This study aimed to assess the impact of the implementation of a rapid multiplex molecular FilmArray Respiratory Panel (FRP) on the medical management of immunocompromised patients from a community general hospital. We conducted a single-center, retrospective, and before–after study. Two periods were evaluated: before the implementation of the FRP (pre-FRP) from April 2017 to May 2018 and after the implementation of the FRP (post-FRP) from January to July 2019. The inclusion criteria were immunocompromised patients over 18 years of age with suspected acute respiratory illness tested by conventional diagnostic methods (pre-FRP) or the FilmArray™ Respiratory Panel v1.7 (post-FRP). A total of 142 patients were included, 64 patients in the pre-FRP and 78 patients in the post-FRP. The positive detection rate was significantly higher in the post-FRP (63% vs. 10%, p<0.01). There were more patients receiving antimicrobial treatment in the pre-FRP compared with the post-FRP period (94% vs. 68%, p<0.01). A decrease in beta-lactam (89% vs. 61%, p<0.01) and macrolide (44% vs. 13%, p<0.01) prescriptions were observed in the post-FRP. No differences were observed in oseltamivir use (22% vs. 13%, p=0.14), changes in antimicrobial treatment, hospital admission rate, days-reduction in droplet isolation precautions, hospital length of stay (LOS), admission to intensive care unit (ICU), LOS in ICU, treatment failure and 30-day mortality. The implementation of the FRP impacted patient care by improving diagnostic yield and optimizing antimicrobial treatment in immunocompromised adult patients.
El objetivo de este estudio fue evaluar el impacto de la implementación del panel respiratorio FilmArray® (FRP), un sistema automatizado de PCR multiplex, en el estándar de cuidado de pacientes adultos inmunocomprometidos en un hospital general. Es un estudio retrospectivo de un único centro con diseño antes/después. Los periodos evaluados fueron abril 2017-mayo 2018, previo a la implementación del FRP (pre-FRP), y enero 2019-julio 2019, luego de la implementación (post-FRP). Los criterios de inclusión fueron pacientes mayores de 18 años inmunocomprometidos con sospecha de infección respiratoria aguda a los que se les realizó, en pre-FRP, diagnóstico por métodos convencionales, y en post-FRP, el panel respiratorio FRP versión 1.7. Se incluyeron un total de 142 pacientes, 64 en pre-FRP y 78 en post-FRP. La tasa de positividad fue significativamente mayor en post-FRP frente a pre-FRP (63 vs. 10%, p<0,01). Hubo más pacientes con tratamiento antimicrobiano en pre-FRP que en post-FRP (94 vs. 68%, p<0,01). En pre-FRP hubo más pacientes tratados con betalactámicos (89 vs. 61%, p<0,01) y macrólidos (44 vs. 13%, p<0,01). No se observaron diferencias significativas en el uso de oseltamivir (22 vs. 13%, p=0,14), cambios en los tratamientos, número de hospitalizaciones, uso de aislamientos, duración de la estadía hospitalaria, ingreso a la unidad de cuidados intensivos, estadía en dicha unidad, falla de tratamiento y mortalidad a 30 días. El uso de FRP contribuyó a la atención del paciente mejorando el rendimiento diagnóstico y optimizando la terapia antimicrobiana en pacientes adultos inmunocomprometidos.
Respiratory viruses and some atypical bacteria can cause both upper respiratory tract infections and severe pneumonia, especially in immunocompromised hosts, and are common causes of hospitalization in adults1. Rapid and definitive diagnosis is critical in the clinical management of viral respiratory infections and contributes to the timely isolation of infected patients. Moreover, overlapping clinical presentations hamper clinicians’ ability to predict causative pathogens (i.e., bacterial or viral) and may lead to unnecessary antimicrobial use9.
The BioFire FilmArray Respiratory Panel (FRP) is a rapid molecular multiplex test designed for the qualitative detection of nucleic acid targets of viruses and bacteria in nasopharyngeal swab specimens. The current evidence on the effect of rapid molecular testing on clinical outcomes and hospital resource use is heterogeneous and inconclusive. Most published studies focus on immunocompetent1,2,6,9,11,12 or pediatric patients2,4,5,10, and not on immunocompromised patients who could be the most favored in our setting.
In hematopoietic stem cell and solid organ transplant recipients, respiratory viruses are a serious cause of morbidity and mortality. These patients are severely immunocompromised, making them highly susceptible to infectious pathogens. Although immunocompromised patients have similar etiologies of acute respiratory tract infection compared to immunocompetent patients, they experience more complications during the disease15. Since the manifestations of influenza-like illness in such patients are frequently less characteristic than in immunocompetent patients, laboratory testing is essential14.
This retrospective study assessed whether a rapid molecular respiratory panel (FilmArray™, v1.7) improved the standard of care in immunocompromised patients with suspected acute respiratory tract infections in a general hospital.
Materials and methodsThis was a single-center, retrospective, before–after study conducted at the Hospital Alemán of Buenos Aires, Argentina, a 254-bed general hospital.
Inclusion criteria were: patient older than 18 years old, immunocompromised at the time of presentation due to a solid organ or hematopoietic stem cell transplantation, active oncological disease, HIV, and/or chronic use of immunosuppressive medication, with suspected upper or lower acute respiratory tract infections exposed to at least one of the following diagnostic tests:
- -
Before the implementation of FilmArray (Pre-FRP):
- (a)
Direct immunofluorescence (IFD) to detect adenovirus, influenza A and B, respiratory syncytial virus, metapneumovirus, and parainfluenza 1, 2, and 3.
- (b)
Mycoplasma pneumoniae and or Chlamydia pneumoniae antibodies.
- (c)
Influenza (H1N1 and A), adenovirus, Mycoplasma, and or Chlamydia pneumoniae real-time PCR.
- -
After the implementation of FilmArray (post-FRP):
- (a)
FilmArray™ Respiratory Panel v1.7.
Upper acute respiratory tract infections included acute nasopharyngitis, acute sinusitis, acute pharyngitis, acute tonsillitis, acute laryngopharyngitis, and acute upper respiratory infections of multiple and unspecified sites; lower respiratory tract infections included bronchiolitis, bronchitis, pneumonia, and influenza, according to the International Classification of Diseases 11th revision (ICD-11) of the World Health Organization.
Study periods were from April 2017 to May 2018, before the implementation of FilmArray (Pre-FRP), and from January 2019 to July 2019, after the implementation of FilmArray (post-FRP).
Clinical and demographic data were collected from the electronic medical record system, including age, sex, type of immunocompromise, neutropenic status, and upper or lower respiratory tract infection. Patients with incomplete medical records were excluded.
This study was approved by the Ethics Committee of Hospital Alemán, Buenos Aires, Argentina.
The primary outcome was the reduction in antimicrobial prescription (beta-lactams, macrolides, and oseltamivir). Secondary outcomes were the reduction in days of antimicrobial treatment, changes in antimicrobial treatment (appropriate antibiotic de-escalation or escalation within the first 72h), hospital admission rate, days-reduction in droplet isolation precautions, hospital length of stay (LOS), admission to intensive care unit (ICU), LOS in ICU, treatment failure, 30-day mortality, and use of complementary resources such as medical imaging (chest/paranasal sinus tomography and X-ray) and additional microbiological tests (blood culture, sputum culture, and bronchoalveolar lavage culture).
The BioFire FilmArray® Respiratory Viral Panel (BioFire Diagnostics, Salt Lake City, a bioMérieux company, Marcy, l’Etoile, France) is an FDA-approved panel for detecting respiratory viruses including influenza A (influenza A H1, influenza A H1 2009, and influenza A H3 viruses) influenza B, parainfluenza virus types 1–4, human metapneumovirus, human rhinovirus/enterovirus (without specifying which), 4 coronaviruses (OC43, 229E, HKU1, and NL63), adenovirus, respiratory syncytial virus (RSV) as well as 3 bacteria (Bordetella pertussis, Chlamydia pneumoniae, and Mycoplasma pneumoniae). Nasopharyngeal swabs were processed as soon as possible after reception from Monday to Saturday.
The direct immunofluorescence kit detects adenovirus, influenza A and B, respiratory syncytial virus, metapneumovirus, and parainfluenza 1, 2, and 3. Nasopharyngeal swabs were processed in batches with a processing time ranging from 2 to 6h, from Monday to Saturday.
Mycoplasma pneumoniae and Chlamydia pneumoniae antibodies (IgM or IgG) were detected by the indirect immunofluorescence assay. They were processed in a batch once a week.
Influenza (H1N1 and influenza A), adenovirus, Mycoplasma, and Chlamydia pneumoniae were also detected by real-time polymerase chain reaction (PCR) and processed in batches once or twice a week.
Sample size was based on the reduction in antimicrobial agent prescription (primary outcome). According to Brendish et al.’s1 estimation, we assumed that about 80% of patients in post-FRP would be treated with antibiotics. To detect a 30% reduction in antibiotic use with a statistical power of 80% and a significance level of 0.05, 116 patients must have been recruited. Baseline characteristics were expressed as median and interquartile range or mean and standard deviation. Nominal or ordinal qualitative variables were summarized by percentages for each category, with their 95% confidence interval. We compared differences in proportions using the Chi-square test or Fisher's exact test, as appropriate. For continuous data, either T-tests or Mann–Whitney U tests were used, based on the distribution of the observed data. To determine if the distribution could be assumed as normal, the following tests were used: the relation between mean and median, histogram inspection, probability plot normal inspection, and Wilk–Shapiro test. A p-value less than or equal to 0.05 was considered statistically significant. Bivariate and multivariate analyses were performed to investigate the possible association between clinical variables. In multivariate logistic regression, those variables that in the bivariate analysis had shown a p-value less than 0.1 in addition to age were chosen for the model. Microsoft Excel, Info Stat, and STATA software were used for data storage and analysis.
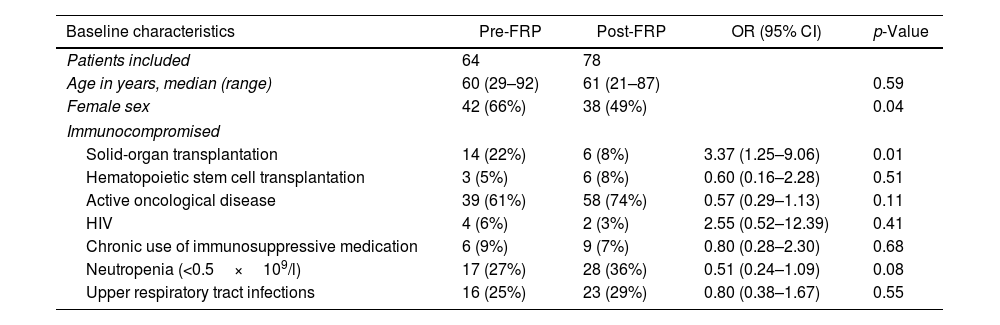
Results142 immunocompromised patients were included, 64 in the pre-FRP group and 78 in the post-FRP group. Baseline characteristics stratified by testing period are listed in Table 1. The distributions of patient demographics in the different exposure groups were similar in terms of age, with a median of 60 and 61 years, neutropenic status (27% and 36%, p 0.08), and type of acute respiratory illness (upper respiratory tract infections 25% and 29%, p 0.55). There were more females (66% vs. 49%, p 0.04) in the pre-FRP group than in the post-FRP group. With regard to the distributions in the categories of immunosuppression, there were more patients with solid organ transplantation in the pre-FRP group (22% vs. 8%, p 0.01) with no differences in other categories (Table 1).
Baseline characteristics.
| Baseline characteristics | Pre-FRP | Post-FRP | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Patients included | 64 | 78 | ||
| Age in years, median (range) | 60 (29–92) | 61 (21–87) | 0.59 | |
| Female sex | 42 (66%) | 38 (49%) | 0.04 | |
| Immunocompromised | ||||
| Solid-organ transplantation | 14 (22%) | 6 (8%) | 3.37 (1.25–9.06) | 0.01 |
| Hematopoietic stem cell transplantation | 3 (5%) | 6 (8%) | 0.60 (0.16–2.28) | 0.51 |
| Active oncological disease | 39 (61%) | 58 (74%) | 0.57 (0.29–1.13) | 0.11 |
| HIV | 4 (6%) | 2 (3%) | 2.55 (0.52–12.39) | 0.41 |
| Chronic use of immunosuppressive medication | 6 (9%) | 9 (7%) | 0.80 (0.28–2.30) | 0.68 |
| Neutropenia (<0.5×109/l) | 17 (27%) | 28 (36%) | 0.51 (0.24–1.09) | 0.08 |
| Upper respiratory tract infections | 16 (25%) | 23 (29%) | 0.80 (0.38–1.67) | 0.55 |
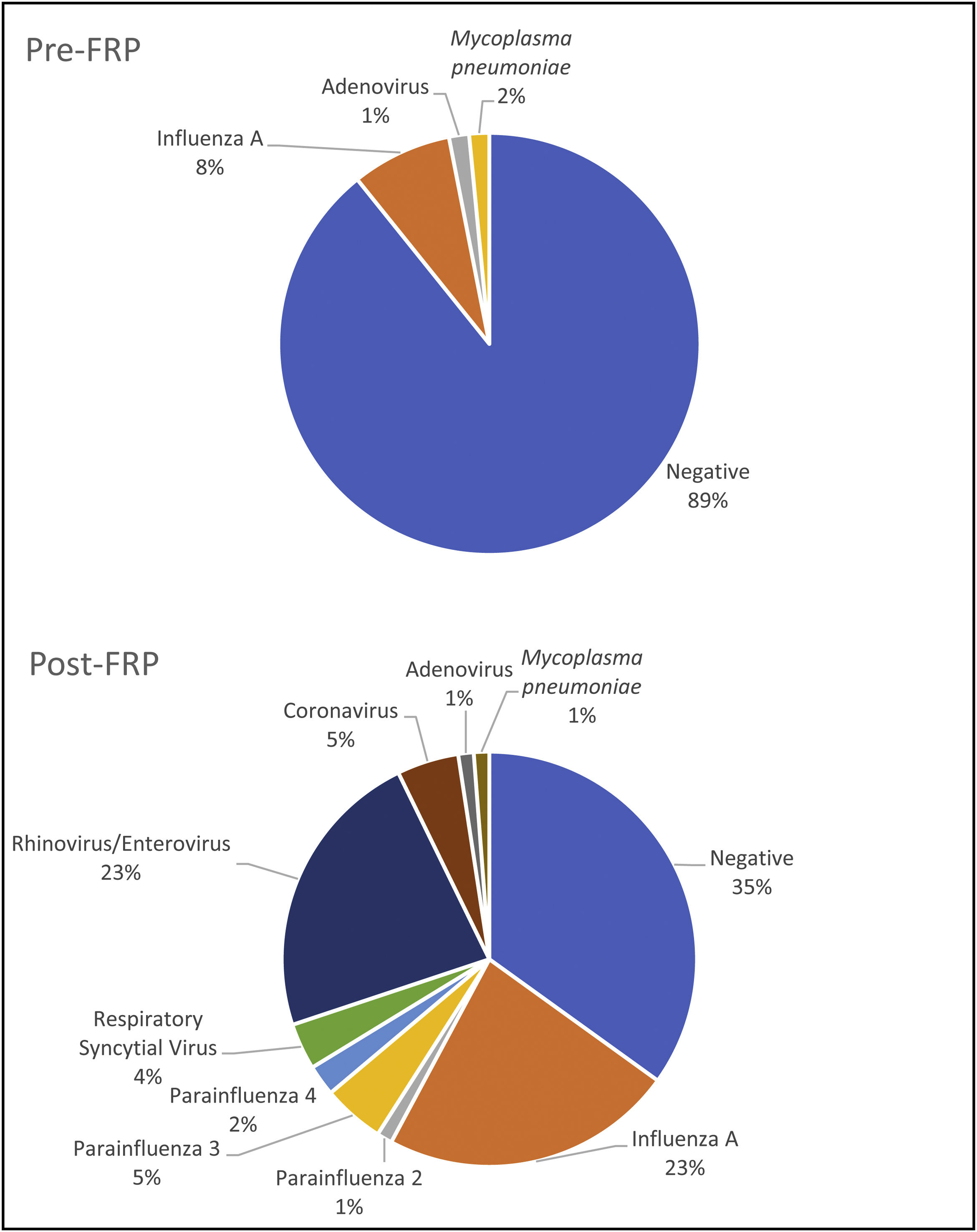
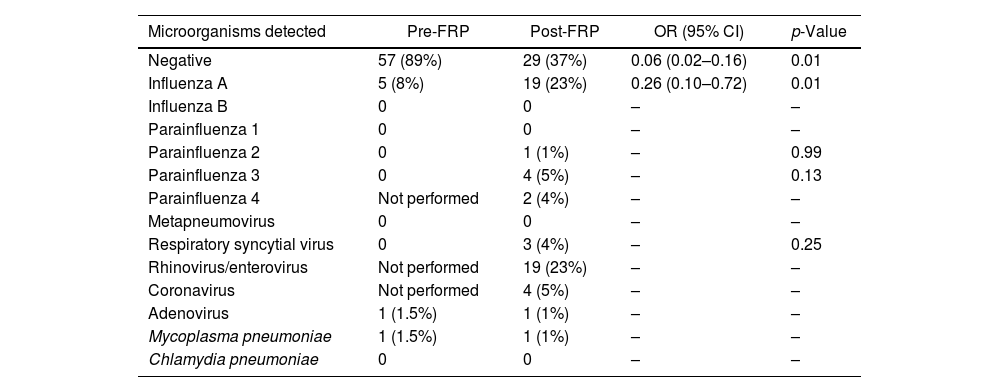
The positive detection rate was higher in the post-FRP group than in the pre-FRP group (63% vs. 11%, p<0.01), mainly due to human rhinovirus/enterovirus and influenza A (Table 2 and Fig. 1). There were more influenza A cases in the post-FRP than in the pre-FRP group (23% vs. 8%, respectively, p 0.01), and 19 human rhinovirus/enterovirus cases detected in the post-FRP (this target was not included in the pre-FRP group). Viruses detected in the post-FRP group and not detected in the pre-FRP group were parainfluenza 2 (n=1), parainfluenza 3 (n=4), respiratory syncytial virus (n=3), and coronavirus (n=4, SARS-CoV-2 not included). In both periods there was only one case of adenovirus and one of Mycoplasma pneumoniae.
Viruses and bacteria detected.
| Microorganisms detected | Pre-FRP | Post-FRP | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Negative | 57 (89%) | 29 (37%) | 0.06 (0.02–0.16) | 0.01 |
| Influenza A | 5 (8%) | 19 (23%) | 0.26 (0.10–0.72) | 0.01 |
| Influenza B | 0 | 0 | – | – |
| Parainfluenza 1 | 0 | 0 | – | – |
| Parainfluenza 2 | 0 | 1 (1%) | – | 0.99 |
| Parainfluenza 3 | 0 | 4 (5%) | – | 0.13 |
| Parainfluenza 4 | Not performed | 2 (4%) | – | – |
| Metapneumovirus | 0 | 0 | – | – |
| Respiratory syncytial virus | 0 | 3 (4%) | – | 0.25 |
| Rhinovirus/enterovirus | Not performed | 19 (23%) | – | – |
| Coronavirus | Not performed | 4 (5%) | – | – |
| Adenovirus | 1 (1.5%) | 1 (1%) | – | – |
| Mycoplasma pneumoniae | 1 (1.5%) | 1 (1%) | – | – |
| Chlamydia pneumoniae | 0 | 0 | – | – |
In the post-FRP group, 5 patients (6%) had viral coinfections. Two patients with rhinovirus/enterovirus and influenza A, one patient with influenza A and parainfluenza 3, one patient with adenovirus and parainfluenza 3, and one patient with rhinovirus/enterovirus and parainfluenza 3. No coinfections were detected in the pre-FRP group.
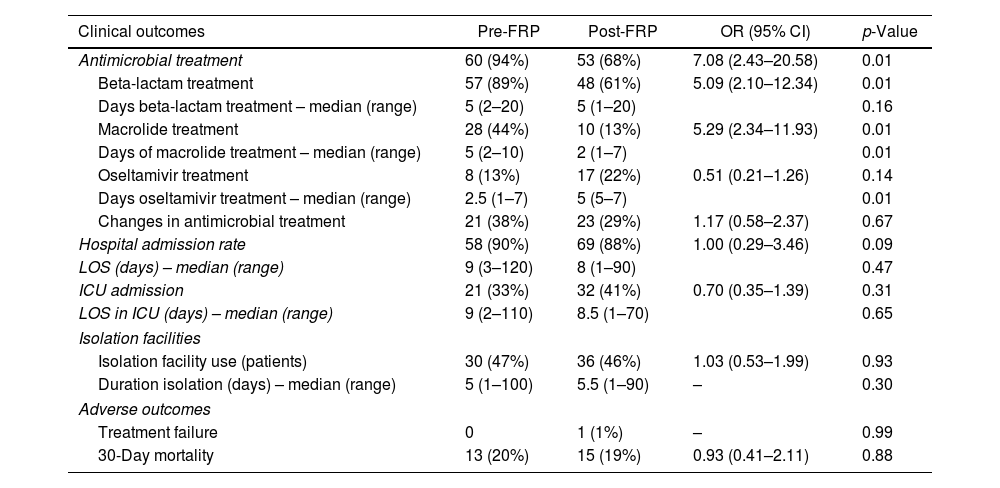
There were more patients treated with antimicrobial agents in the pre-FRP group vs. the post-FRP group (94% vs. 68%, p<0.01). In the pre-FRP period, there were more patients treated with beta-lactams (89% vs. 61%, p<0.01) and macrolides (44% vs. 13%, p<0.01), and there were no differences in patients treated with oseltamivir (22% vs. 13%, p 0.14) (Table 3).
Comparison of clinical outcomes.
| Clinical outcomes | Pre-FRP | Post-FRP | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Antimicrobial treatment | 60 (94%) | 53 (68%) | 7.08 (2.43–20.58) | 0.01 |
| Beta-lactam treatment | 57 (89%) | 48 (61%) | 5.09 (2.10–12.34) | 0.01 |
| Days beta-lactam treatment – median (range) | 5 (2–20) | 5 (1–20) | 0.16 | |
| Macrolide treatment | 28 (44%) | 10 (13%) | 5.29 (2.34–11.93) | 0.01 |
| Days of macrolide treatment – median (range) | 5 (2–10) | 2 (1–7) | 0.01 | |
| Oseltamivir treatment | 8 (13%) | 17 (22%) | 0.51 (0.21–1.26) | 0.14 |
| Days oseltamivir treatment – median (range) | 2.5 (1–7) | 5 (5–7) | 0.01 | |
| Changes in antimicrobial treatment | 21 (38%) | 23 (29%) | 1.17 (0.58–2.37) | 0.67 |
| Hospital admission rate | 58 (90%) | 69 (88%) | 1.00 (0.29–3.46) | 0.09 |
| LOS (days) – median (range) | 9 (3–120) | 8 (1–90) | 0.47 | |
| ICU admission | 21 (33%) | 32 (41%) | 0.70 (0.35–1.39) | 0.31 |
| LOS in ICU (days) – median (range) | 9 (2–110) | 8.5 (1–70) | 0.65 | |
| Isolation facilities | ||||
| Isolation facility use (patients) | 30 (47%) | 36 (46%) | 1.03 (0.53–1.99) | 0.93 |
| Duration isolation (days) – median (range) | 5 (1–100) | 5.5 (1–90) | – | 0.30 |
| Adverse outcomes | ||||
| Treatment failure | 0 | 1 (1%) | – | 0.99 |
| 30-Day mortality | 13 (20%) | 15 (19%) | 0.93 (0.41–2.11) | 0.88 |
More days of oseltamivir treatment (media of days 5 vs. 2.5) and fewer days of macrolide treatment (media of days 2 vs. 5) were observed in the post-FRP group and no differences in the number of days with beta-lactam treatment.
With regard to clinical outcomes, there was no significant difference in changes in antimicrobial treatment, hospital admission rate, days-reduction in droplet isolation precautions, hospital length of stay (LOS), admission to intensive care unit (ICU), LOS in ICU, treatment failure, and 30-day mortality (Table 3).
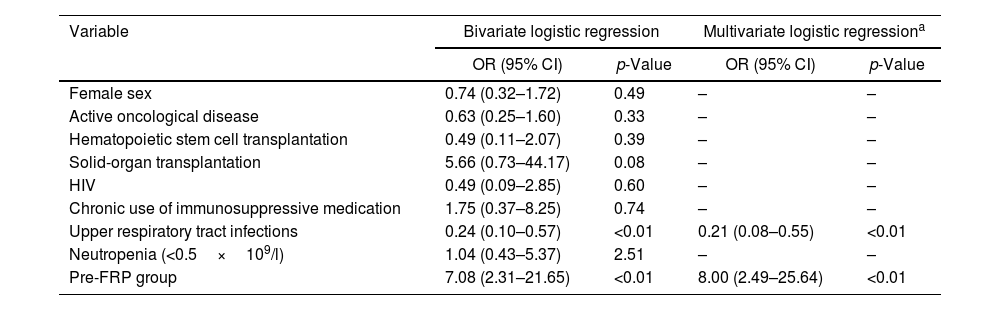
A multivariate logistic regression analysis was performed (Table 4). The only variable that was independently related to receiving treatment was being in the pre-FRP group (OR 8 95% CI 2.49–25.64, p<0.01), whereas the only variable that was independently related to not receiving treatment was having an upper respiratory tract infection (OR 0.21 95% CI 0.08–0.55, p<0.01). Therefore, performing conventional testing (pre-FRP) would increase the risk of receiving treatment and an upper respiratory tract infection would reduce this risk.
Bivariate and multivariate logistic regressions for treatment outcome.
| Variable | Bivariate logistic regression | Multivariate logistic regressiona | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Female sex | 0.74 (0.32–1.72) | 0.49 | – | – |
| Active oncological disease | 0.63 (0.25–1.60) | 0.33 | – | – |
| Hematopoietic stem cell transplantation | 0.49 (0.11–2.07) | 0.39 | – | – |
| Solid-organ transplantation | 5.66 (0.73–44.17) | 0.08 | – | – |
| HIV | 0.49 (0.09–2.85) | 0.60 | – | – |
| Chronic use of immunosuppressive medication | 1.75 (0.37–8.25) | 0.74 | – | – |
| Upper respiratory tract infections | 0.24 (0.10–0.57) | <0.01 | 0.21 (0.08–0.55) | <0.01 |
| Neutropenia (<0.5×109/l) | 1.04 (0.43–5.37) | 2.51 | – | – |
| Pre-FRP group | 7.08 (2.31–21.65) | <0.01 | 8.00 (2.49–25.64) | <0.01 |
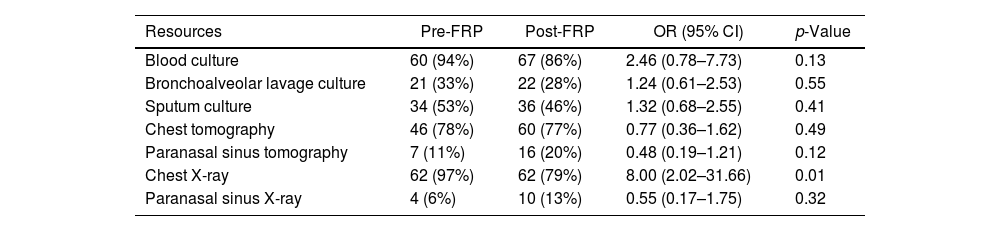
The use of complementary resources was similar in both groups (blood culture, bronchoalveolar lavage culture, sputum culture, chest tomography, paranasal sinus tomography, and paranasal sinus X-ray) and a significant difference was observed only in the chest X-ray resource. In the pre-RFP, 97% of patients had at least one chest X-ray ordered vs. 79% in the post-FRP (p 0.01) (Table 5).
Use of complementary resources.
| Resources | Pre-FRP | Post-FRP | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Blood culture | 60 (94%) | 67 (86%) | 2.46 (0.78–7.73) | 0.13 |
| Bronchoalveolar lavage culture | 21 (33%) | 22 (28%) | 1.24 (0.61–2.53) | 0.55 |
| Sputum culture | 34 (53%) | 36 (46%) | 1.32 (0.68–2.55) | 0.41 |
| Chest tomography | 46 (78%) | 60 (77%) | 0.77 (0.36–1.62) | 0.49 |
| Paranasal sinus tomography | 7 (11%) | 16 (20%) | 0.48 (0.19–1.21) | 0.12 |
| Chest X-ray | 62 (97%) | 62 (79%) | 8.00 (2.02–31.66) | 0.01 |
| Paranasal sinus X-ray | 4 (6%) | 10 (13%) | 0.55 (0.17–1.75) | 0.32 |
This study provided evidence of the effect of the implementation of FRP on antibiotic and oseltamivir treatment and other clinical outcomes in immunocompromised patients with suspected upper or lower acute respiratory illness.
There have been several studies demonstrating high clinical and analytical sensitivity and specificity, and a significant decrease in laboratory turnaround time (TAT) of FRP compared to standard diagnostic testing algorithms1,3–5,9,11,12. The higher sensitivity of the rapid diagnosis may change the current modes of diagnosis and management3. In this study, the detection rate was significantly higher in the post-FRP group. There were more influenza A cases detected in the post-FRP than in the pre-FRP group showing the higher sensitivity of the FilmArray panel and suggesting that many cases of influenza were missed and remained undiagnosed in the pre-FRP group, although in the pre-FRP group, 58% of the patients were requested real-time PCR for influenza (H1N1 and A). Other pathogens not included in the assays performed in the pre-FRP period, such as human rhinovirus/enterovirus, parainfluenza 4, and coronaviruses, also contributed to the higher detection rate in the post-FRP. The positivity rate was similar to that reported by other authors after FilmArray implementation1,2,5,6,9–12.
One of the potential benefits of the molecular multiplex panel for viruses in hospitals includes a reduction in unnecessary antimicrobial use and directed therapy rather than empirical antiviral and antibiotic use1. Antimicrobial resistance is arguably one of the greatest threats to global human health and is driven by the overuse of antibiotics. Antibiotics are prescribed to most hospitalized patients with acute respiratory illness, even when viruses are strongly implicated as the cause. Neuraminidase inhibitors are recommended by the Infectious Diseases Society of America (IDSA) guidelines for the treatment of influenza A and B in hospitalized patients with documented or suspected influenza, and in outpatients who are at high risk of complications (including immunocompromised patients)14. Implementing a rapid viral panel may improve antimicrobial therapy, minimizing unnecessary antiviral and antibiotic exposure and drug-related adverse events. In this study, the diagnosis by FRP of acute respiratory infections in immunocompromised patients was independently associated with fewer prescriptions of antimicrobial treatments. In other studies, the impact of FRP on the reduction in antibiotic prescriptions was not conclusive. Vos et al. and Saarela et al. proved that rapid molecular testing for respiratory viruses did not reduce antibiotic prescriptions11,15. The population studied was immunocompromised and immunocompetent adult patients respectively. Rogers et al. reported an impact on reduction in the duration of antibiotic use; however, that study did not include results of atypical bacteria detected in the FilmArray Respiratory Panel10. Brendish et al. did not observe a reduction in the duration of antibiotics overall, but more patients in the FilmArray group received single doses or brief courses of antibiotics, mainly in patients with asthma and acute exacerbation of chronic obstructive pulmonary disease1. Similar results were reported by Qian et al. stating that the FilmArray group had significantly lower antimicrobial DDDs, compared with the control cohort8. Shengchen et al. showed a shorter duration of intravenous antibiotic treatment in adults with lower respiratory tract illness throughout four consecutive seasons12. Echavarría et al. reported a decrease in antibiotic prescriptions in adult and pediatric patients in an Argentine university hospital2. Another study in pediatric patients proved that the rapid multiplex PCR reduced the days of antimicrobial therapy. Different antibiotics were analyzed separately and a reduction in cephalosporins, macrolides, and tetracyclines was detected5. In the present study, fewer prescriptions in antimicrobial treatment were observed in the post-FRP group, optimizing antimicrobial stewardship. The main impact was observed in the reduction of macrolide prescription (44% vs. 13%, p<0.01), but we also observed a reduction in beta-lactam prescription (89% vs. 61%, p<0.01). The reduction in macrolide prescriptions can be directly explained by the rapid negative result of atypical bacteria detected in the FRP. In the pre-FRP group, as they had a longer TAT, empirical treatment was instituted until a negative result for Mycoplasma pneumoniae and Chlamydia pneumoniae (antibodies or PCR) was obtained. Moreover, there was a reduction in three days of macrolide antibiotics. The reduction in beta-lactam prescriptions could be due to a reduction in empirical therapies with either a positive result for a viral agent or a negative panel result. These findings show a need to develop different antimicrobial stewardship strategies to adapt antibiotic prescribing to the clinical settings.
In other studies, routine rapid multiplex molecular panels for respiratory viruses were associated with an increased rate of detection of influenza cases and an improvement in antiviral use1,2,15. Early initiation of antiviral therapy was associated with the best outcomes14, and Brendish et al. suggested that the shortest TAT reduces the time to administration of the first dose1. Vos et al. and Echavarría et al. demonstrated that the adequate use of oseltamivir improved in immunocompromised and immunocompetent patients respectively, with fewer prescriptions in influenza-negative patients and more in influenza-positive patients2,15. In our study, there was no difference in neuraminidase inhibitor prescriptions within both groups. However, the use of oseltamivir could have been improved because the median of days of treatment in the pre-FRP group was 2.5 days and in the post-FRP group was 5 days (p 0.01). This suggests that there were more completed treatments. However, there were not many cases of influenza to assess this outcome.
The impact of reduced clinical TAT with FilmArray on hospital LOS is controversial. Some studies demonstrated shorter LOS1,4,5,9,10,15, other studies showed no difference in LOS2,6, and one study reported a longer LOS8. Tickoo et al. showed a statistically significant reduction in LOS only among patients with positive test results13. The reason for these differences among studies is not clear. No differences in hospital admission rate, days-reduction in droplet isolation precautions, hospital LOS, admission to the intensive care unit, LOS in ICU, changes in antimicrobial treatment, treatment failure, and 30-day mortality were observed in this study. The use of complementary resources such as medical imaging (chest/paranasal sinus tomography and X-ray) and additional microbiological tests (blood culture, sputum culture, and bronchoalveolar lavage culture) was similar in both groups, with the only exception that there were more chest X-rays in the pre-FRP group. In agreement with other authors7, we consider that clinical decision-making regarding FRP results is influenced by the clinical context and other factors related to the underlying disease such as neutropenic status and comorbid conditions in addition to acute respiratory illness.
This study has several limitations. The first is its retrospective and nonrandomized design. Given the before/after design of the study, outcomes could be biased due to residual confounders. Second, our study was a single-center study, and clinical outcomes such as antibiotic and antiviral prescriptions might be influenced by local protocols and guidelines, making results potentially less generalizable to other settings. Third, we analyzed data for almost two years without assessing seasonal variation in viral influenza-like illnesses; however, we assume that the variation in the prevalence of respiratory infections would not affect antibiotic prescription in immunocompromised patients. According to IDSA recommendations, immunocompromised individuals who present acute onset of respiratory symptoms with or without fever should be tested for influenza during high and low influenza activity14. Lastly, this study was conducted before the coronavirus disease 2019 (COVID-19) pandemic and the FRP panel utilized in this study did not include Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2).
In conclusion, the FilmArray Respiratory Panel impacted patient care by improving diagnostic yield and optimizing antimicrobial treatment in immunocompromised adult patients. This study showed the importance of using these rapid molecular multiplex tests in immunocompromised adults, who could be the most favored patients by these new technologies. Further studies are needed to confirm these findings.
FundingBiomerieux Argentina partially supported this work by providing free panels. The sponsor was not involved in the conduct of the study or the analysis of the data.
Conflict of interestAuthors report no conflict of interest.