Four species of entomophthoroid fungi, Pandora neoaphidis (Entomophthorales: Entomophthoraceae), Zoophthora radicans (Entomophthorales: Entomophthoraceae), Entomophthora planchoniana (Entomophthorales: Entomophthoraceae) and Neozygites fresenii (Neozygitales: Neozygitaceae) were found to infect Aphis craccivora, Therioaphis trifolii, and Acyrthosiphon pisum and unidentified species of Acyrthosiphon on lucerne in Argentina. Samples were collected from five sites (Ceres, Rafaela, Sarmiento, Monte Vera and Bernardo de Irigoyen) in the province of Santa Fe. In this study, Zoophthora radicans was the most important pathogen and was recorded mainly on Acyrthosiphon sp. Zoophthora radicans was successfully isolated and maintained in pure cultures. This study is the first report of entomophthoroid fungi infecting lucerne (Medicago sativa L.) aphids in Argentina.
Se encontraron cuatro especies de hongos Entomophthorales, Pandora neoaphidis, Zoophthora radicans, Entomophthora planchoniana (Entomophthorales: Entomophthoraceae) y Neozygites fresenii (Neozygitales: Neozygitaceae) infectando a Aphis craccivora, Therioaphis trifolii, Acyrthosiphon pisum y a especies no identificadas pertenecientes al género Acyrthosiphon en cultivos de alfalfa (Medicago sativa L.), en la Argentina. Los muestreos fueron realizados en cinco sitios (Ceres, Rafaela, Sarmiento, Monte Vera y Bernardo de Irigoyen) de la provincia de Santa Fe. Zoophthora radicans fue el patógeno más importante registrado principalmente en Acyrthosiphon sp. Zoophthora radicans fue exitosamente aislado y mantenido en cultivos puros. Este estudio documenta por primera vez en la Argentina la presencia de hongos Entomophthorales infectando áfidos en alfalfa.
Aphids (Hemiptera: Aphididae) are among the most successful families of insects and many represent serious agricultural pests11. Four of the ten species of aphids in the world that infect lucerne2 are considered serious pests in Argentina, these being Aphis craccivora Koch, Acyrthosiphon pisum (Harris), Acyrthosiphon kondoi Sinji and Therioaphis trifolii (Monell)13. Aphids feed on phloem sap via extremely thin maxillary stylets that penetrate phloem sieve tubes, greatly reducing the possibility of these insects to ingest viruses, bacteria or protozoa from plant surfaces. Aphids became relevant due to their capacity of transmission of several viruses like Alfalfa Mosaic Virus (AMV) and other potyviruses, which limit the performance and persistence of plants3. Entomophthoralean fungi can cause lethal infections of various aphid species and they belong to the group of most effective control agents of natural aphid colonies. The only record of Entomophthoroid fungi of aphids on lucerne in South America was found in Uruguay1. Limited research efforts have been devoted to investigating the entomopathogenic fungi as agents of natural mortality of aphids in lucerne crops in Argentina. The aim of this paper was to identify and to isolate entomophthoroid fungi of aphid pests on M. sativa in the Argentina Pampas. The study was not intended to provide quantitative data. The taxonomy of entomophthoroid fungi used here is in accordance with the new molecular-based classification of these fungi, including it in a newly described phylum, Entomophthoromycota6.
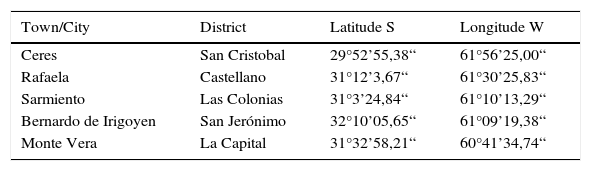
The survey covered the west of Santa Fe province, in the Argentine Pampas (situated between 28–40° S and 68–57° W). The Argentine Pampa is a vast region of 52 million ha of suitable land for agriculture and cattle production. Samplings of insects were conducted in five sites from April 2010 to June 2012 (Table 1). Surveys were occasionally carried out in Ceres, Sarmiento and in Bernardo de Irigoyen, and weekly in Rafaela and Monte Vera. Sampled areas did not exceed 500m2 per site. No insecticides or fungicides were applied to the parts of the fields where collections were made during the course of the study. Fifteen (15) lucerne stems (from 30 to 50cm each) were collected along both diagonals of each field. Stems sustaining aphids were placed in labeled plastic bags and transported to the laboratory as described by Zumoffen et al.14. Lucerne stems were checked to evaluate the presence of healthy or infected aphids. The plants were later discarded. Samples of healthy living aphids were collected and transferred into plastic cups with lids (150cm3) from where subsamples were transferred to microcentrifuge tubes (Eppendorf; 1.5cm3). These subsamples were preserved in 70% ethanol for further identification to species level, according to Blackman & Eastop's keys2.
Location of study sites.
| Town/City | District | Latitude S | Longitude W |
|---|---|---|---|
| Ceres | San Cristobal | 29°52’55,38“ | 61°56’25,00“ |
| Rafaela | Castellano | 31°12’3,67“ | 61°30’25,83“ |
| Sarmiento | Las Colonias | 31°3’24,84“ | 61°10’13,29“ |
| Bernardo de Irigoyen | San Jerónimo | 32°10’05,65“ | 61°09’19,38“ |
| Monte Vera | La Capital | 31°32’58,21“ | 60°41’34,74“ |
Dead aphids with evidence of external fungal growth (showing sporulation) were examined under a stereo microscope and an optic microscope to evaluate the presence of rhizoids, cystidia, and/or spores. Dead aphids without external mycosis signs were placed in Petri dishes (60mm diam) containing a filter paper moistened with a few drops of distilled water (humid chambers), which was maintained at 20°C for 24–72h to allow the development of overt mycoses. Living aphids with apparent infection signs were also disposed in humid chambers and maintained under the same conditions detailed above until they showed an infection development, finally checking that aphid mortality was caused by Entomophthoralean fungi. Fungal structures were mounted in lactophenol-aceto-orcein (LPAO) (1:1) or stained with 1% aceto-orcein plus glycerine for semipermanent mounts. Measurements of fungal structures were made to enable specific identification. Fungal species were identified according to taxonomic keys and monographs of Humber6 and Keller7,8.
In order to obtain pure cultures, infected aphids were placed on a moistened piece of sterile filter paper attached with double coated tape to the lid of a sterile 60mm Petri dish, which was then inverted over the bottom of a sterile Petri dish containing SEMA (80% Sabouraud dextrose agar + 1% yeast extract and 20% of a mixture of egg yolk and skim milk)4 plus 40.000 units/ml penicillin G (Merck®, Germany) and 80.000 units/ml streptomycin (Parafarm®, Argentina). This assembly was left 12h in the dark at 22±1°C. A sterile lid replaced the lid with the attached aphids after 12h. All isolates were incubated at 22±1°C with a photoperiod of 16:8 (L: D).
Only one of the species of Entomophthoroid fungi was successfully isolated and maintained in pure cultures. Zoophthora radicans isolates were deposited in the Mycological Culture Collection at Centro de Estudios Parasitológicos y de Vectores (CEP, La Plata, Argentina) and at USDA-ARS Collection of Entomopathogenic Fungal Cultures (ARSEF, Ithaca, New York) under access numbers CEP 362 and ARSEF 11859 CEP, respectively. Herbarium materials such as dried infected specimens and microscope slides were deposited in the Mycological Culture Collection at Centro de Estudios Parasitológicos y de Vectores (CEP, La Plata, Argentina).
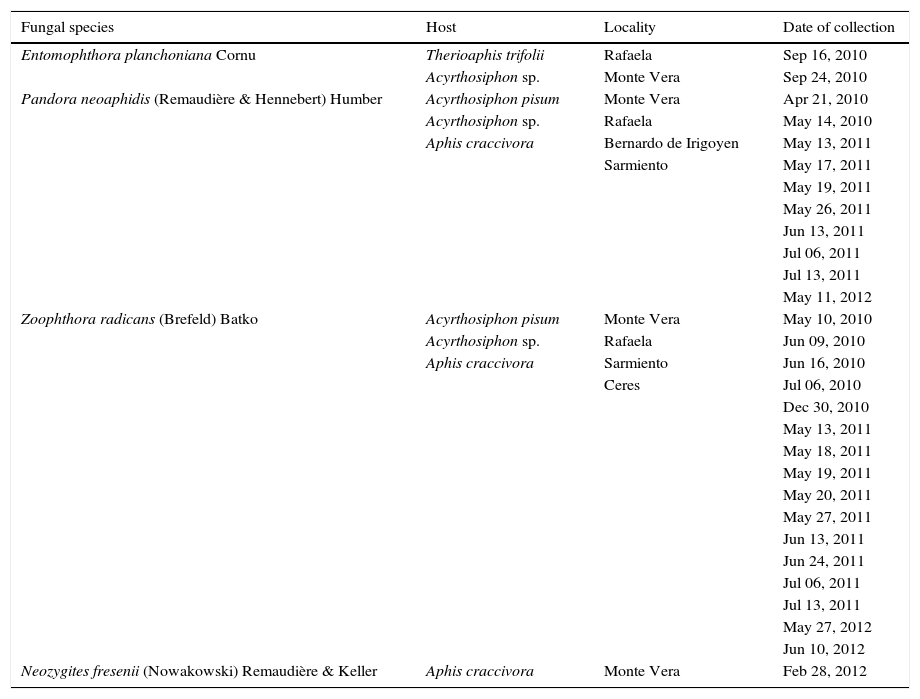
Three of the species of aphids observed were infected by entomophthoroid fungi: A. craccivora, A. pisum, T. trifolii and unidentified species of the genus Acyrthosiphon. Four species of entomophthoralean fungi were indentified in these aphids: Pandora neoaphidis (Remaudière & Hennebert) Humber, Zoophthora radicans (Brefeld) Batko, Entomophthora planchoniana Cornu (Entomophthorales: Entomophthoraceae), and Neozygites fresenii (Nowakowski) Remaudière & Keller (Neozygitales: Neozygitaceae) (Table 2). Fungal infections occurred mainly between May and July and, to a lesser extent, during April, September and December 2010 and February 2012. Previous studies on the phenology of entomophthoroid fungi in populations of insects other than aphids recorded that fungal infections were more common during autumn-winter in Argentina (in the Southern hemisphere, from March to September)9.
Entomophthoralean fungi recorded from M. sativa during 2010–2012.
| Fungal species | Host | Locality | Date of collection |
|---|---|---|---|
| Entomophthora planchoniana Cornu | Therioaphis trifolii | Rafaela | Sep 16, 2010 |
| Acyrthosiphon sp. | Monte Vera | Sep 24, 2010 | |
| Pandora neoaphidis (Remaudière & Hennebert) Humber | Acyrthosiphon pisum | Monte Vera | Apr 21, 2010 |
| Acyrthosiphon sp. | Rafaela | May 14, 2010 | |
| Aphis craccivora | Bernardo de Irigoyen | May 13, 2011 | |
| Sarmiento | May 17, 2011 | ||
| May 19, 2011 | |||
| May 26, 2011 | |||
| Jun 13, 2011 | |||
| Jul 06, 2011 | |||
| Jul 13, 2011 | |||
| May 11, 2012 | |||
| Zoophthora radicans (Brefeld) Batko | Acyrthosiphon pisum | Monte Vera | May 10, 2010 |
| Acyrthosiphon sp. | Rafaela | Jun 09, 2010 | |
| Aphis craccivora | Sarmiento | Jun 16, 2010 | |
| Ceres | Jul 06, 2010 | ||
| Dec 30, 2010 | |||
| May 13, 2011 | |||
| May 18, 2011 | |||
| May 19, 2011 | |||
| May 20, 2011 | |||
| May 27, 2011 | |||
| Jun 13, 2011 | |||
| Jun 24, 2011 | |||
| Jul 06, 2011 | |||
| Jul 13, 2011 | |||
| May 27, 2012 | |||
| Jun 10, 2012 | |||
| Neozygites fresenii (Nowakowski) Remaudière & Keller | Aphis craccivora | Monte Vera | Feb 28, 2012 |
Entomopthoralean fungal infections were most frequently observed in Acyrthosiphon spp. than in the rest of the aphid species collected. Entomophthora planchoniana, P. neoaphidis and Z. radicans were identified infecting Acyrthosiphon spp. In our study Z. radicans was the most important pathogen recorded from aphid pests on M. sativa and it was successfully isolated from A. pisum. On the other hand, Alzugaray et al.1 reported P. neoaphidis as the principal mortality agent of aphids in lucerne crops in Uruguay. In this study P. neoaphidis was secondary to Z. radicans in occurrence and the first was identified among three aphid pest species. Entomophthoralean fungi were reported from other Leguminoseae plants related to lucerne, as for example N. fresenii that was recorded from A. craccivora on faba bean plants12. Entomophthtora species have been recorded to infect A. pisum and T. trifolii on legumes in Australia10. In the present research T. trifolii was only infected by E. planchoniana. There are previous records of E. planchoniana as pathogen of T. trifolii and A. kondoi in New Zealand5.
Pandora neoaphidis, Z. radicans, E. planchoniana and N. fresenii were found to infect A. craccivora, T. trifolii, A. pisum and unidentified species of Acyrthosiphon ssp. The present study is a preliminary record of Entomophthoralean fungi causing infections in natural populations of aphids on lucerne crops in the Argentina Pampas.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We thank Dr. Richard Humber for confirming the taxonomic identification of fungal species and for allowing us to deposit fungal cultures for preservation at the USDA-ARS Collection of Entomopathogenic Fungal Cultures (ARSEF). We also thank the National Research Council (CONICET) of Argentina for the partial financial support of this research.