A novel bioreactor system (low cost and easily scaled-up) is presented for dye decolorization applying filamentous fungi. In this two-phase bioreactor, dyes were decolorized at 28°C in a first phase by immobilized fungi in spherical cartridges prepared with a high-density plastic polyethylene mesh and filled with wheat bran as substrate for growth. In a second phase the capacity of the ligninolytic enzymes (laccase and Mn-peroxidase) present in the extracellular extracts from the solid residues was exploited for decolorization at 50°C. Each sphere behaved as a small-scale bioreactor for cell-culture. This system allowed the decoupling of growth (sterile condition) and decolorization (non-sterile condition) stages. The ability to decolorize the azo dye xylidine and the triphenylmethane Malachite Green by two Argentinean strains of Trametes versicolor was evaluated. The highest decolorization rates were displayed by T. versicolor BAFC 2234. When both dyes were applied together in the bioreactor, after a first phase (100min) 73.5% of Malachite Green and 40% of xylidine decolorization was attained, while at the end of the second phase (240min) a 97% and 52% decolorization was observed. Laccase activity was detected in the decolorized solution, but no Mn-peroxidase activity. The easy change of the cartridges allows the continuous use of the bioreactor in the non-sterile decolorization of dye-containing effluents.
Se presenta un novedoso sistema de biorreactor de dos fases, de bajo costo y fácil ampliación, para la decoloración de colorantes aplicando hongos filamentosos. Durante la primera fase, los colorantes estuvieron en contacto con los hongos inmovilizados en cartuchos esféricos preparados con una malla de polietileno plástico de alta densidad y rellenos con salvado de trigo como sustrato para el crecimiento a 28°C. En una segunda fase, se explotó la capacidad de las enzimas ligninolíticas (lacasa y Mn-peroxidasa) presentes en los extractos de los residuos sólidos para la decoloración a 50°C. Cada esfera se comportó como un biorreactor a pequeña escala. Este sistema permitió desacoplar las etapas de crecimiento en esterilidad de las de decoloración en condiciones no estériles. Se evaluó la capacidad de decolorar el tinte azoico xilidina y el trifenilmetánico verde de malaquita por dos cepas argentinas de Trametes versicolor. T. versicolor BAFC 2234 mostró las tasas de decoloración más altas. Cuando ambos colorantes se aplicaron juntos en el biorreactor, después de la primera fase (100min) se alcanzó una decoloración del 73,5% para el verde de malaquita y del 40% para la xilidina, mientras que al final de la segunda fase (240min) se observó una decoloración del 97 y 52%, respectivamente. Se detectó actividad lacasa en la solución decolorada, pero no actividad Mn-peroxidasa. El sistema mostró fácil operatividad de los cartuchos y permitió un uso continuo del biorreactor para la decoloración no estéril de efluentes coloreados.
Dyes are broadly used in the textile, food, pharmaceutical, cosmetic, printing and leather industries. Effluents containing 5-10% of dyestuffs are usually discharged into natural water bodies and may affect water transparency and gas solubility and cause severe environmental pollution because of their carcinogenicity and toxicity. Traditional technologies for dye treatment are not always effective or may not be environmentally friendly. This has encouraged the search for alternative technologies such as biodegradation with fungi9,20. Fungal biomass is used as a sorbent and/or producer of enzymes involved in biodegradation/biotransformation. White-rot fungi and their nonspecific oxidative ligninolytic enzymes (laccases and peroxidases) have been reported to be responsible for the decolorization and detoxification of different synthetic dyes. Thus, their use can provide alternative methods to replace or complement the current technologies for dye removal8,12,21,26,28,37. The major drawback of using an enzyme preparation is that once the enzymes become inactivated, activity decreases. However, with whole cell cultures, the enzymes could be continually replenished. Furthermore, growing biomass could supply other enzymes and mediators for dye transformation.
The immobilization of white-rot fungi has been successfully applied to the treatment of dye-containing effluents15,20,33. This technique allows to use the microbial cells repeatedly and continuously and facilitates separation of cells from the liquid reaction medium. Immobilized cultures tend to show a higher level of enzymatic activity and more resilience to environmental perturbations such as shear damage and pH/toxic shock26. Moreover, it was found in experiments on dye degradation by white-rot fungi that the transport of the dye through the matrix facilitates constant biodegradation by maintaining a low dye concentration near the fungus17,22. When high pollutant concentrations are treated, this system will keep the microorganism viable for a longer period. Nevertheless, there are few designs available in the literature for bioreactors operating in solid-state fermentation (SSF) conditions, mainly due to the difficulty in the control of parameters such as pH, temperature, aeration and oxygen transfer, moisture and agitation. These disadvantages have promoted the necessity of developing new bioreactor configurations10,19,33.
Azo dyes dominate the worldwide market of dyestuffs26. The biggest problem with their enzymatic cleavage under anaerobic conditions is the formation of toxic amines, many of which are mutagenic and/or carcinogenic. Malachite green is a widely used, though highly toxic triphenylmethane dye, which is employed in aquaculture as a parasiticide/fungicide and in the industrial dyeing of fabrics, colored ceramics and paper18. Laccases involved in aerobic azo dye decolorization by white-rot fungi repeatedly proved to be effective for their detoxification2,14. Malachite Green was also detoxified by Pleurotus florida29, and Ganoderma sp.31 laccases.
In this work, a novel bioreactor system is presented for dye decolorization applying ligninolytic fungi. The design is based on Diorio et al.’s6 two-phase bioreactor, where dyes were decolorized by immobilized fungi in the first phase and in a second phase by their extracellular extracts, without contact between the dye and the fungus. In that work a cylindrical cartridge built with stainless steel twilled weave was employed. In the present work spherical cartridges were prepared with a high-density plastic polyethylene mesh and filled with wheat bran as support material for fungal growth. This is a low cost lignocellulosic agro-residue that offers the nutrients needed for fungal growth and ligninolytic enzyme production34. SSF which proceeds in the absence or near absence of free liquid, employing inert or natural substrates (especially agro-industrial materials) as solid supports, is particularly appropriate to produce enzymes by filamentous fungi since it reproduces the conditions under which these fungi grow in nature7.
The ability to decolorize xylidine and malachite green by Trametes versicolor BAFC 2234 and T. versicolor (syn. Coriolus versicolor f. antarcticus, BAFC 266) was evaluated in this novel bioreactor system. T. versicolor BAFC 266 was selected among 58 basidiomycetes from Argentina for its capacity to decolorize Azure B and Poly R-478 in solid medium17. Its culture filtrates with laccase and manganese peroxidase (MnP) ligninolytic activities proved capable of decolorizing various dyes with different chemical structures16. T. versicolor BAFC 2234 was identified in a screening of Argentinean white-rot fungi tested for their tolerance toward high concentrations of different phenolic compounds. This fungus immobilized on natural plant sponge could remove up to 15mM phenol and decrease the phytotoxicity of treated samples5.
Materials and methodsMicroorganismsT. versicolor BAFC 2234 (Culture Collection of the Department of Biological Sciences, Faculty of Exact and Natural Sciences, University of Buenos Aires) and T. versicolor (BAFC 266) were maintained at 4°C on malt-extract agar slants (1.3% malt extract, 1% glucose, 2% agar).
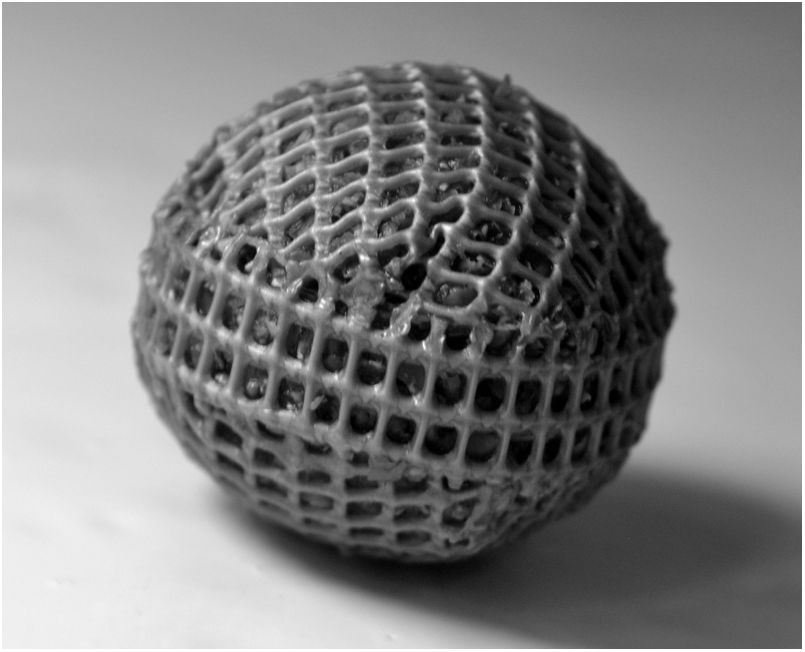
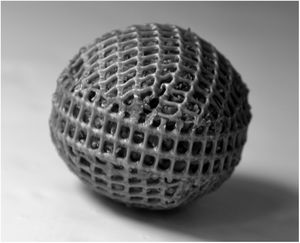
Cartridge preparationSpherical cartridges were prepared with a high-density plastic polyethylene mesh (mesh size: 1.5 mm x 1.5mm) (Fig. 1). Squares of 5 cmcm x 5cm were heat unstiffened and molded over solid glass balls to obtain hemispheres that were assembled with a strip of mesh of 8 cm x 1cm, heat sealing the ends. Before closing the spheres, they were packed with dried wheat bran up to a concentration of 0.159g/cm3. The diameter of the filled spheres was 2.7±0.05cm The spheres were moisturized by introducing them slowly into distilled water to avoid the formation of bubbles. They were hydrated overnight and drained 1h up to a moisture content of 73%, and then autoclaved at 121°C 20min The inoculum consisted of two 0.5cm2 agar plugs placed in opposite sides of the spheres, cut out from the margin of 5-day old colonies grown on malt-extract/agar medium (with 1.8% agar to facilitate inoculum adherence). The inoculated spheres were incubated at 28°C in 16cm Petri plates with a beaker of water inside and Parafilm® sealed to prevent moisture loss.
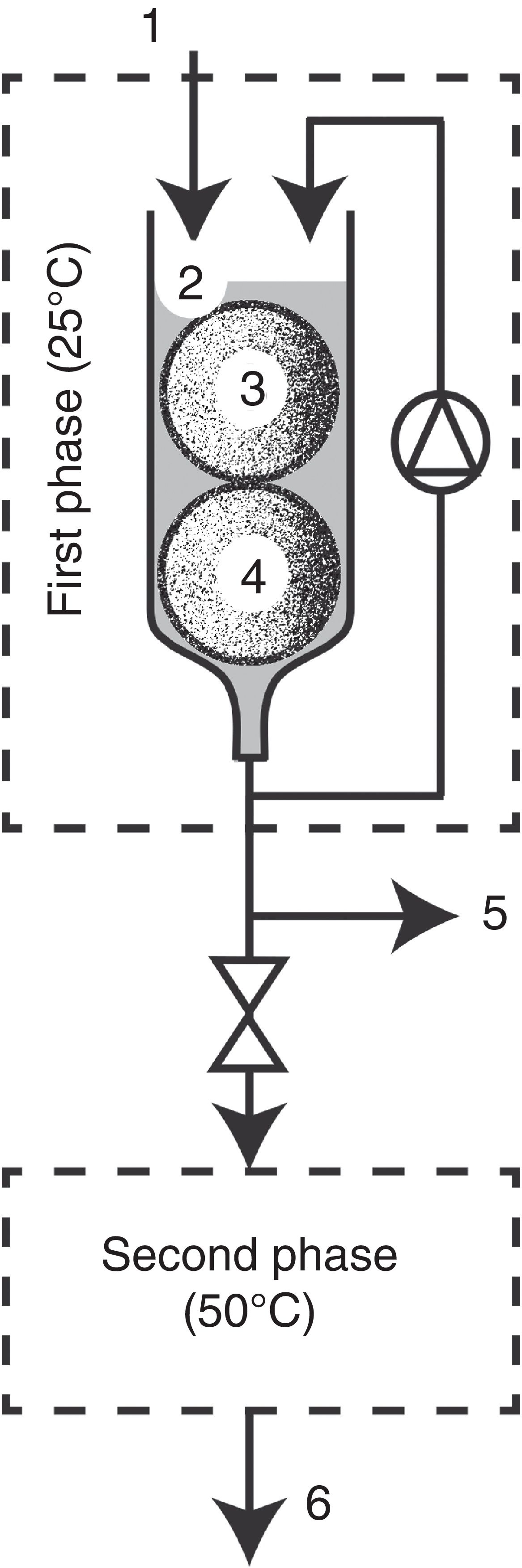
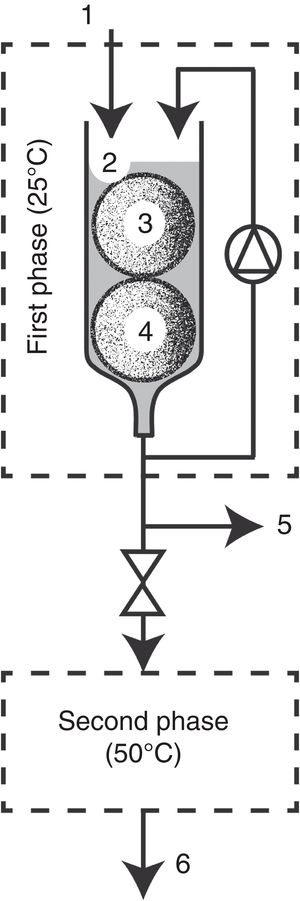
Bioreactor configurationBioreactor configuration (Fig. 2) was based on the two-phase bioreactor described by Diorio et al.6. In this work, first phase configuration consisted of a glass column of 15cm height and 3cm diameter that was kept in an incubator at 28°C. All the elements of the device were autoclaved at 121°C for 20min. Eighteen-days pre-grown T. versicolor cartridges (1, 2, 3 or 4) were introduced into the glass column, and it was overflowed respectively with 17, 30, 43 or 50ml of an aqueous non-sterile solution of the dye (20μM). Then it was homogenized with a flow rate of 1ml/min throughout the decolorization process. Samples were taken from the base of the bioreactor to determine pH, enzymatic activities and decolorization. Controls were made with non-inoculated cartridges. Second phase configuration: after a period of either 25, 40, 100 or 110min, where the extract was subjected to decolorization in the first phase, it was overturned to the second phase that consisted of a stainless steel coil of 6.35mm diameter and a total length of 6.65 mm, placed in a thermostatic bath at 50°C. Samples were taken at the termination of the coil (at the end of the first and second phases) and they were used for pH determination, enzymatic activities and assessment of the decolorization rate.
Analytical determinationsEstimation of fungal growth: the fungus growth inside the cartridge was estimated by measuring its chitin content. The chitin content of dried samples and mycelium from malt-extract liquid cultures was determined by measuring N-acetylglucosamine (NAGA) released from chitin after hydrolysis with 6N HCl23. The mycelium that covered the spheres was removed with a scalpel to estimate the dry weight of the biofilm. Weight losses were determined based on the initial and final dry weights, drying the content of each sphere to constant weight at 80°C. Dried samples were ground in a mortar and stored until they were used for chitin determination. Polyethylene meshes were washed, dried and weighed as well to determine potential damage. For enzyme extraction, crude extracts were obtained by adding 12ml of distilled water to 2g wet solid, stirring for 20min at 120rpm, followed by filtration and centrifugation. Enzyme activities were also determined in aqueous extracts obtained by stirring 1 sphere in 30ml of distilled water for 20min. The extracts were stored at -20°C until needed. Laccase was evaluated using 5mM 2,2-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) as substrate in 0.1M sodium acetate buffer (pH 3.6) at 420nm (ɛ420=1/36mM cm)17. MnP was determined using 0.01% phenol red as substrate in 50mM succinate buffer (pH 4.5) at 610nm (ɛ610=1/36mM cm)17. All enzyme activities were measured at 30°C and expressed in units (U) defined as the amount of enzyme required to produce 1μmol of product per min per ml of aqueous extract or g of dry residue (substrate plus mycelium) extracted. Reducing sugars were quantified by the Somogyi-Nelson method32, using glucose as standard.
Decolorization assaysDecolorization capacity of aqueous solutions of the azoic dyes xylidine and Congo Red, the triphenylmethane-type dyes Malachite Green and Gentian Violet, the anthraquinonic Remazol Brilliant Blue R (RBBR) and the indigoid Indigo Carmine, was assessed measuring the decrease of dye absorbance at their maximum visible wavelength, 505, 490, 615, 590, 590 and 609nm respectively. Decolorization was expressed as percentage over the initial values8. Samples obtained from the bioreactor solution were evaluated spectrophotometrically after being appropriately diluted. The remnant dye retained in the cartridge after the decolorization processes was extracted with ethanol-water (1:1) for 24h.
Statistical analysisThe data presented are the average of the results of three replicates with a standard error of less than 5%. Analysis of variance was tested by the software Minitab v.13.0. The significant differences between treatments were compared by Tukey's multiple comparison tests at 5% level of probability.
ChemicalsMalachite Green and Congo Red were provided by Mallinckrodt (Phillipsburg, N.J., USA) and Indigo Carmine by ICN (Costa Mesa, California, USA). All other chemicals were from Sigma (St. Louis, Mo., USA).
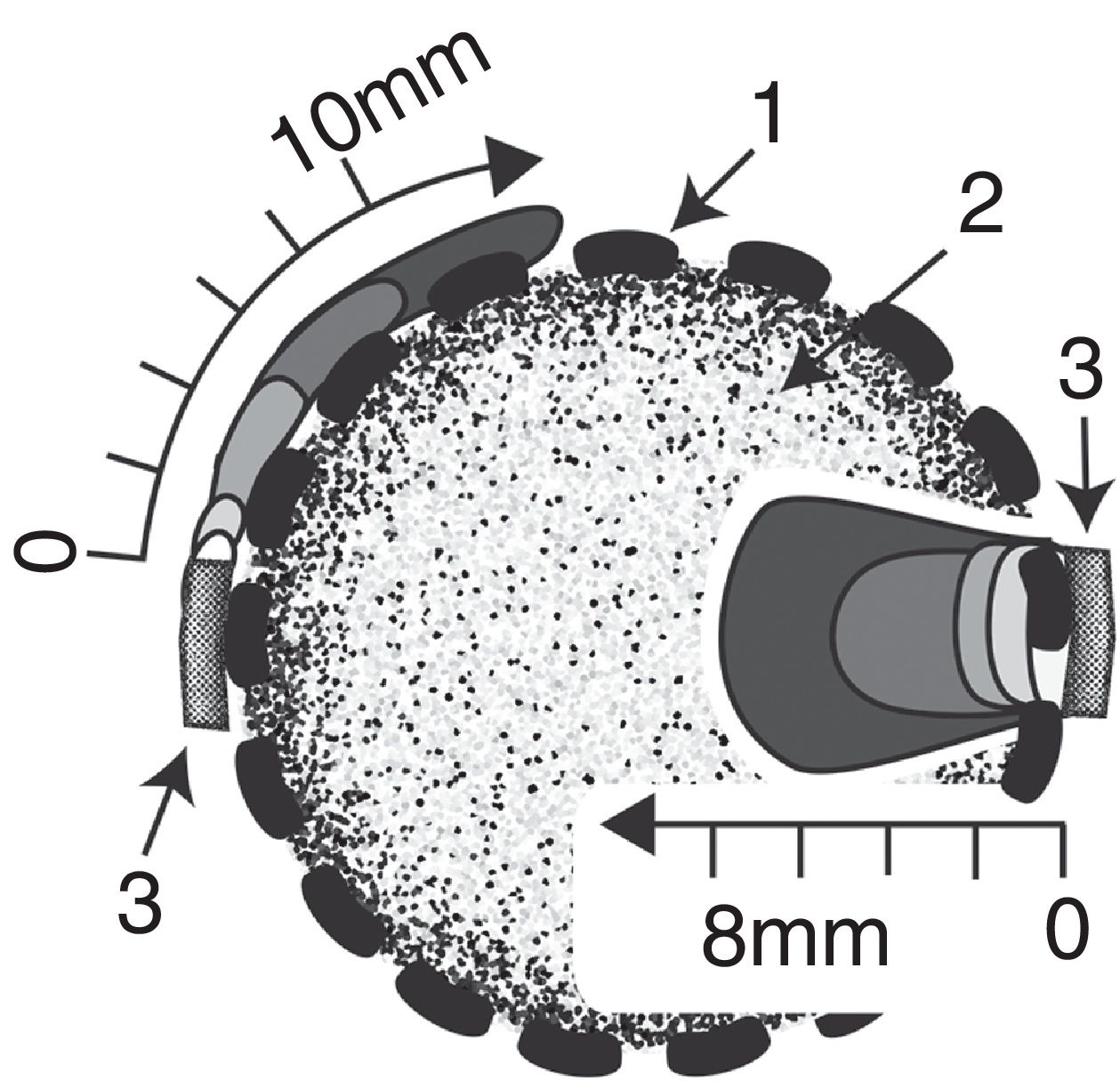
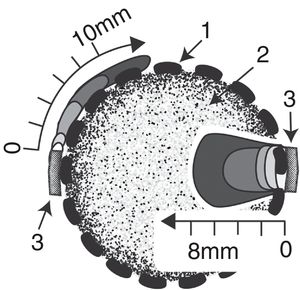
Results and discussionGrowth of T. versicolor in the spherical cartridgesA novel bioreactor system was developed for dye decolorization applying ligninolytic basidiomycetes, based on Diorio et al.’s6 two-phase bioreactor, where dyes were decolorized by immobilized fungi in the first phase and by their extracellular extracts in a second phase. Each sphere behaved as a small-scale bioreactor for cell-culture. Figure 3 depicts fungal colonization of the spherical cartridges. Growth was monitored by measuring the chitin content in the dried substrate. The ratio of glucosamine to dry mass in pure mycelium was determined by culturing T. versicolor in malt-extract liquid medium, and was used to estimate fungal biomass in the cartridges. T. versicolor (BAFC 266) mycelium not only totally invaded the substrate contained in the spherical cartridges but completely covered the surrounding mesh forming a biofilm after only 7 days as well. During the first 7 days most of the fungal biomass consisted of invasive mycelium (83%) but biomass of the fungal biofilm increased, representing 41% of the total at day 18 (Fig. 4a). Possibly the spherical shape of the cartridge allowed a better development of the biofilm, and permitted a more delimited separation between this micro reactor and its surrounding exterior. The reducing sugars measured inside the spheres attained a maximum on the 4th day of incubation, probably released by fungal hydrolytic enzymes (not determined in this work) but decreased afterwards supporting fungal growth (Fig. 4b). Temperature increased inside the cartridges (0.8±0.10°C after 16-22 days of fungal incubation), and pH varied from initial values around 5.0 up to 6.3 at the end of cultivation. Laccase activity was detected inside the cartridges from day 14 of incubation (Fig. 4c), in coincidence with a substantial decrease in the available reducing sugars (highest titers measured on day 18: 5.3 U/g). MnP activity was detected inside the cartridges from day 16 on (approx. 0.18 U/g, data not shown). Very low titers of laccase and MnP were measured in the biofilm as well. The differences in temperature and pH between the interior of the spheres and their exterior, internal accumulation of reducing sugars and ligninolytic enzymes, and the absence of dye adsorption, demonstrates their functionality. Very few articles have considered the effects of morphology on the degree of removal of recalcitrant pollutants such as dyes.30 Bermek et al.3 reported that fungal growth as dispersed filaments can increase the viscosity of the medium (low oxygen levels), limiting mass transfer and causing lower ligninolytic enzyme production. The support material for fungal growth was wheat bran. It is a stable low-cost lignocellulosic agro-residue considering its particle size, water content and physicochemical properties. High titers of laccase production were detected when growing T. versicolor on wheat bran34.
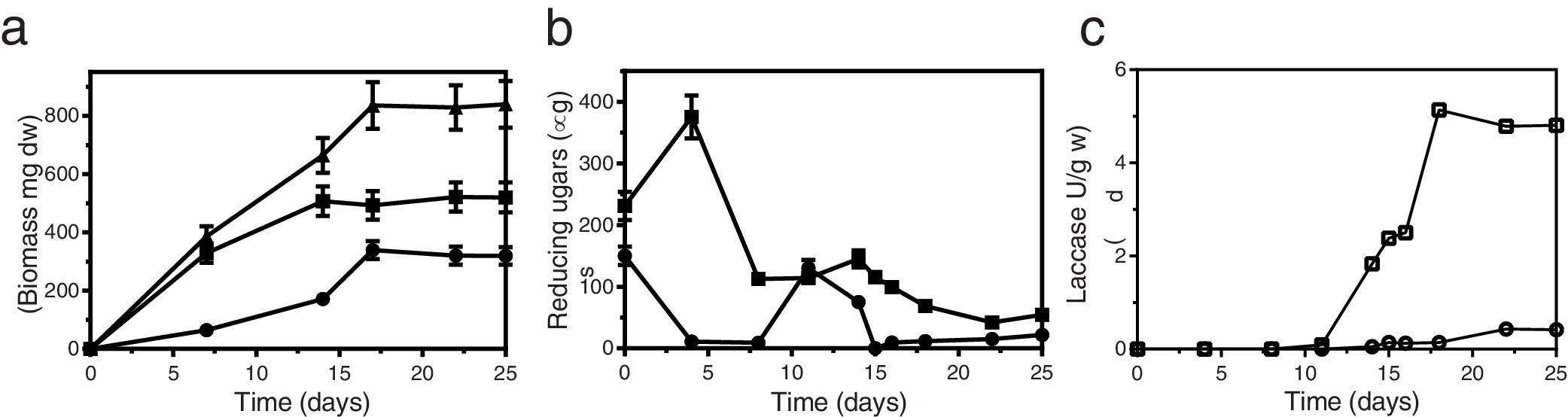
Time course of growth and laccase production by T. versicolor BAFC 266 growing in the spherical cartridge. (a) Biofilm biomass (●), invasive mycelium (■) and total biomass (
). (b) Glucose consumption inside the cartridge (■), and in the biofilm zone (●). (c) Laccase production inside the cartridge (□), and in the biofilm zone (○). Error bars denote SEM.One up to five spheres with T. versicolor (BAFC 266) grown for 18 d in wheat bran were introduced in the bioreactor. The activities of laccase and MnP retained in the interior of the solid medium were 4.7 and 43.7 times higher respectively than those detected in the aqueous extracts, showing not only abundant exoenzyme production by T. versicolor (BAFC 266) under the experimental conditions assayed, but also a significant accumulation inside the bran spheres grown with the fungus, which would therefore allow their reutilization. The bioreactor was flooded with 17, 30, 43 or 50ml respectively of an aqueous non-sterile solution of Malachite Green (20μM). After 40min decolorization reached 32-35%, the best values were attained with 2 spheres. The fungus remained well attached to the carrier during the whole experiment and the medium stayed totally clear. Total decolorization was approx. 95% after 240min of Malachite Green retention. Ligninolytic activities were evaluated in the decolorized solution (respectively among 0.66-0.78 U/ml laccase and 0.09-0.13 U/ml MnP during all the incubation period). Laccase highest values were detected when 2 spheres were applied. The dye was rapidly removed from the medium by physical adsorption, but was later eliminated both from the solution and the surface of the carriers, as a consequence of enzymatic degradation, and Malachite Green retained (adsorbed) in the spheres was not detected, at the end of the assay. Similar results were obtained by Aretxaga et al.1 when comparing dye adsorption by dead pellets of T. versicolor and dye removal by enzymatic degradation. Likewise, sorption accounted for less than 3% of dye removal by ligninolytic (dye-decolorizing) cultures of Pycnoporus sanguineus24.
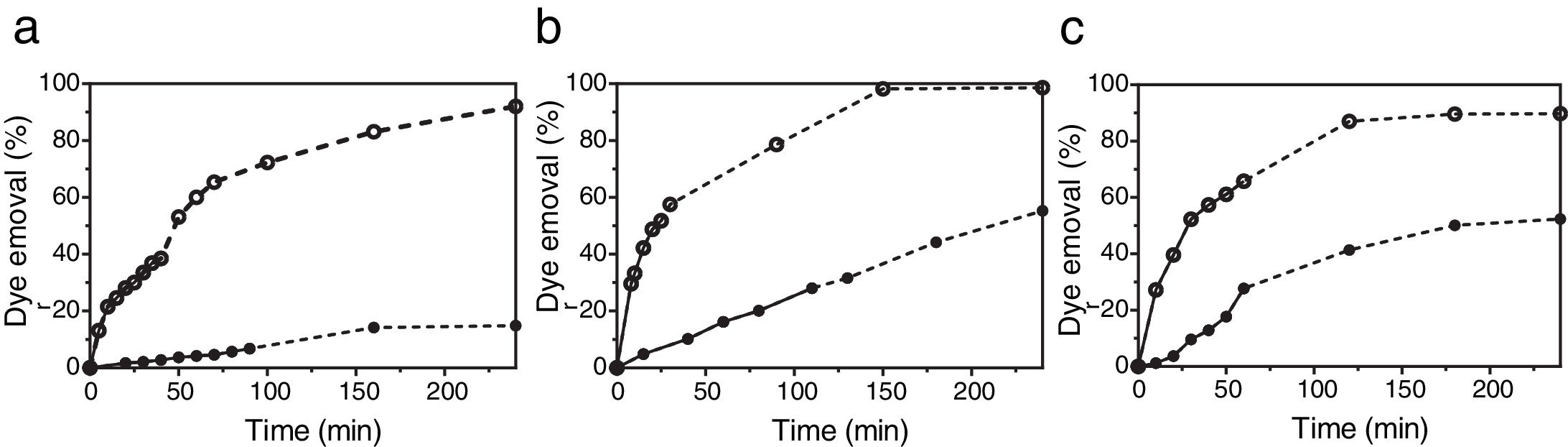
The bioreactor filled with two bran spheres colonized by T. versicolor BAFC 266 was also applied to the decolorization of several dyes with different chemical structures. This fungus was able to decolorize 50% of Gentian Violet, Congo Red, RBBR and Indigo Carmine (each 20μM) in the bioreactor after 53, 118, 141 and 14min respectively. The pH of the extracts did not vary throughout the entire experience, thus decolorization cannot be attributed to pH-dependent color variation25. The respective percentages of decolorization after 240min were 70, 54, 54 and 80%. Dye decolorization of Malachite Green, xylidine or a mixture of both dyes (each 20μM) was assayed as well. After 240min approx. 15% xylidine degradation was detected (Fig. 5a). Laccase and MnP activities evaluated in the xylidine decolorized solution after 90min were lower than those measured in the presence of Malachite Green (0.22 U/ml laccase and 0.02 U/ml MnP). In a previous work, submerged cultures of T. versicolor (BAFC 266), which only displayed laccase activity, decolorized 28% of xylidine (24mg/l), 30% of Poly-R 478 (75mg/l), 43% of Remazol Brilliant Blue R (9mg/l), 88% of Malachite Green (6mg/l) and 98% of Indigo Carmine (23mg/l), in 1h.17 The efficiency of Malachite Green decolorization was not affected by increasing the dye up to 100μM6. T. versicolor (BAFC 2234) grown for 18 d in two bran spheres was also evaluated in the bioreactor. Malachite Green decolorization rates were higher than those attained by T. versicolor (BAFC 266) not only during the first phase in the bioreactor but also in the second one: 52% decolorization was registered after 25min and 98.2% after 150min (Fig. 5b). Laccase but no MnP activity was detected in the decolorized solution (0.09 U/ml), as previously recorded during Malachite Green decolorization in liquid cultures of other Trametes species11. T. versicolor (BAFC 2234) could also decolorize 28% of xylidine after 110min and 55.3% after 240min. T. versicolor BAFC 2234 xylidine decolorization rate (2.9mg/l h) resembles the previously described for other strain of the same species in relation to different azo dyes (3mg/l h)35. Both ligninolytic activities tested were detected in the decolorized solution after 110min (respectively 0.22 U/ml laccase and 0.12 U/ml MnP). When Malachite Green and xylidine were applied together in the bioreactor using T. versicolor BAFC 2234 (Fig. 5c), after a first phase that lasted 100min 73.5% of Malachite Green and 40% of xylidine was decolorized. At the end of the second phase (240min) 96.6% of Malachite Green and 51.7% of xylidine were decolorized. Laccase activity was detected in the decolorized solution (0.16 U/ml), but no MnP activity. In a preceding work when cylindrical cartridges filled with wheat bran were used to support fungal growth, 4 up to 20% of Malachite Green was retained inside the cartridges6. In this work dye adsorption was not noticed at the end of the assay. The spherical design might allow a better development of the biofilm surrounding the cartridge and impeded dye adsorption by the substrate.
Profile of xylidine (20μM) (●) and Malachite Green (20μM) (○) dye removal attained by solid-state cultures of T. versicolor BAFC 266 (a) and T. versicolor BAFC 2234 (b) when the dyes were applied individually in the bioreactor. (c) xylidine and Malachite Green dye removal by T. versicolor BAFC 2234 when the dyes were applied together. First phase (●; ○) and second phase (···●···; ···○···).
Previous studies have shown that many enzymes are involved in Malachite Green decolorization, and that these enzymes are often species-specific; however, the main enzymes responsible for Malachite Green decolorization and detoxification by Pseudomonas bacteria, and by the filamentous fungi Penicillium pinophilum and Myrothecium roridum, proved to be laccase and malachite green-reductase11. Low-molecular-weight factors might be involved in dye decolorization as well35. Mycelial biomass may supply other intracellular or mycelial-bound enzymes, or other compounds that favor decolorization. Significant differences in the ability to decolorize Malachite Green among strains of Trametes were previously detected, but in most of the studied strains there was a correlation between laccase production and decolorization capacity.4 Malachite Green decolorization by T. versicolor (BAFC 266)6 was accompanied by detoxification. Co-cultivation of laccase-producing strains is a natural way to induce laccase production, in the form of either yield increase or induction of new isozymes. Microbial interactions with laccase inducing effects vary with the strain, but the structure of inducing metabolites and the mechanism involved remain basically unknown.36 Co-cultivation of Ganoderma lucidum and T. versicolor resulted in a noticeable increase in laccase activity along with more efficient decolorization and detoxification of Malachite Green13. In the present work, the bioreactor was alternatively filled with two bran spheres, each with one of T. versicolor isolates. Nevertheless, similar results were obtained; 89.8% of Malachite Green and 52.4% of xylidine were decolorized after 4h (data not shown).
Fungal dye decolorization under non-sterile conditions has been scarcely investigated up till now27,30. The application of white-rot fungi in continuous bioreactors for dye wastewater treatment has been so far hindered by difficulties such as excessive growth of fungi causing reactor-clogging, bacterial contamination inhibiting fungal decolorization, and loss of extracellular enzymes and mediators essential for dye degradation with treated effluents30. Nevertheless, this system allows decoupling of growth (sterile condition) and decolorization (non-sterile condition) stages.
In conclusion, a novel bioreactor system (low cost, eco-friendly, and easily scaled-up) is presented for dye decolorization applying ligninolytic basidiomycetes. In this two-phase bioreactor, dyes were decolorized in the first phase by fungi immobilized in spherical plastic polyethylene mesh cartridges filled with wheat bran, and in a second phase by their extracellular extracts. The change of the cartridges is easy and quick, allowing a continuous use of the bioreactor in the decolorization process. Moreover, they can be used as sources of laccase for additional applications. Immobilized cell systems make easy to separate cells from the liquid medium, which simplifies subsequent downstream processes. The white-rot fungus T. versicolor BAFC 2234 immobilized in this bioreactor decolorized the recalcitrant dyes xylidine and Malachite Green with high efficiency. In addition, no operational problems were detected during cultivation. Based on these results, this system may have good prospects for application in industrial wastewater treatment.
Financial supportPIP11220170100283 and UBACYT 2020020170100163.
Conflict of interestThe authors declare that they have no conflicts of interest.
To Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET and Universidad de Buenos Aires (UBA), Argentina, for financial support.