Lower acute respiratory infections (ARI) are a frequent cause of morbidity and mortality in infants, respiratory viruses being the major causative agents. The aim of this work was to determine the respiratory pathogen frequency, the clinical characteristics and the outcome in infants <2 months old hospitalized with ARI. A retrospective study was performed during a five-year period (2008–2011, 2014–2016). Respiratory viruses and atypical bacteria were studied using the FilmArray-Respiratory Panel. Demographic and clinical characteristics, hospitalization course and outcomes were evaluated. Of the 137 infants <2 months old hospitalized with ARI studied, a 94.9% positivity rate as determined in 117 infants with community-acquired infection and 20.0% in 20 infants who acquired the infection during their birth hospitalization in the neonatal intensive care units (NICU) (nosocomial ARI) (p<0.001). In infants with community-acquired infection, Respiratory syncytial virus (RSV) (52.1%) and Rhinovirus/Enterovirus (RV/EV) (41.0%) were the most frequent detected pathogens. Coinfections were determined in one quarter of the infants, RSV-RV/EV being the most frequent combination. In infants with nosocomial infection, RV/EV, RSV or Parainfluenza-3 were detected as single pathogens. Most infants with community-acquired infection presented lower ARI (81.2%) while most infants in the NICU had upper ARI (55.0%). The median length of stay (LOS) in infants with community-acquired ARI was 4 days (IQR: 2–6). Positive infants with nosocomial infection had longer median LOS (71 days [IQR:42–99]) compared to negative infants (58 days [IQR: 49–71]) (p=0.507). Respiratory viruses were detected as the major causative agents of community-acquired infection in hospitalized infants <2-months old, RSV and RV/EV being the most frequently detected. Although a low pathogen positivity rate was observed in infants with nosocomial infection, they may prolong the LOS.
Las infecciones respiratorias agudas (IRA) son una causa habitual de morbimortalidad en infantes y los virus respiratorios son los agentes causales más frecuentes. El objetivo de este trabajo fue determinar la frecuencia de patógenos respiratorios, sus características y la evolución clínica de infantes menores de dos meses internados por IRA. Se realizó un estudio retrospectivo que comprendió 5 años (2008-2011, 2014-2016). Se estudiaron los virus respiratorios y las bacterias atípicas utilizando el panel respiratorio FilmArray. Se evaluaron las características demográficas y clínicas, así como la evolución durante la internación. En 137 infantes menores de dos meses internados por IRA se determinó una positividad del 94,9% en 117 lactantes con infección adquirida en la comunidad (IRA-C) y del 20,0% en 20lactantes que adquirieron la infección durante su hospitalización en la unidad de cuidados intensivos neonatal (IRA-N) (p<0,001). En los infantes con IRA-C los virus más frecuentes fueron sincicial respiratorio (RSV) (52,1%) y rinovirus/enterovirus (RV/EV) (41,0%). Se determinaron coinfecciones en un cuarto de los infantes, la combinación RSV-RV/EV fue la más frecuente. En los infantes con IRA-N se detectó RV/EV, RSV o parainfluenza-3 como únicos patógenos. La mayoría de los infantes con IRA-C presentaron IRA baja (81,2%), mientras que más de la mitad de los infantes en UCIN tenían IRA alta (55,0%). La mediana de la duración de la internación en infantes con IRA-C fue de 4días (RIC: 2-6). Los infantes positivos con IRA-N tuvieron una mediana de internación más larga (71 días [RIC: 42-99]) en comparación con los infantes negativos (58 días [RIC: 49-71]) (p=0,507). Los virus respiratorios fueron los principales agentes causales de IRA adquirida en la comunidad en infantes menores de dos meses que requirieron internación; los virus más frecuentes fueron RSV y RV/EV. Aunque se observó una baja tasa de positividad de patógenos en infantes con infección nosocomial, estos agentes podrían prolongar la duración de la internación.
Acute respiratory infections (ARIs) are the most frequent cause of morbidity in children, while lower respiratory tract infections (LRTIs) are a common cause of hospitalization and mortality in young children. In Argentina, LRTIs are the third cause of death in infants less than one year old and the second cause in children from 1 to 5 years old, respectively18. Upper respiratory tract infections (URTIs) can be frequent in neonates17.
Preterm infants are at an increased risk of perinatal morbidity and mortality and serious LRTIs impose an additional burden of illness causing hospitalization and leading to a higher admission rate to neonatal intensive care units (NICUs) and increased need for oxygen and mechanical ventilation compared to term infants9.
Respiratory virus detection in newborn during birth hospitalization can range from 4% to 52%, Rhinovirus (RV), Respiratory syncytial virus (RSV), Parainfluenza (PIV) and Human coronavirus (HCoV) being the most frequently detected respiratory viruses1,4,17,19. These infections may exacerbate underlying clinical conditions leading to increased oxygen requirement, even mechanical ventilation in severe cases, and extend the length of stay (LOS) in hospital.
Molecular methods have significantly improved viral diagnosis and some commercial multiplexed nested PCR assays allow the simultaneous detection of several respiratory pathogens. The purpose of this study was to determine the frequency of 17 respiratory viruses and 3 atypical bacteria using a molecular respiratory panel (FilmArray®) in infants less than 2 months old hospitalized with ARI and to evaluate clinical characteristics, hospitalization course and outcomes.
Material and methodsInfants included in this investigation were part of a larger prospective multicenter study performed at two private hospitals in Buenos Aires City, Argentina, evaluating children <6 years old with ARI from June 2008 to December 201113 and from May 2014 to June 2016. Classical respiratory viruses (RSV, Adenovirus [AdV], Influenza A [FluA] & B [FluB], PIV) were detected by immunofluorescence and RV were detected by PCR. Inclusion criteria were the following: either LRTI and/or URTI, a nasopharyngeal aspirate obtained within 3 days of symptom onset and informed consent signed by parents/tutors. Exclusion criteria included underlying conditions such as cardiopathy, congenital malformation, metabolic or genetic disease or immunosuppression.
Of 1400 enrolled patients, 137 (9.8%) were less than 2 months old. Frozen samples of these 137 patients were further assessed in this study using a rapid molecular assay for 20 pathogens. Specifically, the Respiratory Panel (FilmArray®-RP, BioFire/bioMèrieux, Salt Lake City, UT) can simultaneously detect RSV, FluA, FluA-H1, FluA-H1 pandemic 2009, FluA-H3, FluB, AdV, PIV 1–4, RV/Enterovirus (EV), Human metapneumovirus (HMPV), HCoV-OC43, HCoV-229E, HCoV-NL63, HCoV-HKU1, Bordetella pertussis, Mycoplasma pneumoniae and Chlamydiapneumoniae16.
The demographic and social characteristics, signs and symptoms, clinical diagnosis (in accordance with the International Classification of Diseases: ICD-10) and the course of hospitalization were recorded for each infant in a specifically designed form.
Laboratory diagnosisNasopharyngeal aspirates from patients with ARI that were submitted for viral diagnosis (standard care) were retrospectively tested using the FilmArray®-RP. Blood and urine samples from infants with sepsis suspicion or fever without an apparent source were collected and bacterial cultures were performed.
Statistical analysisAll demographic, clinical and etiological data were entered in MS Excel. Values were given as percentages for categorical variables or as median and interquartile range (IQR) for continuous variables. Coinfection was defined as a sample with a positive test result for two or more respiratory pathogens. Data were analyzed using either the Chi-square test, the Fisher's exact test, the student's T test or the Mann–Whitney test as appropriate. Statistical significance was assumed for p values less than 0.05. Statistical analyses were performed using STATA 12.0 (StataCorp, College Station, TX, USA).
ResultsPopulation characteristicsAll 137 infants less than 2 months old hospitalized with ARI studied were Caucasian, well-nourished and belonged to a middle-income population. Seventy-seven infants were male (56.2%), median age was 41 days old (range: 11–60 days) and median birth weight was 3200g (range: 825–4700g) (Table 1). Thirty-nine infants (28.5%) were preterm (gestational age <38 weeks); 2 (1.5%) of them were extremely preterm (<28 weeks), 13 (9.5%) very preterm (28–31 weeks) and 24 (17.5%) late preterm (32–37 weeks). Most infants had updated schedules of mandatory vaccines (BCG and Hepatitis B vaccines) (92.7%) and were breastfed (94.9%).
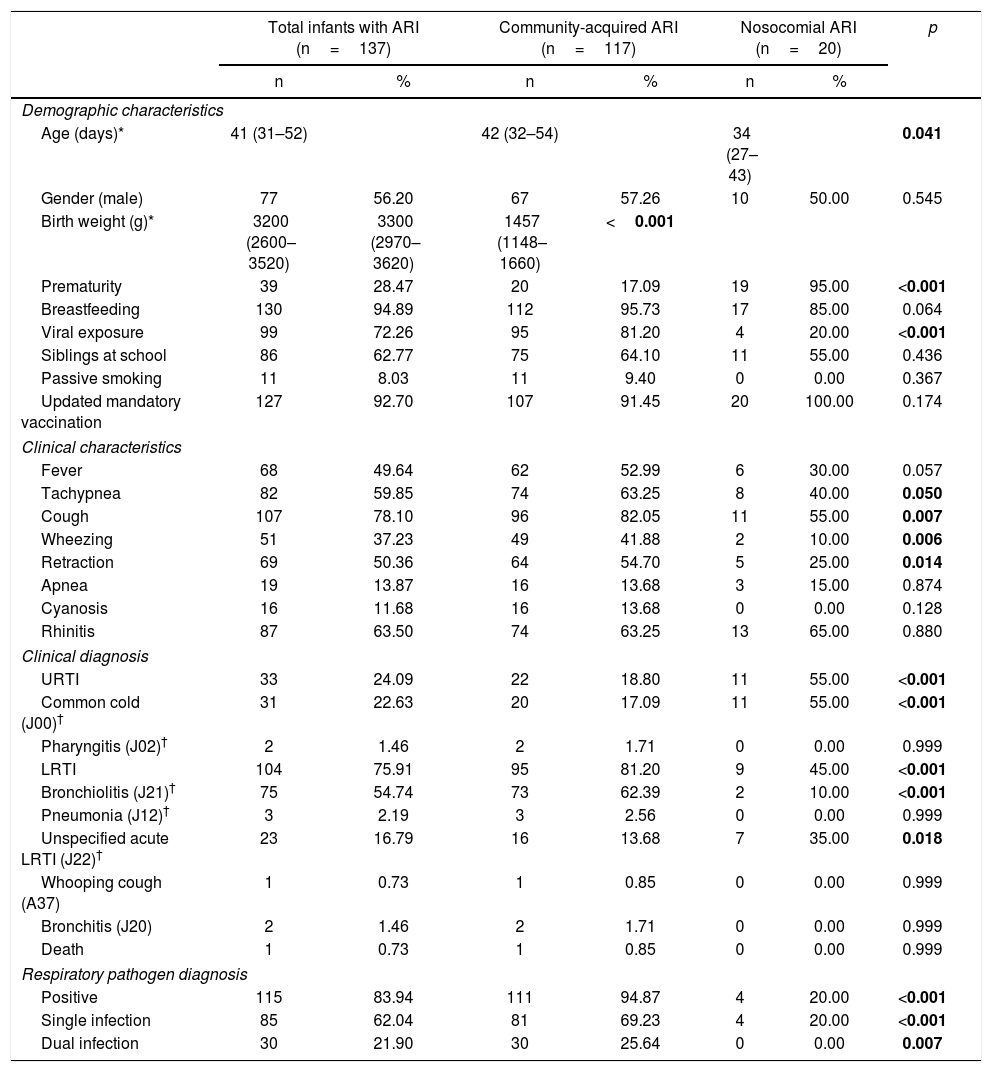
Demographic and clinical characteristics and diagnosis in 137 infants less than 2 months old hospitalized with ARI, by site of ARI acquisition.
| Total infants with ARI (n=137) | Community-acquired ARI (n=117) | Nosocomial ARI (n=20) | p | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Demographic characteristics | |||||||
| Age (days)* | 41 (31–52) | 42 (32–54) | 34 (27–43) | 0.041 | |||
| Gender (male) | 77 | 56.20 | 67 | 57.26 | 10 | 50.00 | 0.545 |
| Birth weight (g)* | 3200 (2600–3520) | 3300 (2970–3620) | 1457 (1148–1660) | <0.001 | |||
| Prematurity | 39 | 28.47 | 20 | 17.09 | 19 | 95.00 | <0.001 |
| Breastfeeding | 130 | 94.89 | 112 | 95.73 | 17 | 85.00 | 0.064 |
| Viral exposure | 99 | 72.26 | 95 | 81.20 | 4 | 20.00 | <0.001 |
| Siblings at school | 86 | 62.77 | 75 | 64.10 | 11 | 55.00 | 0.436 |
| Passive smoking | 11 | 8.03 | 11 | 9.40 | 0 | 0.00 | 0.367 |
| Updated mandatory vaccination | 127 | 92.70 | 107 | 91.45 | 20 | 100.00 | 0.174 |
| Clinical characteristics | |||||||
| Fever | 68 | 49.64 | 62 | 52.99 | 6 | 30.00 | 0.057 |
| Tachypnea | 82 | 59.85 | 74 | 63.25 | 8 | 40.00 | 0.050 |
| Cough | 107 | 78.10 | 96 | 82.05 | 11 | 55.00 | 0.007 |
| Wheezing | 51 | 37.23 | 49 | 41.88 | 2 | 10.00 | 0.006 |
| Retraction | 69 | 50.36 | 64 | 54.70 | 5 | 25.00 | 0.014 |
| Apnea | 19 | 13.87 | 16 | 13.68 | 3 | 15.00 | 0.874 |
| Cyanosis | 16 | 11.68 | 16 | 13.68 | 0 | 0.00 | 0.128 |
| Rhinitis | 87 | 63.50 | 74 | 63.25 | 13 | 65.00 | 0.880 |
| Clinical diagnosis | |||||||
| URTI | 33 | 24.09 | 22 | 18.80 | 11 | 55.00 | <0.001 |
| Common cold (J00)† | 31 | 22.63 | 20 | 17.09 | 11 | 55.00 | <0.001 |
| Pharyngitis (J02)† | 2 | 1.46 | 2 | 1.71 | 0 | 0.00 | 0.999 |
| LRTI | 104 | 75.91 | 95 | 81.20 | 9 | 45.00 | <0.001 |
| Bronchiolitis (J21)† | 75 | 54.74 | 73 | 62.39 | 2 | 10.00 | <0.001 |
| Pneumonia (J12)† | 3 | 2.19 | 3 | 2.56 | 0 | 0.00 | 0.999 |
| Unspecified acute LRTI (J22)† | 23 | 16.79 | 16 | 13.68 | 7 | 35.00 | 0.018 |
| Whooping cough (A37) | 1 | 0.73 | 1 | 0.85 | 0 | 0.00 | 0.999 |
| Bronchitis (J20) | 2 | 1.46 | 2 | 1.71 | 0 | 0.00 | 0.999 |
| Death | 1 | 0.73 | 1 | 0.85 | 0 | 0.00 | 0.999 |
| Respiratory pathogen diagnosis | |||||||
| Positive | 115 | 83.94 | 111 | 94.87 | 4 | 20.00 | <0.001 |
| Single infection | 85 | 62.04 | 81 | 69.23 | 4 | 20.00 | <0.001 |
| Dual infection | 30 | 21.90 | 30 | 25.64 | 0 | 0.00 | 0.007 |
Age and birth weight are given as median (IQR). † Clinical diagnosis is named as diagnosis (CID-10 code). Data were analyzed using either the Chi-square test, Fisher's exact test, student's T test or Mann–Whitney test as appropriate. Statistical significance was assumed for p values less than 0.05.
Two groups of infants were identified in this population: 20 (14.6%) who had never been discharged since they were born and developed a respiratory infection while being in the NICU, and 117 (85.4%) who developed the respiratory infection at home and required hospitalization. Therefore, the first patients were defined as having a nosocomial (hospital-acquired) infection and the latter patients as having a community-acquired infection.
Of the 117 infants with community-acquired infection, most were term-born (82.1%) and had normal birth weight (90.6%). The median age of respiratory infection onset was 42 days (IQR: 32–54). Among them, 81.2% reported a viral exposure at home, 64% had siblings at school, 10% were exposed to passive smoking and none attended day care. In contrast, almost all infants in the NICU were preterm (95%), all had low birth weight (<2.500g) and the median age of respiratory infection onset was 34 days (IQR: 27–43), significantly lower than in the community-acquired group (p=0.041).
Clinical findingsMost infants with community-acquired infection presented a LRTI (81.2%). The most frequent diagnosis was bronchiolitis (62.4%),followed by common cold (17.1%), unspecified acute LRTI (13.7%), pneumonia (2.6%), pharyngitis (1.7%), bronchitis (1.7%), and whooping cough (0.9%) (Table 1). Of 20 infants with ARI in the NICU, 55.0% had URTI, 35.0% had unspecified acute LRTI and only 10.0% had bronchiolitis.
Almost all patients (99%) recovered from the respiratory infection but one 45-day-old infant with community-acquired pneumonia died after 9 days of hospitalization. RSV was the only detected pathogen; bacterial cultures from blood, urine, cerebrospinal and pleural fluids were negative.
EtiologyThe positivity rate was significantly different between both infants’ groups (Table 1). Infants with community-acquired respiratory infection had a 94.9% positivity rate, while infants in the NICU with nosocomial infection had a low positivity rate (20.0%) (p<0.001).
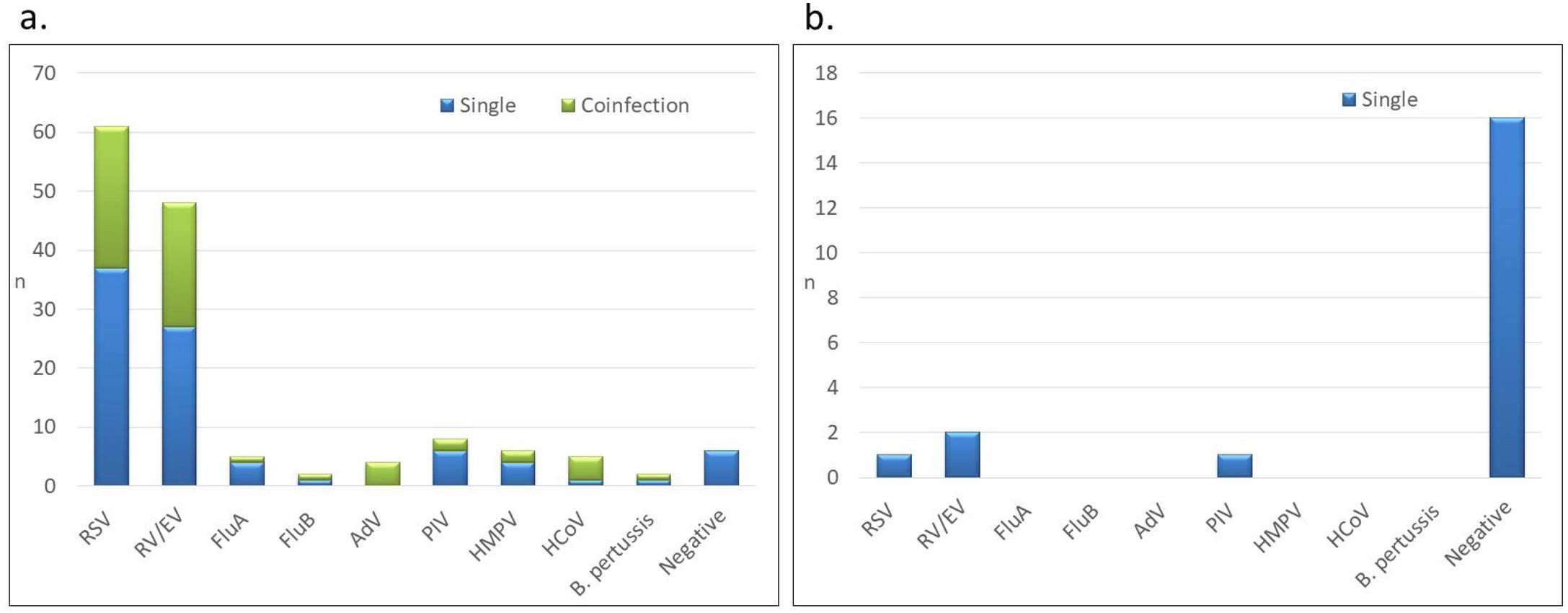
In infants with community-acquired infection, RSV was the most frequent virus (n=61, 52.1%) followed by RV/EV (n=48, 41.0%) (Fig. 1). Other respiratory pathogens, including PIV (n=8, 6.8%), HMPV (n=6, 5.1%), HCoV (n=5, 4.3%), FluA (n=5, 4.3%), FluB (n=2, 1.7%), AdV (n=4, 3.4%) and B. pertussis (n=2, 1.7%), were also detected. Interestingly, RSV was the most prevalent pathogen both in premature infants (42%) and term-born infants (53%) with community-acquired ARI.
Respiratory pathogens detected in hospitalized infants less than 2 months old by site of ARI acquisition: (A) community-acquired ARI (n=117), (B) nosocomial ARI (n=20). RSV: respiratory syncytial virus; RV/EV: rhinovirus/enterovirus; FluA: influenza A; FluB: influenza B; PIV: parainfluenza; AdV: adenovirus; HMPV: human metapneumovirus; HCoV: human coronavirus; B. pertussis: Bordetella pertussis.
All coinfections (n=30) were detected in infants with community-acquired ARI. RSV, RV/EV, FluA, PIV and HMPV were more frequently detected as single infections than in coinfections (Fig. 1). In contrast, all AdV, PIV-4 and HCoV were detected in coinfections, except for one infant in whom HCoV-HKU1 was present as a single infection. B. pertussis was detected in 2 infants (1.7%), as the only agent in one patient and in coinfection with RV/EV in the second case. The most frequent viral coinfection was RSV with RV/EV (n=16, 53.3%) (Supplemental table). Other viral coinfections were detected at lower frequencies and included RSV with either FluA, FluB, AdV, PIV-4 or HCoV; RV/EV with either AdV, HMPV, HCoV or B. pertussis; and HMPV with PIV-3.
Most of the 20 infants in the NICU with ARI were negative for any of the respiratory pathogens, and only 4 (20.0%) had a positive pathogen detection: 2 infants had RV/EV, 1 infant had RSV and 1 infant had PIV-3. No coinfections were detected in these patients.
HospitalizationThe median LOS in infants with community-acquired infection was 4 days (IQR 2–6). Of 117/31 (26.5%) were in the intensive care unit for a median of 5 days (IQR 2–9) and 2.6% required mechanical ventilation for a median of 7 days (IQR 4–9).
In contrast, the median LOS in infants in the NICU who developed ARI was 62 days (IQR 49–75); 75.0% required oxygen therapy for a median of 3 days (IQR 2–7); 75.0% were in intensive care for a median of 60 days (IQR30-72) and 45.0% needed mechanical ventilation for a median of 7 days (IQR 3–18). This prolonged LOS, higher oxygen-therapy and mechanical ventilation assistance was related to their prematurity. Interestingly, infants with a positive viral respiratory diagnosis had a longer LOS (median: 71 days [IQR 42–99]) with respect to infants with a negative viral respiratory diagnosis who had a median LOS of 58 days (IQR 49–71) (p=0.507).
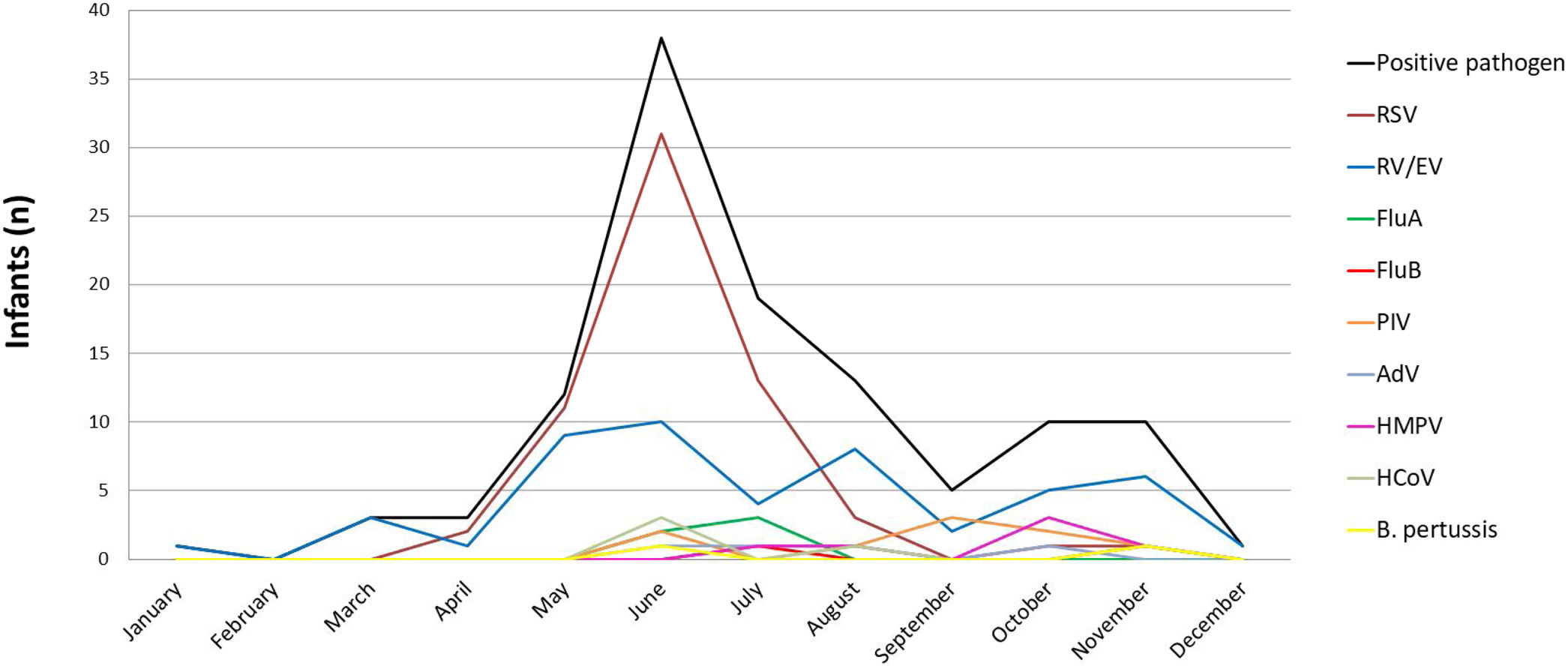
Viral seasonalityMost infants developed their respiratory infection during the autumn and winter seasons (from March to September) (91.4% and 82.2% positive diagnosis, respectively). During spring and summer, positivity was 77.8% and 57.1%, respectively. RV/EV was detected throughout the year. RSV detection started in April and its characteristic peak was observed in June. FluA and FluB were detected in June and July while PIV, HMPV, HCoV and AdV were detected at a low frequency in winter and spring (Fig. 2).
Viral seasonality in 137 infants less than 2 months old hospitalized with ARI, Buenos Aires, Argentina. Period: June 2008 to August 2011 and May 2014 to June 2016. RSV: respiratory syncytial virus; RV/EV: rhinovirus/enterovirus; FluA: influenza A; FluB: influenza B; PIV: parainfluenza; AdV: adenovirus; HMPV: human metapneumovirus; HCoV: human coronavirus; B. pertussis: Bordetella pertussis.
We retrospectively studied respiratory viruses and atypical bacteria in 137 infants less than 2 months old hospitalized with ARI in Buenos Aires, Argentina. We found a high positivity rate of respiratory pathogens (95%) in infants who were discharged after birth, went home and developed a community-acquired ARI that required hospitalization. Even though this population was very young, the rate was as high as that previously reported in older children (<5 years old) from our institution when using the same molecular methodology (92%)12 and was also similar to the rates described in US hospital-based studies (97%)10.
In contrast, a low positivity rate (20%) was detected in hospitalized infants less than 2 months old who had remained hospitalized since birth and acquired the infection in the hospital. This data was consistent with other reports from Austria (15%) and Finland (18%)8,19.
Our results demonstrated that viral exposure in the community increases the possibility of acquiring a respiratory pathogen, as compared to a protected neonatology environment. These findings highlight the importance of recognizing the high transmissibility of respiratory pathogens in this vulnerable age group and suggest the need for a rapid viral diagnosis and a stronger awareness in the improvement of hygiene measures among parents and relatives.
The viral burden in infants with community-acquired infection was broad and included not only classical but also emerging respiratory viruses as well as B. pertussis. RSV remains one of the most frequent respiratory viruses in children and can lead to fatal outcomes, as it was observed in the 45-day-old infant with community-acquired infection mentioned in our study. Although the vaccine for B. pertussis is mandatory for pregnant women since 2013, we detected this bacterium in two infants with community-acquired ARI in 2008. One of them in coinfection with RV/EV, stressing the need for a bacterial diagnosis, even in the presence of a positive viral result, when there is a compatible clinical diagnosis of whooping cough.
In those infants in the NICU with nosocomial infection, RSV was not the only detected virus; RV/EV and PIV were also found. Other authors reported the presence of other respiratory viruses in infants during their birth hospitalization in the NICU, including HMPV, Flu and HCoV; however, RSV, RV and PIV remained the most frequently detected viruses1,4,9.
Coinfection was only detected in infants with community-acquired infection, RSV-RV/EV being the most frequent combination, as previously described5,13,14. The coinfection rate was similar to our previous results in outpatients less than 5 years old (31%) when using the same diagnostic method6. All AdV, PIV-4 and most HCoV were detected in coinfection with another virus. Interestingly, one mixed viral–bacterial coinfection (B. pertussis with RV/EV) was detected. Although the association of B. pertussis with RSV is the most frequently reported virus-bacterium coinfection3,15, the coinfection of B. pertussis with RV was described in children with community-acquired infection and prolonged coughing20.
Viral seasonality observed during the studied period was similar to our previous reports and the national program of virus surveillance, exhibiting the highest frequency during the autumn and winter months2,13. Nevertheless, viral detection occurs throughout the year at lower frequencies.
The most common clinical diagnosis in patients with community-acquired ARI was bronchiolitis and unspecified acute LRTI while the most frequent diagnosis was upper RTI in infants in the NICU. The prolonged LOS, intensive care and mechanical ventilation requirements in infants that remained in the NICU since birth is mainly related to their premature birth condition that can be possibly prolonged due to a respiratory infection. In our study, neonates with a positive viral diagnosis had a longer LOS and mechanical ventilation requirement compared to those with a negative result. This difference did not achieve statistical significance probably due to the low number of studied infants. Bennet et al. also demonstrated that preterm infants in the NICU with a positive respiratory virus had a significantly longer LOS and prolonged ventilation support compared to those who were negative1. Generally, viral respiratory infection in preterm neonates can cause mild or atypical symptoms but can also trigger a clinical worsening leading to oxygen therapy requirement, including mechanical ventilation7. Furthermore, we have recently described a respiratory outbreak in the NICU associated with RV worsening the patients’ underlying clinical conditions and extending their LOS11.
One strength of this study was the inclusion of infants less than 2 months old with either nosocomial or community-acquired infection, throughout the year, and for 6 non-consecutive years. These results allowed a better understanding of the viral burden through different years. A significant strength was the simultaneous detection of 20 classical and emerging respiratory pathogens.
However, our study has limitations. The number of infants who remained hospitalized since birth and acquired a nosocomial infection at our institution was small. Possible respiratory infection sources (healthcare workers or relatives) were not investigated. The molecular assay (FilmArray-RP) is unable to distinguish between RV and EV and does not detect typical bacteria such as Streptococcus pneumoniae, Haemophilusinfluenzae either Chlamydia trachomatis.
In conclusion, a high detection rate of respiratory pathogens was observed in hospitalized infants <2 months old with community-acquired ARI, RSV and RV/EV being the most frequent identified agents. In contrast, a significant lower detection rate was observed in new born infants who had remained hospitalized in the NICU since birth and developed ARI. These findings highlight the role of respiratory pathogens in these young infants associated with high morbidity and even mortality, stress the need for a rapid viral diagnosis and reinforce the impact of hygiene and infection control measures not only in hospital settings but also among parents and relatives at home.
Ethical approvalThis work was approved by the Ethics Committees from Hospital Universitario CEMIC and Sanatorio Mater Dei (No. 00001745 and IORG 001315).
FundingThis work was supported by the National Scientific and Technologic Agency (ANPCYT) [PICT 2006-650 to M.E.]. The FilmArray-respiratory panels were provided by Biofire/BioMèrieux, USA, as part of a larger study.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank all pediatricians, neonatologists, residents and physical therapists from both hospitals for the patients’ enrollment and sample collection; Carmen Ricarte and the Virology Laboratory for technical assistance.