In the central area of Argentina, the epidemiological and molecular characteristics of Chlamydophila pneumoniae infections in reptiles are still unknown. A nested polymerase chain reaction of the rpoB gene was used to detect C. pneumoniae in cloacal swab samples from 19 reptiles at a recreational area. Eleven (57.89%) reptiles were positive; the sequencing and phylogenetic analysis confirmed the presence of this bacterium. Neither C. pneumoniae DNA in the caregivers pharynges nor IgM antibodies anti-C. pneumoniae in their serum samples were detected; however, caregivers presented very high titers of IgG anti-C. pneumoniae. The detection of C. pneumoniae DNA in reptiles demonstrated the circulation of this agent in the recreational area and could be responsible for the exacerbated immune response of the personnel handling the reptiles, which suggests a potential zoonotic cycle. This is the first report of the detection of C. pneumoniae in reptiles in Argentina.
En la región central de Argentina, las características epidemiológicas y moleculares de las infecciones por Chlamydophila pneumoniae en reptiles son desconocidas. Para detectar C. pneumoniae, se usó la reacción en cadena de la polimerasa anidada que amplifica el gen rpoB en muestras de hisopado cloacal de 19 reptiles. Once (57,89 %) reptiles resultaron positivos. La secuenciación y el análisis filogenético corroboraron la presencia de esta bacteria. No se detectó ADN de C. pneumoniae en la faringe ni IgM anti-C. pneumoniae en el suero de los cuidadores; sin embargo, ellos presentaron títulos muy elevados de IgG anti-C. pneumoniae. La detección de ADN de C. pneumoniae en los reptiles demostró la circulación de este agente en el centro recreativo donde se realizó este estudio, lo que podría explicar la exacerbada respuesta inmunitaria en los cuidadores; este hallazgo sugiere la presencia de un potencial ciclo zoonótico. Se reporta aquí por primera vez la detección de C. pneumoniae en reptiles en Argentina.
Chlamydia pneumoniae is a widespread pathogen responsible for upper and lower respiratory tract infections, in addition to a wide range of human and animal diseases9. The range of hosts known to be infected by C. pneumoniae has expanded to several species of reptiles, such as puff adders (Bitis arietans), flap-necked chameleons (Chameleo dilepis), green turtles (Chelonia mydas), Nile crocodiles (Crocodylus niloticus), green iguanas (Iguana iguana) and Burmese pythons (Python molurus bivittatus)1–3,7,9. Lesions are varied and include histiocytic granulomas (puff adders), granulomatous inflammation (flap necked chameleons), necrotizing enteritis (green iguanas), necrotizing myocarditis (green turtles), and proliferative pneumonia (Burmese pythons)7. However, in humans, C. pneumoniae infections range from asymptomatic to severe respiratory diseases, including pneumonia. Previous studies have reported seroprevalence values of 50% by the age of 20 years and 80% in the elderly3.
Genome characterization of the C. pneumoniae sequence detected in Australian reptiles and amphibians showed 99% homology with the human sequence, revealing their potential role in the zoonotic transmission from reptiles to humans11. Recently, at a Recreation Center in Cordoba province, a specimen of reticulated python (Python reticulata) presented symptoms compatible with proliferative pneumonia, which led to the development of this investigation. The aim of this study was to describe the presence of C. pneumoniae in several species of reptiles in Córdoba and in their caregivers, and to evaluate the presence of specific antibodies against C. pneumoniae in the personnel.
We analyzed 27 cloacal swab samples from 19 reptiles at a recreational area using a specific nested-PCR for C. pneumoniae8. In May 2011, we obtained 1 cloacal swab from the reticulated python with symptoms compatible with proliferative pneumonia and 18 samples of reptiles living together. In December 2011, resampling of only 8 of these reptiles could be conducted.
The cotton swabs were placed in 1ml sucrose-phosphateglutamate (SPG) and 200μl was subjected to DNA extraction using the Accuprep Genomic DNA Extraction Kit (BIONEER, Alameda, CA, USA) according to the manufacturer's instructions. DNA AR39 C. pneumoniae (ATCC53592) was used as positive control. Negative controls (deionized H2O instead of template DNA) were included in all PCR reaction sets. First, 5μl of DNA extract was used to amplify a fragment of RNA polymerase beta subunit gene (rpoB) using primers HL1 (GTT GTT CAT GAA GGC CTA CT)/ HR1 (TGC ATA ACC TAC GGT GTG TT) and N1 (AGT TGA GCA TAT TCG TGA GG)/ N2 (TTT ATT TCC GTG TCG TCC AG) as previously described by Mass et al.8.
Positive samples were sequenced to corroborate the species. For sequence analysis, the nested polymerase chain reaction products were purified with the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA, USA) and subjected to direct nucleotide sequencing reaction in both directions using an ABI automatic sequencer (Applied Biosystems, Foster City, CA, USA). C. pneumoniae identification was confirmed by phylogenetic analysis.
Human pharyngeal swabs were also analyzed by a nested- PCR of C. pneumoniae8. Furthermore, sera from the 6 healthy caregivers were tested for C. pneumoniae antichlamydial immunoglobulin G (IgG) and IgM using the microimmunofluorescence assay (MIF), according to the manufacturer's instructions (Bion Enterprises®, France). In this test, C. pneumoniae elementary bodies were purified by removal of genus-reactive LPS. Since this technique has very low cross-reactivity with other Chlamydophila species, it is recommended for the differential diagnosis of these agents in humans.
The rpoB sequences obtained were submitted to the GenBank (accession numbers are shown in Table 1) and alignment using the ClustalX program was performed (Conway Institute UCD Dublin, Dublin, Ireland)12. The maximum likelihood tree was constructed with the PhyML 3.0 software (Université de Montpellier, Montpellier, France)5. The model of nucleotide substitution for the data set analyzed was selected according to the Akaike Information Criterion implemented in the ModelTest 3.7 software (Universidad de Vigo, Galicia, Spain)10.
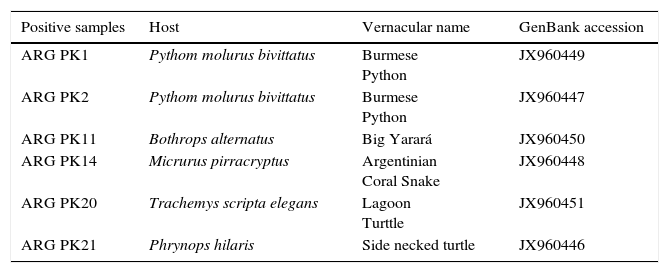
Characteristics of C. pneumoniae positive samples analyzed.
| Positive samples | Host | Vernacular name | GenBank accession |
|---|---|---|---|
| ARG PK1 | Pythom molurus bivittatus | Burmese Python | JX960449 |
| ARG PK2 | Pythom molurus bivittatus | Burmese Python | JX960447 |
| ARG PK11 | Bothrops alternatus | Big Yarará | JX960450 |
| ARG PK14 | Micrurus pirracryptus | Argentinian Coral Snake | JX960448 |
| ARG PK20 | Trachemys scripta elegans | Lagoon Turttle | JX960451 |
| ARG PK21 | Phrynops hilaris | Side necked turtle | JX960446 |
Among the cloacal swabs samples of 19 reptiles studied, 11 (57.89%) tested C. pneumoniae positive by nested-PCR [Pythom reticulata (n=2), Python molurus bivittatus (n=2), Bothrops alternatus (n=1), Crotalus atrox (n=1), Trachemis scripta elegans (n=2); Phrynops hilaris (n=2); Micrurus pirracryptus (n=1)]. DNA of C. pneumoniae was not detected in samples from the symptomatic reticulated python, probably because at the time of collection, this reptile was isolated and had already received specific antibiotic treatment. PCR products suitable for sequencing were obtained from 6 reptiles. Sequence analysis confirmed that the amplicons obtained belonged to C. pneumoniae (Fig. 1). These findings demonstrated that C. pneumoniae is capable of infecting several species of snakes and turtles, broadening the range of reptiles known to be infected by this primarily human pathogen. After confirmation of infection, reptiles were subjected to specific antibiotic treatment. All the caregivers’ pharyngeal swabs tested negative for C. pneumoniae, excluding active human infection.
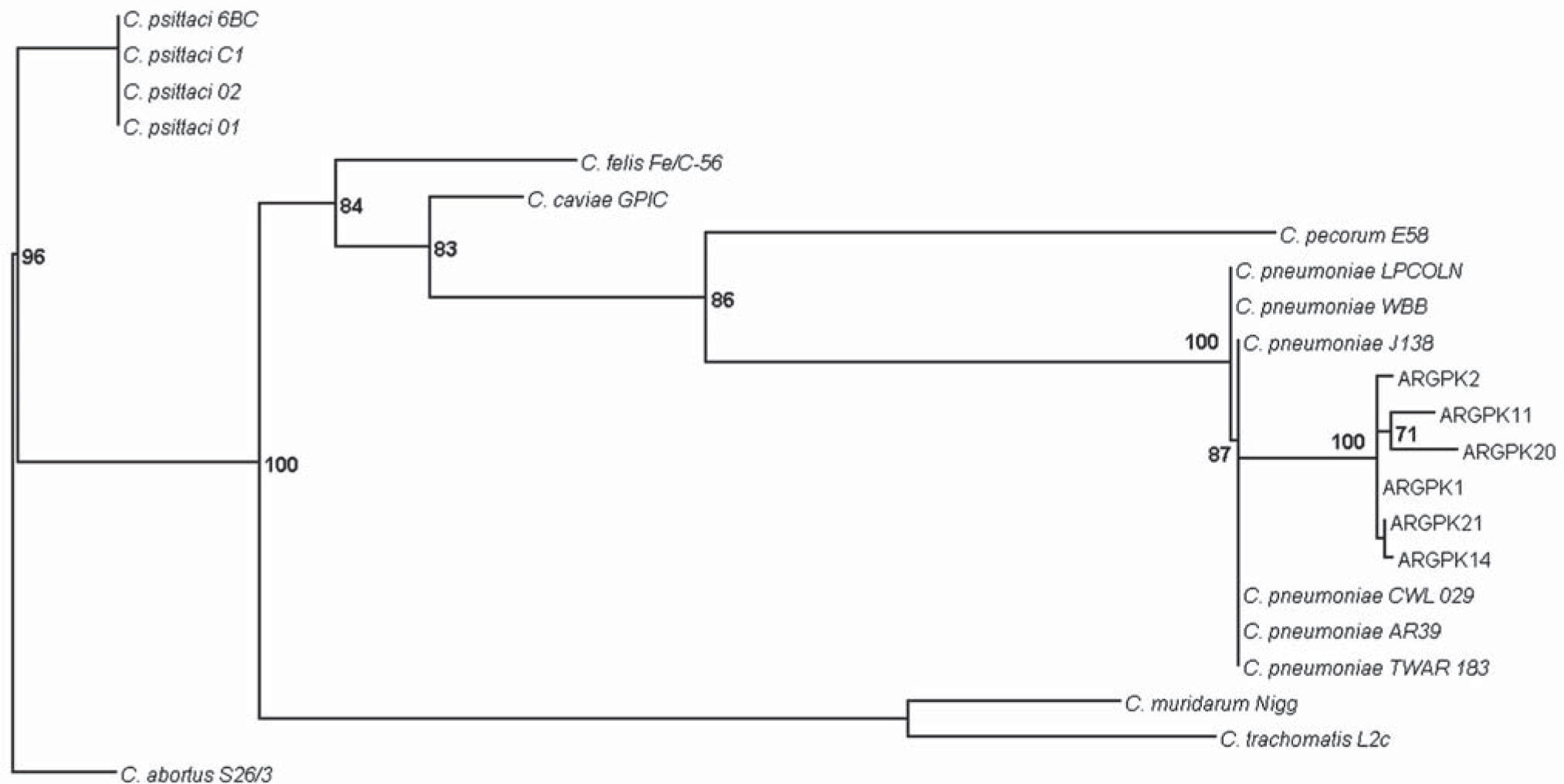
Maximum likelihood tree for the rpoB gene (441 nt) constructed using GTR+G as model of nucleotide substitution, with parameters suggested by ModelTest 3.7 (PhyML software). Sequences belonging to this study are initiated by ARG PK. Numbers above branches: bootstrap values over 2000 bootstrap pseudo replicates. Only bootstrap values >50% are shown at nodes. C. abortus S26/3 is used as out-group. C. pneumoniae LPCOLN: isolated from a koala. C. pneumoniae WBB: isolated from a bandicoot. C. pneumoniae TWAR 183, CWL029, AR39, J138: isolated from humans. C. psittaci 01: isolated from a pig. C. psittaci 02: isolated from cattle. C. psittaci C1: isolated from a sheep. C. psittaci 6BC: isolated from a parakeet. C. felis Fe/C–56: isolated from a cat. C. caviae GPIC: isolated from a guinea pig. C. pecorum E58: isolated from cattle. C. muridarum Nigg: isolated from a mouse. C. trachomatis L2c: isolated from humans. C. abortus S26/3: isolated from a sheep.
In the second visit to the recreational centre in December, all cloacal and pharyngeal swabs analyzed were negative for C. pneumoniae.
In terms of immune response, IgM antibodies anti-C. pneumoniae were not detected in the caregivers’ sera tested in May. One of the caregivers who maintained sporadic contact with the animals presented IgG titers of 1:512. Three caregivers with periodic contact showed IgG titers of 1:2048. One susceptible caregiver [IgG (−)] was the only worker who always used protection when handling the animals or cleaning their excreta. In December, the caregiver who used protective equipment remained susceptible while IgG levels remained elevated in the others (1:2048). The immune status of a new caregiver who had reported pneumonia in September showed high titers of IgG anti-C. pneumoniae (1:4096); however specific IgM was not detected.
The presence of C. pneumoniae strains in reptiles is important because this bacterium is a common cause of human pneumonia and bronchitis throughout the world; in addition, it has recently been associated with several chronic diseases, including coronary heart disease, Alzheimer's disease, and multiple sclerosis3,4,13. Although C. pneumoniae DNA or specific IgM was not detected in the caregivers, the detection of C. pneumoniae DNA in the animals demonstrated the circulation of this agent in the recreational park and supports the exacerbated immune response in people handling the reptiles, which suggests a potential zoonotic cycle. Respiratory diseases and excretion of this bacterium in the reptiles were attenuated by specific antibiotic treatment. The implementation of biosecurity measures is essential to prevent transmission, as demonstrated by our results.
In our study, as well as in other reports in a range of reptiles (snakes, iguanas, chameleons) and amphibians (frogs, turtles), the relationship of our sequences with other reference data sets of chlamydiae indicates that although our sequences belonged to the C. pneumoniae branch, they are quite distinct from the human TWAR strain (Fig. 1)1,6,11.
Even though a limited amount of sequences were analyzed, we found that all C. pneumoniae sequences clustered among them and revealed segregation with sequences of human strains. Although transmission among humans is believed to occur from person to person through respiratory secretions3,13, until more is known about the epidemiology, pathogenicity, and transmission of C. pneumoniae strains recovered from captive and wild populations of cold-blooded animals, their zoonotic potential must be considered. Moreover, because of the increasing ownership of reptiles as pets, caution must be exercised during direct handling of these animals.
Isolation and characterization of reptile C. pneumoniae strains are required to assess their clinical impact and possible zoonotic potential.
Ethical responsibilitiesProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This study was partially supported by Mincyt-Cba 1427/09, Mincyt-Cba 113/2011. This letter has been prepared in the context of the collaboration promoted by the Secretaría de Ambiente de la provincia de Córdoba, Argentina. V. Ré is a scientific member of CONICET, Argentina.