In 2011, Argentina launched a government-funded national Human papillomavirus (HPV) immunization program incorporating a bivalent HPV vaccine, with a 0–1–6-month schedule, for girls 11 years of age, born after January 2000. Monitoring the changes of HPV infection prevalence among young women has been proposed as an endpoint for early assessment of HPV vaccination programs. However, the data on HPV prevalence at young ages are very limited. The aim of this work was to determine the prevalence of HPV infection and type-specific distribution in sexually active 15–17-year-old non-vaccinated girls. Cervical samples from 1073 adolescents were collected for HPV detection and genotyping using the BSGP5+/GP6+PCR-reverse line blot (RLB) assay. Out of 957 specimens analyzed, 56.3% were positive for any HPV type; 42.2% harbored at least one high-risk HPV (HR-HPV) type and 30.8% low-risk HPV (LR-HPV) types. Multiple and single infections were identified in 36.3% and 20.0% of the samples respectively. The 6 most common HR-HPV types were HPV16 (11.1%), HPV52 (10.8%), HPV56 (8.3%), HPV51 (7.4%), HPV58 (7.3%) and HPV31 (7.1%). The prevalence of HR-HPV-16/18 was 15.2%. In conclusion, results confirm that HPV (particularly HR-types) are very common among sexually active adolescents, and prevalence rises quickly after their sexual debut. Our HPV type-specific prevalence baseline may be used to monitor post-vaccinal longitudinal changes in Argentina.
En 2011 se introdujo en el Calendario Nacional de Vacunación de Argentina una vacuna bivalente contra el virus del papiloma humano (HPV, por sus siglas en inglés) tipo 16 y 18, para ser aplicada en niñas de 11 años. Se propuso la monitorización de los cambios en la prevalencia de infección por este virus HPV en las adolescentes como un criterio de valoración temprano para evaluar programas de vacunación; sin embargo, los datos sobre prevalencia del HPV en la población joven son limitados. El objetivo de este estudio fue determinar la prevalencia de la infección por el HPV y su distribución tipo-específica en mujeres de 15 a 17 años, sexualmente activas y no vacunadas. Se colectaron 1.073 muestras cervicales para la detección y tipificación del HPV mediante la técnica de PCR BSGP5+/GP6+-hibridación reversa en línea (RLB). Sobre 957 muestras analizadas, la frecuencia global de infección fue del 56,3%. El 42,2% de los casos positivos presentó algún genotipo de alto riesgo oncogénico (HPV-AR) y un 30,8% al menos un genotipo de bajo riesgo oncogénico (HPV-BR). El 20,0 y el 36,3% fueron infecciones simples y múltiples, respectivamente. Los HPV-AR más prevalentes fueron HPV16 (11,1%), HPV52 (10,8%), HPV56 (8,3%), HPV51 (7,4%), HPV58 (7,3%) y HPV31 (7,1%). La prevalencia conjunta de HPV16/18 fue del 15,2%. En conclusión, los HPV (en especial los HPV-AR) son muy frecuentes en adolescentes jóvenes sexualmente activas, con un rápido aumento de la prevalencia tras el comienzo sexual. La prevalencia tipo-específica basal registrada podrá servir para monitorizar cambios en la prevalencia de los tipos vacunales y no vacunales en el tiempo.
Genital Human papillomavirus (HPV) is the most common sexually transmitted viral infection29. More than 200 HPV types have been identified and officially recognized by the International Human Papillomavirus Reference Center, located at the Karolinska Institute in Stockholm, Sweden19.
Given their oncogenic potential and clinical relevance, approximately 40 HPV types within the genus Alphapapillomavirus are roughly subdivided into high-risk (HR) and low-risk (LR) HPV types2,34,45,50. According to the classification of the International Agency for Research on Cancer (IARC-WHO), HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 are carcinogenic to humans (Group 1), being etiologically associated with more than 98% of cervical cancers (CCs), 70–90% of anal and vaginal cancers, 40% of vulvar cancers, 47% of penile cancers, and 25 to 30% of oropharyngeal cancers11. HPV 68 is probably carcinogenic to humans (Group 2A). HPV types 26, 30, 34, 53, 66, 67, 69, 70, 73, 82, 85, and 97 are classified as possibly carcinogenic to humans (Group 2B). HPV types 6 and 11, the archetypes of LR-HPVs, are not classifiable as to their carcinogenicity to humans (Group 3), being associated with more than 90% of anogenital warts and laryngeal papillomas (benign lesions)25.
In Argentina, around 4500 new CC cases are diagnosed every year, and almost half of the women affected die23.
The introduction of HPV vaccines offers a valuable tool to prevent CCs. Currently, three prophylactic HPV vaccines are available: a bivalent HPV16/18 vaccine (Cervarix; GlaxoSmithKline Biologicals, Belgium), a quadrivalent HPV6/11/16/18 vaccine (Gardasil/Silgard; Merck & Co., USA/Sanofi Pasteur MSD, France), and a nonavalent HPV6/11/16/18/31/33/45/52/58 vaccine (Gardasil9, Merck & Co.). After the first 13 years of routine use of HPV vaccines, comprehensive clinical trials and real-life data have confirmed their safety and effectiveness47.
The World Health Organization (WHO) recommends the inclusion of the HPV vaccine in national immunization programs primarily targeting girls aged 9–14, prior to becoming sexually active, and as a secondary target, females aged ≥15, or males (only when feasible and cost-effective, and without diverting resources from the primary population vaccination program)47.
To prevent CC, in October 2011 Argentina launched the most comprehensive government-funded national HPV prevention program of Latin America, incorporating a bivalent HPV vaccine, with a 0–1–6-month schedule, for girls 11 years of age, born after January 200014. This intervention also involved reinforcing CC screening in women aged 30–64 as a secondary prevention strategy, while gradually introducing HPV testing3. In 2014, the programme switched to the quadrivalent vaccine, and was extended to males and females aged 11 to 26 living with HIV, and transplanted individuals with a 3-dose schedule (0, 2 and 6 months). In 2015 the number of doses was reduced to two and two years later, vaccination was extended to boys aged 11, born after 200614.
Since the first generation of HPV vaccines (bivalent and quadrivalent) protects against some, but not all the types capable of causing associated cancers, monitoring both the positive and negative effects of these vaccines on other HPV types is critical to evaluate their impact on the population. Data on type-specific HPV distribution may be useful to evaluate the additional benefits of vaccines, e.g. cross protection against non-vaccine types and herd immunity, and to monitor unlikely adverse phenomena such as type replacement.
Because the major benefit of HPV vaccination -the prevention of cervical and other less common HPV-associated cancers-will take decades to be seen, a number of previous outcomes are being monitored toward an earlier assessment of the impact of HPV vaccines, primarily in young adolescents7,49. The short-term evidence of the effectiveness of the vaccine program is crucial to encourage government planners to sustain and improve HPV vaccination services.
Since no data existed on the HPV burden in adolescent women in Argentina, a study was conducted to determine the type-specific HPV prevalence in cervicovaginal samples from sexually active, unvaccinated girls aged 15–17 years. This data provides an important baseline to compare future changes in overall and type-specific HPV prevalence after HPV vaccination.
Materials and methodsStudy designFrom April 2014 to October 2015, a cross sectional study about HPV genotype circulation in unvaccinated girls was conducted by the National and Regional Reference HPV Laboratory (N&R-HPV Lab) (National Institute of Infectious Diseases, ANLIS Malbrán, Buenos Aires).
When girls sought medical advice, particularly about contraception, the gynecologists of the Adolescence Services invited them to join the study. Inclusion criteria was: no HPV vaccine, 15–17 years of age, and sexually active.
Fifteen years of age was established as the lower limit of the reference range since that is the average age at which adolescents start sexual activity in Argentina42.
Due to difficulties related to the target population, a convenience sampling method was designed and used.
For the adolescent population sample to be broad and representative, girls were recruited in six public hospitals from four cities of Argentina: Hospital Evita Pueblo (Berazategui, Province of Buenos Aires; east-central area of Argentina), Hospital Madariaga (Posadas, Province of Misiones; northeastern area of Argentina), Centro Integral de Salud La Banda (La Banda, Province of Santiago del Estero, north-central area of Argentina; Hospital Argerich, Hospital Rivadavia and Hospital Durand (Buenos Aires city, Capital city, east-central area of Argentina).
Recruitment and sample collectionThe girls who agreed to participate signed an informed consent and answered a short questionnaire that included basic information on age, date and place of birth, current address and age of sexual debut. These data are relevant to define certain basic characteristics of the group under investigation, and allow for a valid comparison with data to be obtained from vaccinated girls in the near future.
In order to standardize the collection, storage and transfer of the samples obtained in the different centers, a Standard Operating Procedure (SOP) was developed and shared in the training workshops carried out in each of the centers before launching the project. Audit visits were made to evaluate the progress of these processes.
Cervical samples were obtained from both the endocervix and the ectocervix, using a Cytobrush (hc2 DNA Collection Device, Qiagen) introduced in the cervical canal and rotated 360°, 5 times. The cellular material was placed in a vial containing specimen transport medium (STM, Qiagen) and stored at +4°C until its shipment (within fifteen days) to the N&E-HPV Lab for processing.
DNA extraction from cervical samplesDNA was extracted from 200μl of STM sample using commercial columns (Qiagen) on a robotic system (QIAcube system, Qiagen), following the manufacturer's instructions. DNAs were stored at −20°C until use. For every ten samples, a tube containing only STM medium was included as a control for contamination.
HPV detection and genotypingHPV detection and genotyping was performed by PCR using Broad-Spectrum General Primers (BSGP) 5+/6+ biotin-labeled designed to amplify a highly conserved 140bp fragment of the HPV-L1 gene41,44. DNA amplification was combined with a reverse line hybridization (RLB) which identifies 36 HPV genotypes (6, 11, 16, 18, 26, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 61, 66, 68, 70, 71, 72, 73, 81, 82, 83, 84 and 89), including all the HR-HPV established to date.
Briefly, the denatured biotinylated amplicons were hybridized with genotype-specific oligonucleotide probes immobilized as parallel lines on a Biodyne C membrane (Life Science) using the Miniblotter MN45. The hybrids were treated with alkaline phosphatase-streptavidin conjugate (GE HealthCare) and substrate (ECL Detection Reagents, GE HealthCare), resulting in a chemiluminescent product subsequently detected by exposure to autoradiography film. The β-globin gene (208bp fragment) was co-amplified and its relative abundance detected for each sample to serve as internal control41.
Every year the N&R-HPV Lab participates in the external control of HPV genotyping (HPV DNA typing proficiency panel), provided by the International Papillomavirus Reference Center, which serves as Quality Control of the results obtained in this study16.
Statistical analysesData were entered and analyzed in an especially designed database, and subsequently processed using the SPSS Statistics 23.0.0.3 (IBM) statistical software. The association between proportions was assessed by the chi-square test and the 95% confidence interval (CI) was considered in all cases. For all the data analyses conducted, p values <0.05 were considered statistically significant.
Ethical and legal considerationsThe present study was approved by the ANLIS Malbrán Research Ethics Board and the Ethics Committee of each participating hospital to ensure confidentiality and anonymity. A written informed consent was obtained before enrolling the study participants.
Under the Argentine legal system, adolescents over 14 years of age have the right to be assisted and receive health-related care unaccompanied by an adult or guardian; they may also decide to participate in research projects and sign their consents.
Because the teenage girls invited to participate in the study were not within the age range for CC screening, special care was taken to report that it was only a research study; the opportunity was taken to teach basic concepts about HPV infection and health care which included the age at which screening should begin to avoid generating alarm and/or an inappropriately early screening onset.
In some provinces, social workers gave talks in secondary schools informing about the study within the framework of health care counseling seminars.
ResultsThe study enrolled 1073 unvaccinated, sexually active girls who provided cervical cell samples; 116 were excluded because their ages were not within the established range. The remaining 957 samples were amplifiable for beta-globin, thus being adequate for the PCR analysis; therefore, this analysis refers to them. The distribution of the samples collected in the four cities was as follows: 272 from Berazategui, 172 from Posadas; 215 from La Banda, and 298 from Buenos Aires.
The prevalence of HPV infection of any type was 56.3% (95% CI; 53.2–59.5), 42.2% (95% CI; 39.1–45.3) were infected with at least one HR-HPV genotype and 7.9% (95% CI; 6.2–9.7) with LR-HPV genotypes only.
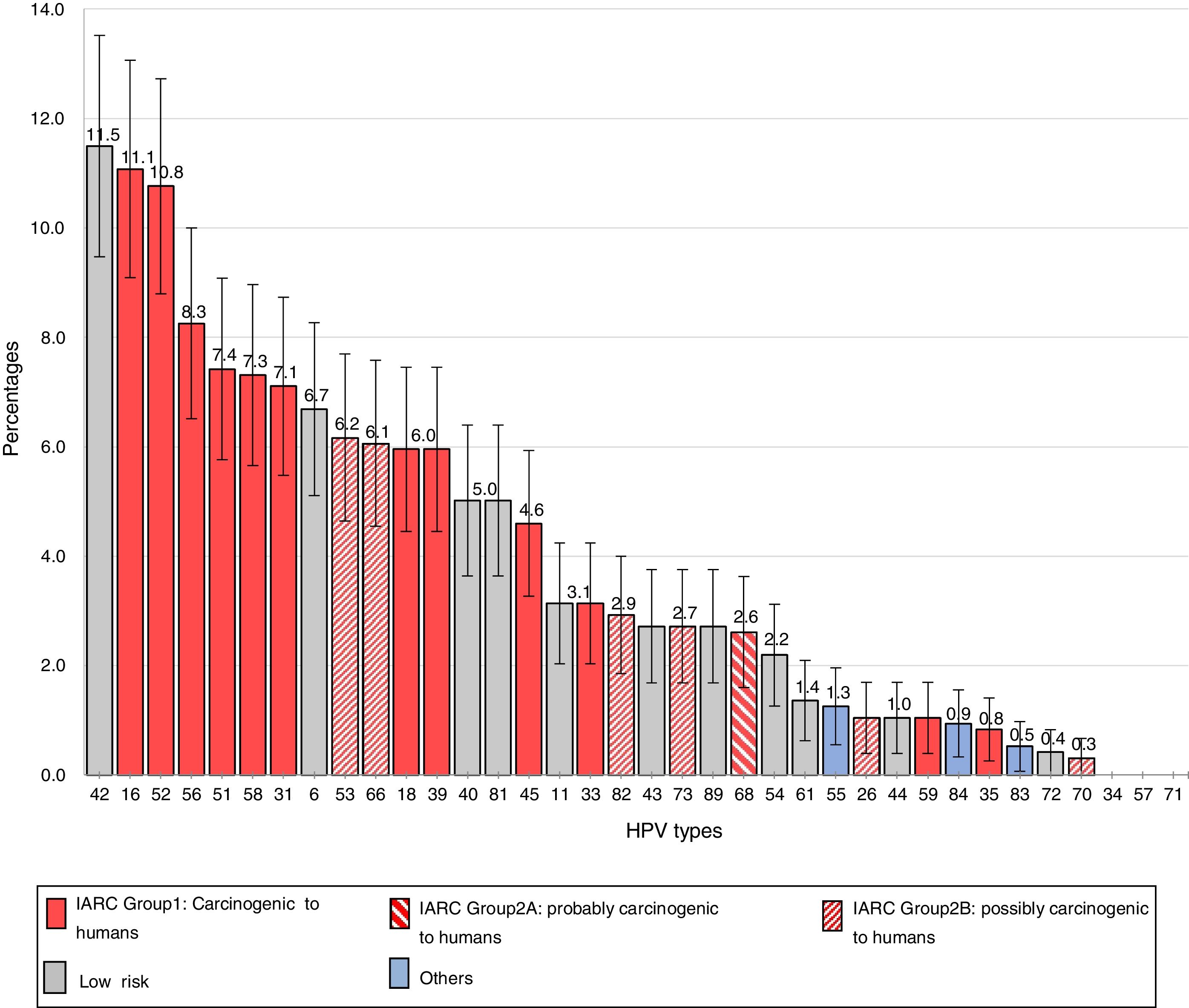
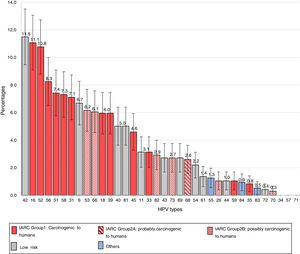
The frequencies of the different genotypes in descending order are presented in Figure 1. The 6 most common HR types were HPV16 [11.1%; (95% CI, 9.1–13.1)], HPV52 [10.8%; (95% CI, 8.8–12.7)], HPV56 [8.3%; (95% CI, 6.5–10.0)], HPV51 [7.4%; (95% CI, 5.8–9.1)], HPV58 [7.3%; (95% CI, 5.7–9.0)] and HPV31 [7.1%; (95% CI, 5.5–8.7)].
Among the LR types, HPV42 had the highest prevalence [11.5% (95% CI, 9.5–13.5)], followed by HPV6 [6.7%; (95% CI, 5.1–8.3)], HPV40 and HPV81 [5.0%; (95% CI, 3.6–6.4)] and HPV11 [3.1%; (95% CI, 2.0–4.2)].
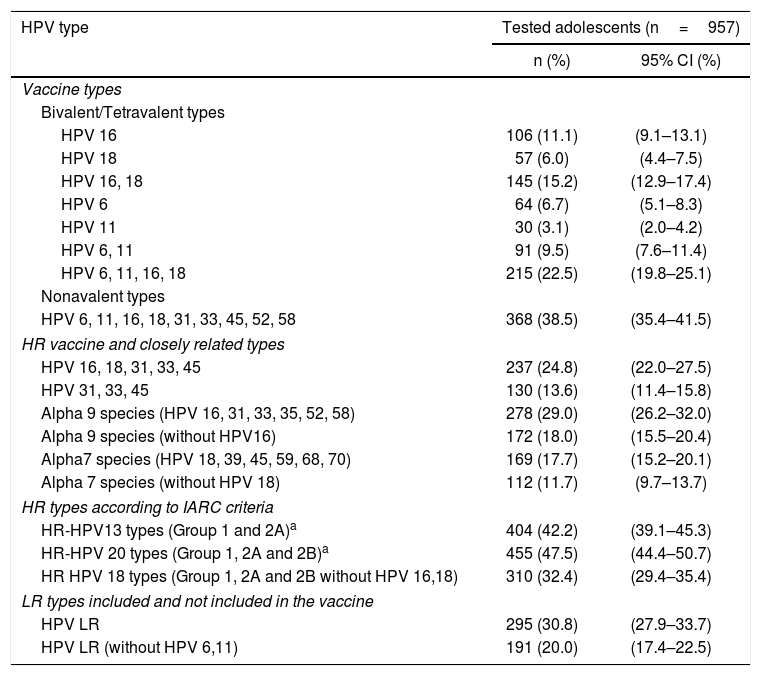
The prevalence of types closely related to the HPV vaccine, types against which cross-protection has been reported in clinical trials, and types according to IARC criteria are shown in Table 1.
Prevalence of selected HPV types and groups of types among sexually active non-vaccinated 15–17-year-old girls from Argentina.
| HPV type | Tested adolescents (n=957) | |
|---|---|---|
| n (%) | 95% CI (%) | |
| Vaccine types | ||
| Bivalent/Tetravalent types | ||
| HPV 16 | 106 (11.1) | (9.1–13.1) |
| HPV 18 | 57 (6.0) | (4.4–7.5) |
| HPV 16, 18 | 145 (15.2) | (12.9–17.4) |
| HPV 6 | 64 (6.7) | (5.1–8.3) |
| HPV 11 | 30 (3.1) | (2.0–4.2) |
| HPV 6, 11 | 91 (9.5) | (7.6–11.4) |
| HPV 6, 11, 16, 18 | 215 (22.5) | (19.8–25.1) |
| Nonavalent types | ||
| HPV 6, 11, 16, 18, 31, 33, 45, 52, 58 | 368 (38.5) | (35.4–41.5) |
| HR vaccine and closely related types | ||
| HPV 16, 18, 31, 33, 45 | 237 (24.8) | (22.0–27.5) |
| HPV 31, 33, 45 | 130 (13.6) | (11.4–15.8) |
| Alpha 9 species (HPV 16, 31, 33, 35, 52, 58) | 278 (29.0) | (26.2–32.0) |
| Alpha 9 species (without HPV16) | 172 (18.0) | (15.5–20.4) |
| Alpha7 species (HPV 18, 39, 45, 59, 68, 70) | 169 (17.7) | (15.2–20.1) |
| Alpha 7 species (without HPV 18) | 112 (11.7) | (9.7–13.7) |
| HR types according to IARC criteria | ||
| HR-HPV13 types (Group 1 and 2A)a | 404 (42.2) | (39.1–45.3) |
| HR-HPV 20 types (Group 1, 2A and 2B)a | 455 (47.5) | (44.4–50.7) |
| HR HPV 18 types (Group 1, 2A and 2B without HPV 16,18) | 310 (32.4) | (29.4–35.4) |
| LR types included and not included in the vaccine | ||
| HPV LR | 295 (30.8) | (27.9–33.7) |
| HPV LR (without HPV 6,11) | 191 (20.0) | (17.4–22.5) |
HR-types: High risk HPV types; LR: low risk HPV types.
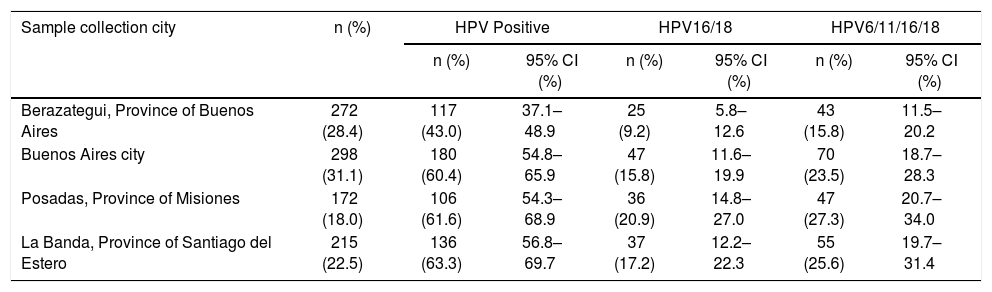
Data about HPV prevalence broken down by city of sample collection are shown in Table 2. Significant differences were observed for the prevalence of HPV infection of any type only between Berazategui and the rest of the cities (p<0.000); the prevalence of HPV16/18 and 6/11/16/18 differed significantly only between Berazategui and Posadas (p=0.001 and p=0.004, respectively).
Prevalence of HPV infection of any type, HPV 16/18, and HPV 6/11/16/18 by city of recruitment, among sexually active non-vaccinated 15–17-year-old girls from Argentina.
| Sample collection city | n (%) | HPV Positive | HPV16/18 | HPV6/11/16/18 | |||
|---|---|---|---|---|---|---|---|
| n (%) | 95% CI (%) | n (%) | 95% CI (%) | n (%) | 95% CI (%) | ||
| Berazategui, Province of Buenos Aires | 272 (28.4) | 117 (43.0) | 37.1–48.9 | 25 (9.2) | 5.8–12.6 | 43 (15.8) | 11.5–20.2 |
| Buenos Aires city | 298 (31.1) | 180 (60.4) | 54.8–65.9 | 47 (15.8) | 11.6–19.9 | 70 (23.5) | 18.7–28.3 |
| Posadas, Province of Misiones | 172 (18.0) | 106 (61.6) | 54.3–68.9 | 36 (20.9) | 14.8–27.0 | 47 (27.3) | 20.7–34.0 |
| La Banda, Province of Santiago del Estero | 215 (22.5) | 136 (63.3) | 56.8–69.7 | 37 (17.2) | 12.2–22.3 | 55 (25.6) | 19.7–31.4 |
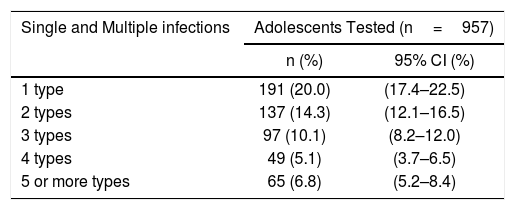
In 36.3% (95% CI: 33.3–39.4) multiple infections were identified and up to 9 different HPV types were found in one sample (Table 3).
Single and multiple human papillomavirus infections among sexually active non-vaccinated 15–17-year-old girls from Argentina.
| Single and Multiple infections | Adolescents Tested (n=957) | |
|---|---|---|
| n (%) | 95% CI (%) | |
| 1 type | 191 (20.0) | (17.4–22.5) |
| 2 types | 137 (14.3) | (12.1–16.5) |
| 3 types | 97 (10.1) | (8.2–12.0) |
| 4 types | 49 (5.1) | (3.7–6.5) |
| 5 or more types | 65 (6.8) | (5.2–8.4) |
Several studies have shown cervical HPV prevalence and type distribution in women older than 18 years of age in different study designs worldwide10,13,22,30,33,36,38; however, there is very scarce information for young adolescents. The present work reports HPV prevalence and genotype distribution among 957 sexually active non-vaccinated girls from Argentina, aged 15–17 years.
In this study, the overall HPV prevalence (56.3%) was similar to previous local data from the subgroup of 15–23-year-old (y) women from Buenos Aires and Santa Fe (Argentina) (46.7% and 64.2%, respectively)9, and also close to that obtained for comparable age groups in other regions like Sweden (60%, subgroup 16 y)37, USA (49% for 11–19 y15; 71% for 14–17 y17), Australia (66.3%; subgroup under 21 y)43, and Colombia (60.3% for 18–25 y35; and 49% for 12–19 y12), but higher than Africa where 36% was reported in non-vaccinated women aged ≤16 years46. Results confirm that this infection is very common among sexually active adolescents, and rises quickly after their sexual debut21,48.
The present study showed a prevalence of 42.2% for HR-HPV types (Group 1 and 2A IARC), which is consistent with previous surveys from Colombia (48% for 12–19 y)12, Denmark (40% for 15–19 y)28, Sweden (over 50% for 16 y)37 and France (35.71%; 15–18 y)4.
The prevalence of HPV infection of any type was similarly high in all the cities of sample collection, with the exception of Berazategui, where a significant smaller value was recorded (Table 2). Although Berazategui is located in the same geographical region as Buenos Aires city, it has very different urban and demographic characteristics since it is a small town (170000 inhabitants). Anyhow, this observation is difficult to explain because we have no information about the participants’ variables related to sexual behavior such as the number of sexual partners. Furthermore, we cannot rule out that these estimates may be unstable due to the limited sample size by city.
A high prevalence of HR-HPV infections in young women does not necessarily mean a high rate of persistent infection, a pre-requisite for the development of precancerous and cancerous lesions, since most of these infections are transient and will be cleared in less than two years24; this is the main reason for CC screening to be discouraged under 25 years of age40. In cross-sectional prevalence studies such as this one, it is not possible to differentiate transient infections from those that will persist; however, premature contact with HR-HPV at the onset of adolescence could increase the risk of cervical disease at an earlier age if a persistent infection is established.
Estimates of type-specific HPV prevalence in cervical samples vary greatly worldwide among different populations according to age range, cytological status, laboratory method used, and the biological interaction between different viruses and the host's immunogenetic factors10. In this study, it was found that 15.2% of the girls were infected with HPV 16/18, the HR types included in the bivalent and quadrivalent vaccines. This rate is close to that reported in Denmark (13.6%) for women aged 15–19 y28, England (11.5%) for 13–15 y22 and USA (14.3%) for 11–19 y15.
It is well known that HPV vaccines do not impact on infections that are present at the time of immunization20. Under the public health plan, the Argentine regular immunization program introduced HPV vaccination in 11-year-old girls to get the maximum benefit. The fact that 15% of the girls participating in this study were already infected by HPV16/18 supports the decision of local sanitary authorities to vaccinate at an early age, before their sexual debut47. However, the vaccines were also approved for older women who were already sexually active; since it is unlikely that they were infected with all the vaccine HPV types, they could benefit from vaccination and speed up the public health impact of vaccination and increase short-term benefits5.
The 6 most common HR types identified in this work were HPV16 (11.1%), HPV52 (10.8%), HPV56 (8.3%), HPV51 (7.4%), HPV58 (7.3%) and HPV31 (7.1%), 4 of which were included in the nonavalent vaccine formula; this could represent a beneficial future scenario if the nonavalent vaccine were used.
In the case of the bivalent and quadrivalent HPV vaccines, the possibility of cross-protection against other HPV types is an extremely important aspect since it could increase the number of prevented cervical cancers. To date, there is evidence on the bivalent and quadrivalent vaccines cross-protection against HPV types 31, 33 and 4547. It was shown that the efficacy in preventing 6-month persistent infection in HPV naive women vaccinated with the current vaccines does not exceed 30% for non-vaccine genotypes, namely HPV 31, 33, 45, 52, 588,31. However, a more recent report from Scotland revealed that immunization with the bivalent vaccine has led to a startling reduction in vaccine and cross-protective HPV types seven years after vaccination; there is also evidence of herd protection against the vaccine-specific and cross-protective types (HPV types 31, 33 and 45)6,26. The data is relevant considering that Argentina started the national immunization program using the bivalent vaccine; local studies on the prevalence and risk of non-vaccine HPV types are important to better assess the effectiveness of vaccination.
In almost 30% of the samples at least one LR-HPV was identified, in line with data from Denmark that show 25% prevalence of these viruses among women aged 15–19 y28. In this study, the prevalence of HPV6/11, the components of the quadrivalent and nonavalent vaccines is 9.5%; a similar amount was previously detected in Sweden (10.1%)37, and a higher percentage in Denmark (16.1%)28; in the three cohorts, the rate of HPV11 prevalence was about one half of that obtained for HPV6, confirming the predominance of the latter.
Notably, HPV42 was the most common type in this group of adolescents (11.5%). In Sweden, HPV42 (6.4%) was the most frequent type among the LR-HPVs; however, the total type-specific prevalence ranking was led by HPV1637. In several studies on cervical samples from healthy women in Denmark and the Netherlands, the prevalence of HPV42 was lower than that of HPV6 and roughly equal to that of HPV1128,32. However, reports from Southern Europe and Africa show that the prevalence of HPV42 exceeds that of HPV6 and HPV111,27. At first, the high prevalence of HPV42 raised the question of whether it would be advantageous to include HPV42 in a vaccine. Nevertheless, a study on HPV types in external genital warts in a group of Argentine women showed that HPV6 and/or 11 were present in 93.3% of the cases, indicating that most of the lesions could be prevented by the quadrivalent HPV vaccine18.
The results of the present study also show that infection with multiple HPV types is frequent in adolescents (36.3%), in accordance with previously published data from Sweden (38%) in a cohort of 15–23-year-old non-vaccinated women37 and Colombia (29%) in 12–19-year-old girls12; while in African girls, the value rises more than 50% for sexually active young women (<25 years of age)46. Multiple cervical HPV infections have been extensively associated with young age, which may be due to a lack of natural immunity to HPV during the first years of sexual activity, but also to the number and characteristics of sexual partners39.
The choice of HPV genotyping method is crucial, particularly due to the extensive variation in available tests; they mainly differ in the viral types that they are able to identify, the limit of detection (LOD) for each genotype, and their cost. The test should exhibit high analytical sensitivity to detect a broad spectrum of HPV types and to have a more accurate picture of their relative prevalence. The BSGP 5+/6+ PCR-RLB method applied in this study was developed and validated for the sensitive detection of 36 HPV types in single as well as multiple infections; it provides a powerful, highly flexible and sensitive tool for a more homogeneous amplification and HPV genotyping, at a considerably lower cost than commercial tests with a similar methodological basis41. This assay allowed to identify all HR-types (IARC groups 1, 2A and 2B), with the only exception of HPV30, 67, 69, 85 and 97, showing a remarkable ability for viral identification. It is important to emphasize that our results are supported by the correct resolution of the Proficiency Panel distributed on an annual basis by the Global HPV LabNet16; it guarantees that at least 5 copies of vaccine viruses are detected (in single and multiple infections) and that all negative controls are correct. This adds strength and confidence to these data.
Although this study was not population-based, data are particularly relevant because they provide information about HPV frequency in a group rarely assessable or included in worldwide epidemiological surveys, mainly due to recruitment difficulties, which adds value to our work.
In conclusion, this is the first report about cervical HPV genotype distribution that involves sexually active 15–17-year-old non-vaccinated girls from Argentina, and one of the few existing in the world focusing exclusively on this group.
It may be used to inform on vaccination policies, as a baseline against which to measure the impact of the national HPV immunization program on the prevalence of vaccine-type and non-vaccine-type HPV infections. We are conducting the same study in vaccinated girls; assessments of the overall reduction in vaccine type infections, changes in related alpha types, and differential reductions based on the number of doses received would be the main points to evaluate and thus help to sustain and improve prevention strategies.
FundingThis study was financially supported by Salud Investiga (Carrillo-Oñativia fellowships), Dirección Nacional de Control de Enfermedades Inmunoprevenibles (Grant 2014–2015) and Instituto Nacional de Enfermedades Infecciosas - ANLIS Malbrán.
Conflict of interestThe authors declare that they have no conflicts of interest
The authors sincerely thank all hospitals and institutions involved in the study from the following cities: Berazategui (Province of Buenos Aires), Posadas (Province of Misiones), La Banda (Province of Santiago del Estero) and Buenos Aires, and the girls who participated in this study for their generosity.
The authors are particularly grateful to the members of the Health Ministry of Santiago del Estero Province: Minister, Dr. Luis Martínez; Undersecretary, Dr. Cesar Monti; Executive Director CISB, Dra. Liliana Garnica; Director of Maternity, Childhood and Adolescence, Dr. Pedro Carrizo; Director of Immunizations, Dra. Florencia Coronel; Coordinator of the Cervical Cancer Program, Dra. Yolanda Martínez; APS Director, Dra. Teresa Santillán and Unidades de ProntaAtención (UPAs); USM Director, José Alzogaray; Departamento de Docencia e Investigación, Dr. Carlos Marrodán, as well as to Dr. Walter Villalba (Director of the Hospital Escuela de Agudos Ramón Madariaga, Posadas, Misiones), and Delia Dominga Motta (nurse), for their support and commitment in patient recruitment.
We are also indebted to Dr Enrique Lamuedra and Marcela Grosso (Centro Nacional de Red de Laboratorios, ANLIS - Malbrán), for their assistance in sample transfer.
Alejandra Giurgiovich (Hospital Evita Pueblo, Berazategui); Gabriela Alzogaray, Ricardo Aboslaiman (Centro Integral de Salud La Banda, Santiago del Estero); Cecilia Chami (Ministerio de Salud de Santiago del Estero); Juan José Carmona, Néstor Tappari, Andrea Morgenstern (Hospital Madariaga, Misiones); María ElinaTotaro (Universidad Nacional de Misiones); Enrique Berner, Viviana Cramer, Sandra Vázquez, Paula Real (Hospital Argerich, Buenos Aires), Carlota Lopez Kaufman, Gabriela Kosoy, Lucía Katabian (Hospital Rivadavia, Buenos Aires); Maria Silvia Severino (Hospital Durand, Buenos Aires).