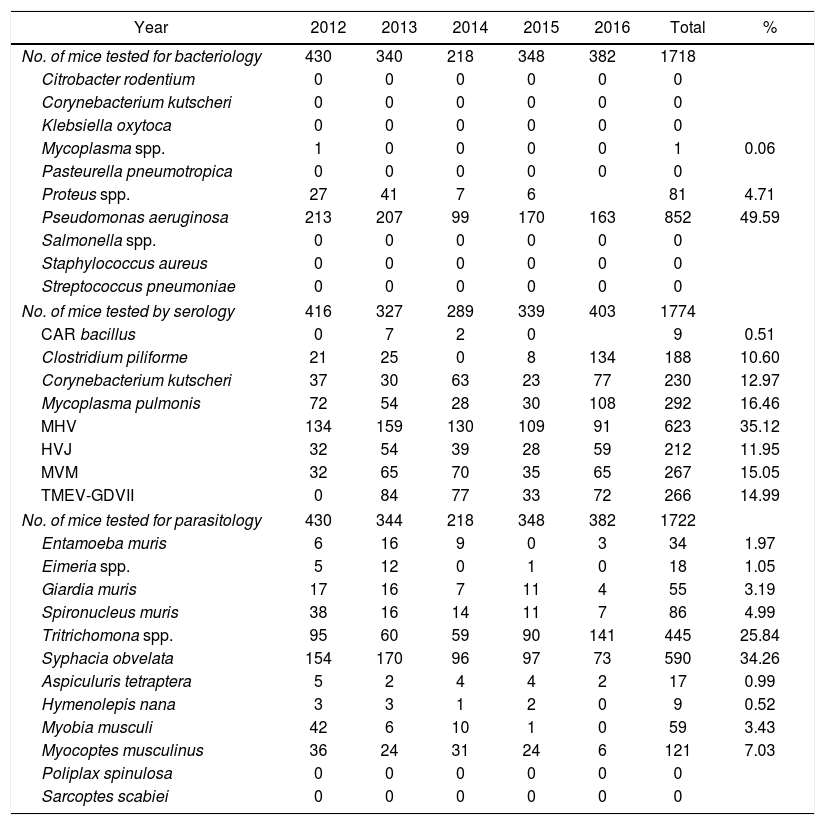
In mice the results showed that the most frequent bacterium isolated was P. aeruginosa (49.59%); the second one was Proteus spp. (4.71%). At the serology, 35.19% of 1774 mice had antibodies against MHV. It was determined the presence of Syphacia obvelata and Tritrichomonas spp. in 34.26% and 25.84% respectively, in the monitored 1722 mice tested (Table 1).
Contaminations in laboratory mice in Argentina during 2012 to 2016 period.
| Year | 2012 | 2013 | 2014 | 2015 | 2016 | Total | % |
|---|---|---|---|---|---|---|---|
| No. of mice tested for bacteriology | 430 | 340 | 218 | 348 | 382 | 1718 | |
| Citrobacter rodentium | 0 | 0 | 0 | 0 | 0 | 0 | |
| Corynebacterium kutscheri | 0 | 0 | 0 | 0 | 0 | 0 | |
| Klebsiella oxytoca | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mycoplasma spp. | 1 | 0 | 0 | 0 | 0 | 1 | 0.06 |
| Pasteurella pneumotropica | 0 | 0 | 0 | 0 | 0 | 0 | |
| Proteus spp. | 27 | 41 | 7 | 6 | 81 | 4.71 | |
| Pseudomonas aeruginosa | 213 | 207 | 99 | 170 | 163 | 852 | 49.59 |
| Salmonella spp. | 0 | 0 | 0 | 0 | 0 | 0 | |
| Staphylococcus aureus | 0 | 0 | 0 | 0 | 0 | 0 | |
| Streptococcus pneumoniae | 0 | 0 | 0 | 0 | 0 | 0 | |
| No. of mice tested by serology | 416 | 327 | 289 | 339 | 403 | 1774 | |
| CAR bacillus | 0 | 7 | 2 | 0 | 9 | 0.51 | |
| Clostridium piliforme | 21 | 25 | 0 | 8 | 134 | 188 | 10.60 |
| Corynebacterium kutscheri | 37 | 30 | 63 | 23 | 77 | 230 | 12.97 |
| Mycoplasma pulmonis | 72 | 54 | 28 | 30 | 108 | 292 | 16.46 |
| MHV | 134 | 159 | 130 | 109 | 91 | 623 | 35.12 |
| HVJ | 32 | 54 | 39 | 28 | 59 | 212 | 11.95 |
| MVM | 32 | 65 | 70 | 35 | 65 | 267 | 15.05 |
| TMEV-GDVII | 0 | 84 | 77 | 33 | 72 | 266 | 14.99 |
| No. of mice tested for parasitology | 430 | 344 | 218 | 348 | 382 | 1722 | |
| Entamoeba muris | 6 | 16 | 9 | 0 | 3 | 34 | 1.97 |
| Eimeria spp. | 5 | 12 | 0 | 1 | 0 | 18 | 1.05 |
| Giardia muris | 17 | 16 | 7 | 11 | 4 | 55 | 3.19 |
| Spironucleus muris | 38 | 16 | 14 | 11 | 7 | 86 | 4.99 |
| Tritrichomona spp. | 95 | 60 | 59 | 90 | 141 | 445 | 25.84 |
| Syphacia obvelata | 154 | 170 | 96 | 97 | 73 | 590 | 34.26 |
| Aspiculuris tetraptera | 5 | 2 | 4 | 4 | 2 | 17 | 0.99 |
| Hymenolepis nana | 3 | 3 | 1 | 2 | 0 | 9 | 0.52 |
| Myobia musculi | 42 | 6 | 10 | 1 | 0 | 59 | 3.43 |
| Myocoptes musculinus | 36 | 24 | 31 | 24 | 6 | 121 | 7.03 |
| Poliplax spinulosa | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sarcoptes scabiei | 0 | 0 | 0 | 0 | 0 | 0 | |
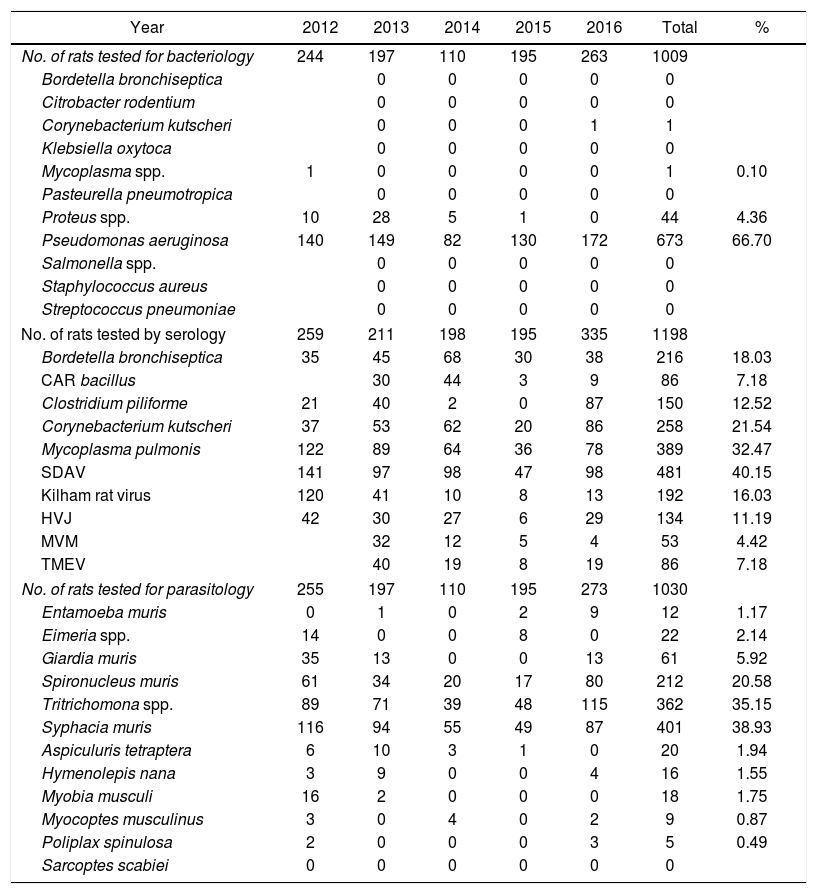
P. aeruginosa and Proteus spp. were the most frequent bacteria isolated in rats with 66.7% and 4.36% respectively. The serology assay showed that 40.15% of the rats present SDAV. Syphacia muris was present in 38.83% and Tritrichomonas spp. in 35.15% of the rats examined (Table 2).
Contaminations in laboratory rats in Argentina during 2012 to 2016 period.
| Year | 2012 | 2013 | 2014 | 2015 | 2016 | Total | % |
|---|---|---|---|---|---|---|---|
| No. of rats tested for bacteriology | 244 | 197 | 110 | 195 | 263 | 1009 | |
| Bordetella bronchiseptica | 0 | 0 | 0 | 0 | 0 | ||
| Citrobacter rodentium | 0 | 0 | 0 | 0 | 0 | ||
| Corynebacterium kutscheri | 0 | 0 | 0 | 1 | 1 | ||
| Klebsiella oxytoca | 0 | 0 | 0 | 0 | 0 | ||
| Mycoplasma spp. | 1 | 0 | 0 | 0 | 0 | 1 | 0.10 |
| Pasteurella pneumotropica | 0 | 0 | 0 | 0 | 0 | ||
| Proteus spp. | 10 | 28 | 5 | 1 | 0 | 44 | 4.36 |
| Pseudomonas aeruginosa | 140 | 149 | 82 | 130 | 172 | 673 | 66.70 |
| Salmonella spp. | 0 | 0 | 0 | 0 | 0 | ||
| Staphylococcus aureus | 0 | 0 | 0 | 0 | 0 | ||
| Streptococcus pneumoniae | 0 | 0 | 0 | 0 | 0 | ||
| No. of rats tested by serology | 259 | 211 | 198 | 195 | 335 | 1198 | |
| Bordetella bronchiseptica | 35 | 45 | 68 | 30 | 38 | 216 | 18.03 |
| CAR bacillus | 30 | 44 | 3 | 9 | 86 | 7.18 | |
| Clostridium piliforme | 21 | 40 | 2 | 0 | 87 | 150 | 12.52 |
| Corynebacterium kutscheri | 37 | 53 | 62 | 20 | 86 | 258 | 21.54 |
| Mycoplasma pulmonis | 122 | 89 | 64 | 36 | 78 | 389 | 32.47 |
| SDAV | 141 | 97 | 98 | 47 | 98 | 481 | 40.15 |
| Kilham rat virus | 120 | 41 | 10 | 8 | 13 | 192 | 16.03 |
| HVJ | 42 | 30 | 27 | 6 | 29 | 134 | 11.19 |
| MVM | 32 | 12 | 5 | 4 | 53 | 4.42 | |
| TMEV | 40 | 19 | 8 | 19 | 86 | 7.18 | |
| No. of rats tested for parasitology | 255 | 197 | 110 | 195 | 273 | 1030 | |
| Entamoeba muris | 0 | 1 | 0 | 2 | 9 | 12 | 1.17 |
| Eimeria spp. | 14 | 0 | 0 | 8 | 0 | 22 | 2.14 |
| Giardia muris | 35 | 13 | 0 | 0 | 13 | 61 | 5.92 |
| Spironucleus muris | 61 | 34 | 20 | 17 | 80 | 212 | 20.58 |
| Tritrichomona spp. | 89 | 71 | 39 | 48 | 115 | 362 | 35.15 |
| Syphacia muris | 116 | 94 | 55 | 49 | 87 | 401 | 38.93 |
| Aspiculuris tetraptera | 6 | 10 | 3 | 1 | 0 | 20 | 1.94 |
| Hymenolepis nana | 3 | 9 | 0 | 0 | 4 | 16 | 1.55 |
| Myobia musculi | 16 | 2 | 0 | 0 | 0 | 18 | 1.75 |
| Myocoptes musculinus | 3 | 0 | 4 | 0 | 2 | 9 | 0.87 |
| Poliplax spinulosa | 2 | 0 | 0 | 0 | 3 | 5 | 0.49 |
| Sarcoptes scabiei | 0 | 0 | 0 | 0 | 0 | 0 | |
This study reports on the microbiological status of laboratory mice and rats housed in 102 conventional facilities in Argentina from 2012 to 2016. One of the major contaminations identified was P. aeruginosa. It was present in 852 mice out of 1718 (49.59%) and 673 rats out of 1009 (66.70%). Although this bacteria is considered an opportunistic pathogen in immunocompetent animals, it produces interferences in the research results when immunodeficient mice are used. In this animal model P. aeruginosa causes severe infections and aggravates this disease resulting in significant morbidity and mortality. The major viral contaminations were MHV in mice facilities; it is considered a fatal pathogen. We have found antibodies against MHV in 623 animals out of 1774 (35.19%). Among fatal pathogens of rats SDAV was present in the colonies in a 40.15%.
Nonpathogenic protozoa were detected in facilities of both mice and rats. The most common were Tritrichomonas spp. and the pinworms S. obvelata and S. muris.
Contamination rates of some tested microorganisms (P. aeruginosa, Proteus spp., M. pulmonis, MHV, Giardia spp., Tritrichomonas spp., Myocoptes musculinus in mice and P. aeruginosa, Proteus spp., Mycoplasma spp., Tritrichomonas spp., S. muris, Spironucleus muris in rats) have increased because the number of mice and rats used in biomedical research in Argentina has increased in the past 15 years.
All the isolated microorganisms have been identified in facilities worldwide, they are common mice and rats pathogens, hence it can be confirmed that the contaminations detected in Argentina do not differ from those in any part of the world.
We also have to consider that in Argentina there are no regulations regarding the care and use of experimental animals and thus no requirements; this fact probably contributes with the poor microbiological quality that have been found in mice and rats colonies. Therefore, it would be important that the facilities in Argentina perform health monitoring programs in order to decrease pathogen contaminations.
In conclusion, the Argentine scientific community should be aware of the role of rats and mice subclinical infectious and of the need to improve laboratory animals microbiological status by establishing SPF barrier facilities and regular health monitoring programs in order to work in compliance with international standards.
FundingThis study was performed with the financial support provided by the UNLP and the Argentine Ministry of Science and Technology.
Authors’ contributionsAll authors contributed to the conception, analysis, or interpretation of data; drafted the manuscript; critically revised the manuscript; and gave final approval.
Conflict of interestThe authors declare that they have no conflicts of interest.