In Argentina, hemolytic uremic syndrome (HUS) caused by Shiga toxin-producing Escherichia coli (STEC-HUS) infection is endemic, and reliable data about prevalence and risk factors have been available since 2000. However, information about STEC-associated bloody diarrhea (BD) is limited. A prospective study was performed during the period October 2018–June 2019 in seven tertiary-hospitals and 18 referral units from different regions, aiming to determine (i) the frequency of STEC-positive BD cases in 714 children aged 1–9 years of age and (ii) the rate of progression of bloody diarrhea to HUS. The number and regional distribution of STEC-HUS cases in the same hospitals and during the same period were also assessed. Twenty-nine (4.1%) of the BD patients were STEC-positive, as determined by the Shiga Toxin Quik Chek (STQC) test and/or the multiplex polymerase chain reaction (mPCR) assay. The highest frequencies were found in the Southern region (Neuquén, 8.7%; Bahía Blanca, 7.9%), in children between 12 and 23 month of age (8.8%), during summertime. Four (13.8%) cases progressed to HUS, three to nine days after diarrhea onset. Twenty-seven STEC-HUS in children under 5 years of age (77.8%) were enrolled, 51.9% were female; 44% were Stx-positive by STQC and all by mPCR. The most common serotypes were O157:H7 and O145:H28 and the prevalent genotypes, both among BD and HUS cases, were stx2a-only or -associated. Considering the endemic behavior of HUS and its high incidence, these data show that the rate of STEC-positive cases is low among BD patients. However, the early recognition of STEC-positive cases is important for patient monitoring and initiation of supportive treatment.
En Argentina, el síndrome urémico hemolítico asociado a Escherichia coli productor de toxina Shiga (STEC-SUH) es endémico y, desde 2000, de notificación obligatoria. Sin embargo, la información sobre diarrea sanguinolenta (DS) asociada a STEC (DS-STEC) es limitada. Se realizó un estudio prospectivo desde octubre de 2018 hasta junio de 2019 en siete hospitales de tercer nivel y 18 unidades de referencia de diferentes provincias argentinas, con el objetivo de determinar la frecuencia de casos de DS-STEC en 714 niños de 1 a 9 años que tuvieron DS (I) y la tasa de progresión de DS a SUH en dicha cohorte (II). También se evaluó el número y distribución regional de casos de STEC-SUH en los mismos hospitales en dicho período. Veintinueve casos de DS (4,1%) fueron STEC-positivos, determinados por Shiga Toxin Quik Chek (STQC) o PCR múltiple (mPCR). Las frecuencias más altas se encontraron en el sur del área relevada (Neuquén, 8,7%; Bahía Blanca, 7,9%), en niños de 12 a 23 meses (8,8%), en verano. Cuatro casos de DS-STEC (13,8%) progresaron a SUH, de tres a nueve días después del inicio de la diarrea. Se registraron 27 niños con STEC-SUH, estos fueron mayoritariamente <5 años (77,8%) del sexo femenino (51,9%). El 44% de estos casos fueron Stx-positivos por STQC y todos por mPCR. Los serotipos más comunes fueron O157:H7 y O145:H28, y el genotipo predominante fue stx2a, solo o asociado, en DS y SUH. Considerando el comportamiento endémico del SUH y su alta incidencia, estos datos muestran que la tasa de casos de DS-STEC es baja. Sin embargo, su reconocimiento temprano es importante para el seguimiento e inicio del tratamiento de sostén.
Shiga toxin-producing Escherichia coli (STEC) are a heterogeneous group of foodborne pathogens and an important cause of morbidity and mortality, with associated loss of life years and diminished health-related quality of life. The clinical manifestations of infection range from symptom-free carriage, diarrhea with and without visible blood, and the hemolytic-uremic syndrome (HUS)11. HUS is a systemic thrombotic microangiopathy, involving acute kidney failure that may cause death or end-stage renal disease, a chronic condition that reduces life expectancy22.
The linkage between STEC infection and the development of HUS was established in the early 1980s15,16. Shiga toxin 1, and/or Shiga toxin 2 have been demonstrated to be the primary virulence traits responsible for human disease35 and many Stx alleles have been described (Stx1a, Stx1c, Stx1d, Stx2a, Stx2b, Stx2c, Stx2d, Stx2e, Stx2f, Stx2g, and the recently described Stx2h, Stx2i, Stx2k)13,35. Strains producing Stx2a, Stx2c, or Stx2d are reported to be more virulent than those strains producing Stx1 subtypes alone or both Stx1 and Stx236. In addition, a mosaic of different virulence traits, comprising several adhesins and other toxins that may play a role in pathogenesis, has also been described30.
STEC isolates belong to a large number of O:H serotypes, but STEC O157:H7 is the most prevalent serotype associated with severe human diseases. However, non-O157 serogroups such as O26, O45, O103, O111, O121, and O145 have been identified as human pathogens10.
The mean incubation period of STEC infection is 3 (range 2–12) days40. The predominant sign of infection is diarrhea, which is bloody in 80% of the cases. Abdominal pain is more severe than in other cases of bacterial gastroenteritis and most patients infected with O157:H7 do not have fever at the time they present to medical facilities8. Diarrhea usually improves in one week, but 15–20% of childhood cases evolve to HUS32.
STEC-HUS is endemic in Argentina. In the last 10 years, the incidence of HUS has ranged from 8 to 10 cases per 100000 children under 5 years of age and the case fatality rate remains between 1 and 4%. In 2018, 339 HUS cases were notified, with a rate of 0.74 cases per 100000 inhabitants. HUS incidence differs markedly according to region (Boletín Integrado de Vigilancia No. 463–SE 34/2019. Available online: https://www.argentina.gob.ar/sites/default/files/biv_463_cuatrisemanal.pdf [accessed on 14 July 2022]).
While there have been reliable data on HUS incidence since its reporting became mandatory in 200032, the frequency of STEC-associated BD, its epidemiology, and the risk of progression to HUS in children remain unknown.
Here, we determine (i) the frequency of STEC-positive in BD cases in children 1–9 years of age; (ii) the rate of progression to HUS in STEC positive-BD cases within 28 days from diagnostic confirmation, and (iii) the epidemiology and regional distribution of STEC-HUS cases in children aged 1–9 years old in the same institutions during the same period.
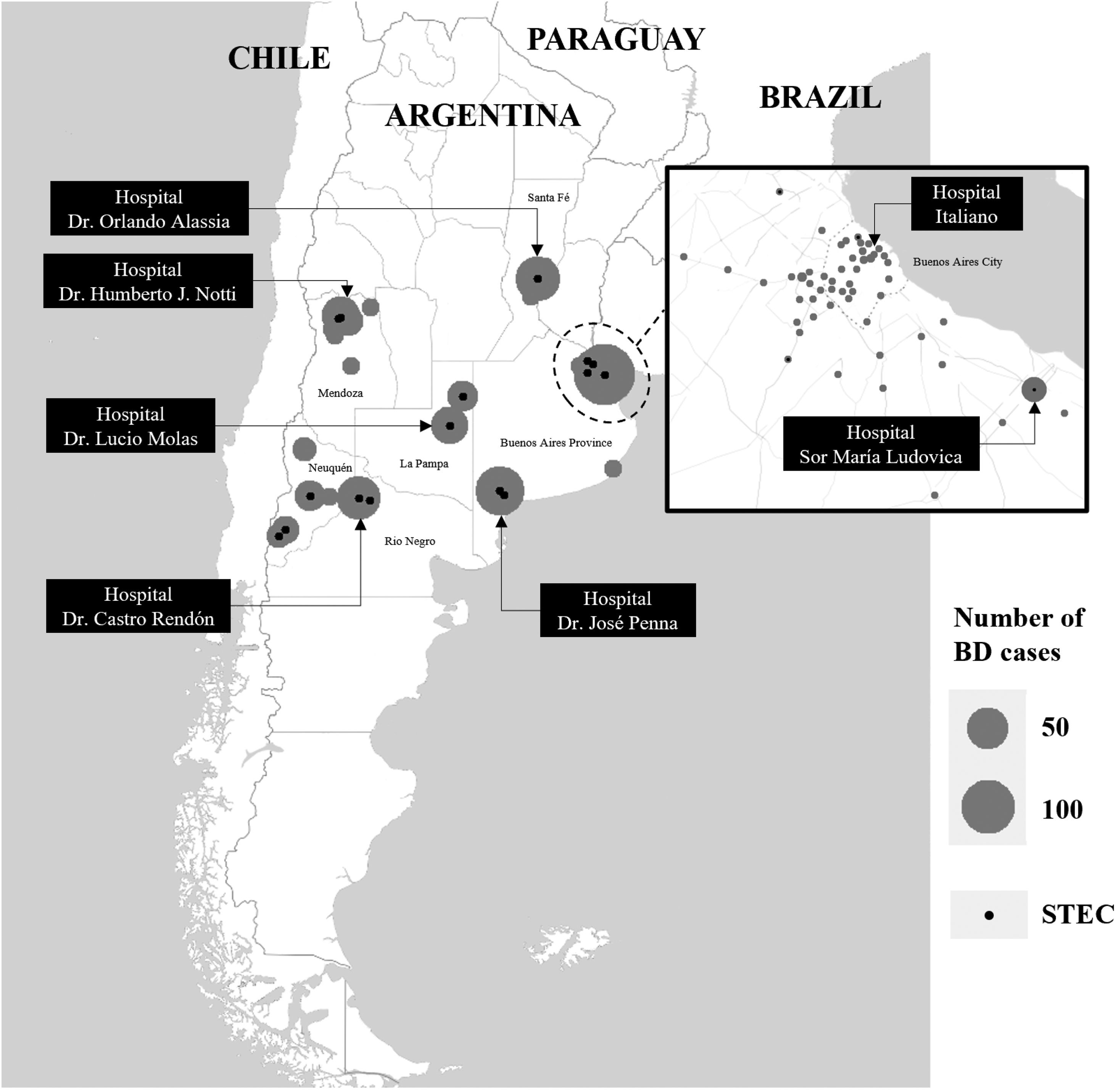
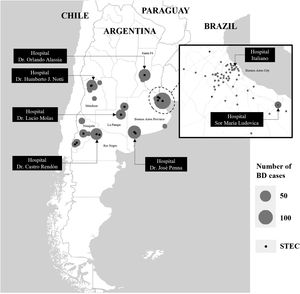
Materials and methodsStudy designA prospective multicenter surveillance study of pediatric patients with acute BD (first cohort) was conducted between October 10, 2018 and June 30, 2019 in seven tertiary-care hospitals (Reference Sites) and their 18 hospitals that treat patients with less complex illnesses (referral units) in Buenos Aires, La Pampa, Mendoza, Neuquén, Río Negro and Santa Fe provinces, and Buenos Aires City, Argentina (Fig. 1). Screening criteria included children of either gender between 1 and 9 years of age who presented acute BD. Acute BD was defined as loose stools by parental/caregiver report, with the presence of visible blood or blood observable only under a microscope, with less than 2 week-duration.
We also studied a second cohort of HUS cases aged 1–9 years, known to be infected with STEC in the same hospitals during the study interval. HUS was defined as the triad: microangiopathic hemolytic anemia (fragmented red blood cells in peripheral blood smear – schistocytes, of non-immune origin [negative direct Coombs test]), thrombocytopenia (platelet count<150000cells/mm3), and renal dysfunction (serum creatinine concentration>2 standard deviations above the upper limit of normal for age).
The demographic, clinical and microbiological data of all cases incorporated were obtained from the medical history and related medical records, according to the standards of the Reference Site. A study-specific electronic Case Report Form (eCRF) was used as the only tool for data collection, storage and reporting. Data entered included age, gender, origin (province/locality), date of onset of bloody diarrhea, date of collection of the stool sample (identified by a code number for microbiological analysis), and history of antibiotic exposure. Progression to HUS within 28 days from the STEC diagnostic confirmation was identified and the date of the diagnosis (i.e., the date that the HUS case definition was fulfilled) was recorded.
The study was performed in compliance with the legal and regulatory requirements, as well as scientific purpose, value and rigor, and the principles established in the Declaration of Helsinki42 and in the Guide for Good Clinical Practices (ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6(R2). November 9th, 2016. URL: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf).
The study protocol was approved by the Institutional Review Boards or the local Ethics Committees as required by the local regulation [Disposición ANMAT No. 6677/10. November 2010. URL: https://www.argentina.gob.ar/normativa/nacional/disposici%C3%B3n-6677-2010-174557/texto] and institutional Standard Operating Procedures. Thus, each Reference Site joined the study as soon as approval was obtained, between October 2018 (Hospital Penna of Bahia Blanca, Buenos Aires Province) and February 2019 (Hospital Molas of Santa Rosa, La Pampa).
Laboratory methodsDates of reception and processing of the stool sample in the local laboratory, type of sample (fresh stool or rectal swab) and result of the microbiological tests were recorded.
All fecal samples were screened using the Shiga Toxin Quik Chek (STQC) test (Abbott™, TechLab®, Blacksburg, VA) according to the manufacturer's instructions and the guidelines established by the National Reference Laboratory (NRL) of Argentina directly on feces (direct method), and after overnight (16–20h) stationary incubation at 37°C in 8ml of Gram Negative (GN) Broth-Hajna (Becton Dickinson, Sparks, MD) (enrichment method). A total of 413/714 fecal samples were processed by an in-house validated multiplex PCR, according to laboratory capacity17. These samples were plated onto sorbitol-MacConkey agar (SMAC, Becton Dickinson) directly, and after enrichment at 37°C for 4h in trypticase soy broth (TSB) (Becton Dickinson), and TSB supplemented with cefixime (50ng/ml) and potassium tellurite (25mg/ml) (CT-TSB). The confluent growth zone and colonies were screened for stx1, stx2, and rfbO157 genes. Isolates positive for stx1 and/or stx2 were confirmed as E. coli by standard biochemical tests.
STEC infection in HUS patients was established by at least detecting (i) Stx1 and/or Stx2 by STQC; (ii) stx genes by mPCR and/or STEC isolation; (iii) detection of free fecal Shiga toxin (FFStx) by neutralization of the cytotoxic effect on Vero cells, using Stx1- and Stx2-specific monoclonal antibodies (MAb 13C4 and BC5BB12, respectively)16; (iv) detection of specific IgM and IgG antibodies against the O-polysaccharide of E. coli O157, O145, O121, and O103 using an indirect enzyme linked immunosorbent assay (CHEMLIS® E. coli Combi Glyco-iELISA kit, Chemtest S.A., Argentina).
Fecal samples, and stx-positive isolates and sera were sent to the NRL for confirmation and further characterization23,31.
Statistical analysisA descriptive and exploratory analysis was performed using R software version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were summarized by mean/standard deviation and median/interquartile range (IQR), whereas frequency and proportions were applied to summarize qualitative variables. Categorical variables were compared using the Pearson chi-square test. All tests were two-sided and p-values less than 0.05 were considered statistically significant.
The following proportions and intervals were estimated: (1) proportion of STEC-positive BD by any method (STQC, direct and/or enriched culture, and mPCR) globally and by Reference Site; (2) cumulative incidence of STEC-positive BD evolving to HUS; (3) interval between the onset of BD and seeking medical attention; (4) interval between the onset of BD symptoms and sample collection; (5) interval between sample collection and processing; and (6) frequency of confirmed HUS cases. The performance of the STQC in comparison to the mPCR was also evaluated.
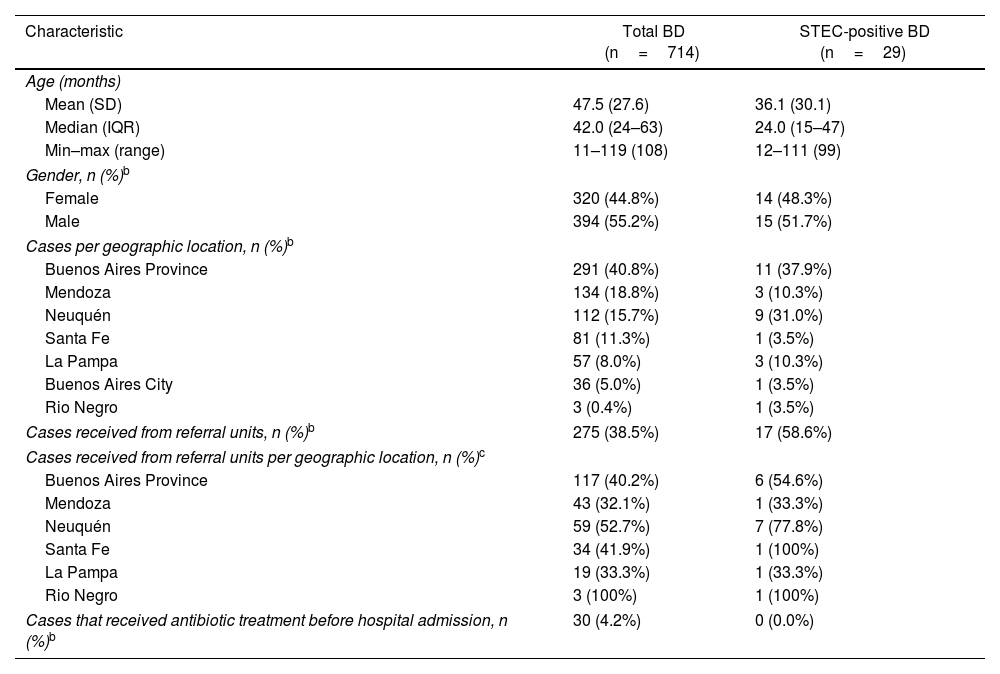
ResultsStudy populationA total of 714 children with BD were included in the study. Median age was 42 months (IQR 24–63; range, 11–119 months), 44.8% (n=320) were female and 4.2% (n=30) received antimicrobial treatment before admission to the hospital. The median interval between BD onset and seeking medical attention was 0 days (IQR 0–1 days; range, 0–17 days).
Twenty-seven STEC-HUS cases were enrolled. Median age was 37 months (IQR 25–59 months; range 16–104 months) and 51.9% (n=14) were female. For this cohort, the median interval between BD onset and STEC-HUS diagnosis was 4 days (IQR 1–6 days; range, 0–10 days).
Distribution of bloody diarrhea cases by Reference SiteOf the 714 patients, Hospital “Dr. José Penna” of Bahía Blanca enrolled 12.4% of the cases, Hospital “Dr. Humberto J. Notti” of Mendoza 18.8%, Hospital “Dr. Castro Rendón” of Neuquén, 16.1%, Hospital “Sor María Ludovica” of La Plata 21.9%, Hospital Italiano of Buenos Aires City 11.6%, Hospital “Dr. Orlando Alassia” of Santa Fe 11.2%, Hospital “Dr. Lucio Molas” of Santa Rosa, La Pampa 8.0%. Of the total, 61.5% patients were assisted in the Reference Sites and 38.5% in the corresponding referral units. The geographic distribution of BD cases is shown in Figure 1.
Microbiological and demographic findingsTwo hundred and eighty-nine (40.5%) of stool samples were collected on the same day of BD onset, 250 (35%) one day later, while the remaining 175 (24.5%) two or more days after BD onset; 593 (83.1%) were processed by STQC within the first 24h of sampling.
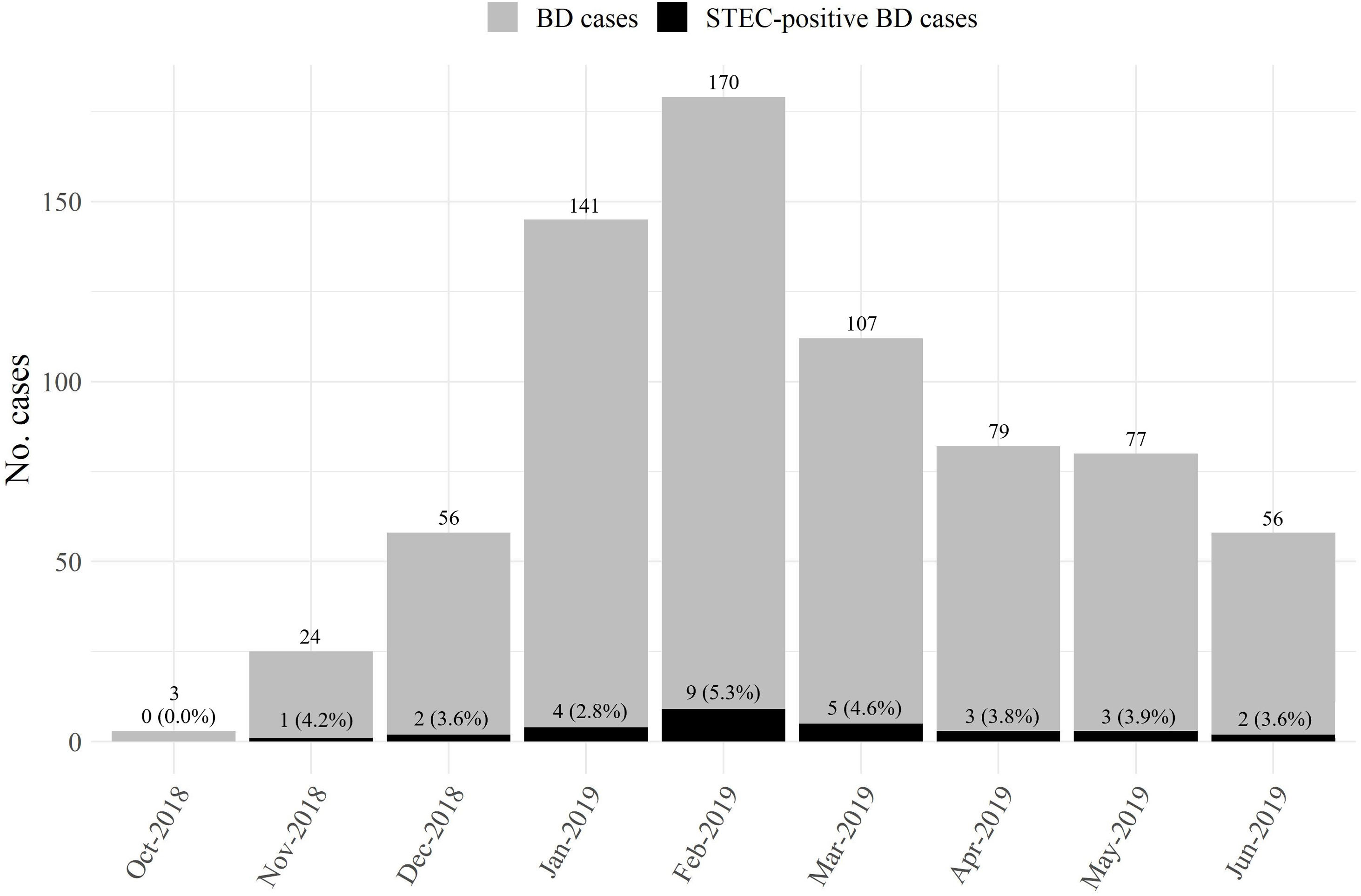
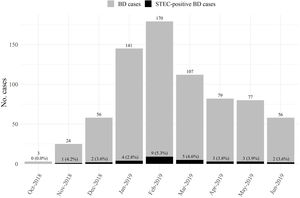
STEC-positive BD cohortA total of 29 cases were confirmed by STQC and/or mPCR, resulting in a cumulative incidence of 4.1% (95% CI: 2.7%–5.8%). Of the 29 STEC-positive BD cases, 21 (72.4%) were detected by the STQC test, 13 (44.8%) by the direct method and 8 (27.6%) by the enrichment method. By mPCR, 28 cases were stx-positive. In the remaining case that tested positive by STQC, mPCR was not performed; however FFStx was detected at the NRL as described by Karmali et al.16 Among the stool samples collected on the same day and one day later of BD onset, the rate of STEC detection was 4.3% (23/539), and 3.4% (6/175) among the samples collected 2 or more days later. The proportion of STEC-positive BD ranged from 1.3% to 8.7% (Fig. 1) and there was a summer peak (Fig. 2). Of the 29 STEC-positive BD cases detected, 17 (58.6%) were cared for in the referral units and 12 (41.4%) in the Reference Sites (Table 1).
Demographic characteristics of BD and STEC-positive BD casesa
| Characteristic | Total BD (n=714) | STEC-positive BD (n=29) |
|---|---|---|
| Age (months) | ||
| Mean (SD) | 47.5 (27.6) | 36.1 (30.1) |
| Median (IQR) | 42.0 (24–63) | 24.0 (15–47) |
| Min–max (range) | 11–119 (108) | 12–111 (99) |
| Gender, n (%)b | ||
| Female | 320 (44.8%) | 14 (48.3%) |
| Male | 394 (55.2%) | 15 (51.7%) |
| Cases per geographic location, n (%)b | ||
| Buenos Aires Province | 291 (40.8%) | 11 (37.9%) |
| Mendoza | 134 (18.8%) | 3 (10.3%) |
| Neuquén | 112 (15.7%) | 9 (31.0%) |
| Santa Fe | 81 (11.3%) | 1 (3.5%) |
| La Pampa | 57 (8.0%) | 3 (10.3%) |
| Buenos Aires City | 36 (5.0%) | 1 (3.5%) |
| Rio Negro | 3 (0.4%) | 1 (3.5%) |
| Cases received from referral units, n (%)b | 275 (38.5%) | 17 (58.6%) |
| Cases received from referral units per geographic location, n (%)c | ||
| Buenos Aires Province | 117 (40.2%) | 6 (54.6%) |
| Mendoza | 43 (32.1%) | 1 (33.3%) |
| Neuquén | 59 (52.7%) | 7 (77.8%) |
| Santa Fe | 34 (41.9%) | 1 (100%) |
| La Pampa | 19 (33.3%) | 1 (33.3%) |
| Rio Negro | 3 (100%) | 1 (100%) |
| Cases that received antibiotic treatment before hospital admission, n (%)b | 30 (4.2%) | 0 (0.0%) |
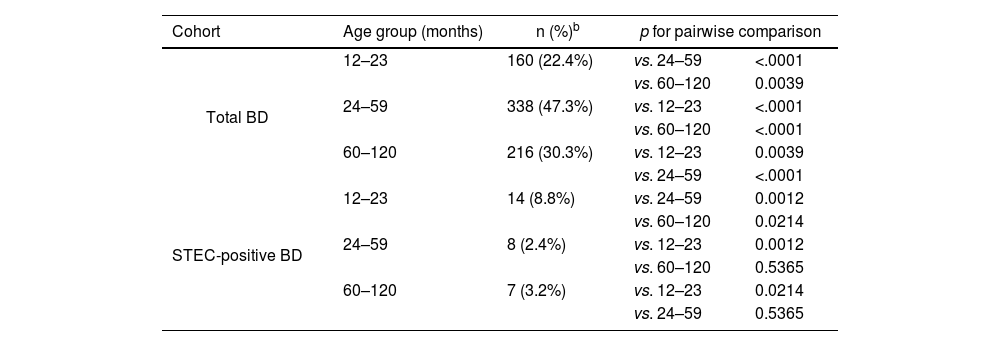
The greatest proportion of STEC-positive BD (8.8%, 14/160) was in children 12–23 months of age, and significant differences were observed among age groups (chi-square test for equal proportions (α=0.05), p=0.0026; pairwise comparisons: 12–23 vs. 24–59, p=0.0012; 12–23 vs. 60–120, p=0.0214; 24–59 vs. 60–120, p=0.5365) (Table 2).
Distribution of BD and STEC-positive BD cases by age groupa
| Cohort | Age group (months) | n (%)b | p for pairwise comparison | |
|---|---|---|---|---|
| Total BD | 12–23 | 160 (22.4%) | vs. 24–59 | <.0001 |
| vs. 60–120 | 0.0039 | |||
| 24–59 | 338 (47.3%) | vs. 12–23 | <.0001 | |
| vs. 60–120 | <.0001 | |||
| 60–120 | 216 (30.3%) | vs. 12–23 | 0.0039 | |
| vs. 24–59 | <.0001 | |||
| STEC-positive BD | 12–23 | 14 (8.8%) | vs. 24–59 | 0.0012 |
| vs. 60–120 | 0.0214 | |||
| 24–59 | 8 (2.4%) | vs. 12–23 | 0.0012 | |
| vs. 60–120 | 0.5365 | |||
| 60–120 | 7 (3.2%) | vs. 12–23 | 0.0214 | |
| vs. 24–59 | 0.5365 | |||
Of the 29 STEC-positive BD cases, 9 (31.0%) and 6 (20.7%) were caused by O157:H7 and O145:H28, respectively. Other serotypes detected were O26:H11 (4), O121:H19 (1), ONT:H2 (1), ONT:HNT (3). In five mPCR-positive cases, STEC was not recovered. Among 24 STEC isolates, the frequency of the stx-genotypes was, stx2a-only (n=12, 50.0%), stx2a/stx2c (n=8, 33.3%), stx1a (n=2, 8.3%), stx1a/stx2a and stx1a/stx2c (n=1, 4.2%, each one). All O157:H7 strains were recovered during warm weather months.
Three (10.7%) STEC-positive BD cases, diagnosed by direct or enriched STQC and confirmed by mPCR in local laboratories from Buenos Aires, Neuquén and Santa Fe developed HUS during follow-up. Additionally, one STEC-negative BD case by direct and enriched STQC tests and mPCR at the local laboratory in Mendoza developed HUS 4 days after admission and STEC O157:H7 stx2a/stx2c, and anti-O157-specific antibodies were detected at the NRL. Thus, with the addition of this latter case, 13.8% of STEC-positive BD patients developed HUS, three of whom were ≤24 months of age. The median interval between BD onset and the progression to STEC-HUS was 4.5 days (IQR 3–5 days; range, 3–5 days) during warm weather months. STEC O157:H7 stx2a/stx2c was isolated from three patients, while a STEC ONT:H2 stx1a/stx2a was identified in the remaining one.
HUS cohortThe 27 HUS patients of the second cohort were attended at the following hospitals: Sor María Ludovica (13), Notti (6), Penna (4), Castro Rendón (2), Italiano (1), Molas (1). The distribution by month was the following: December (2), January (8), February (9), March (5), April (1), May (1) and June (1).
The patient attended at the Hospital Italiano of Buenos Aires City was first STEC-positive in the local laboratory in Chubut Province. Of the remaining 26 cases analyzed by STQC and mPCR, 11 (44%) were Stx-positive by STQC, 5 by the direct method, and 6 after enrichment, while all 26 cases were positive by mPCR. No HUS patient received antibiotic treatment before this outcome ensued.
Among the 27 patients, STEC O157:H7 strains were recovered in 10 (37.0%), O145:H28 in 6 (22.2%). Among the O157:H7 isolates, the stx2a/stx2c genotype prevailed (n=8) and all O145:H28 strains were stx2a-only. Ten HUS cases were stx2-PCR positive without isolation and one was stx1-PCR without isolation. FFStx was detected in 9 of 22 cases analyzed; anti-O157 antibodies were identified in 8 and anti-O145 in 6 of 19 cases studied. The remaining 5 sera samples were negative for the currently available antigens (O157, O145, O121).
DiscussionLópez et al.19 and Rivero et al.33 reported a frequency of 4.1% and 10.1% STEC infections, respectively, in children with watery or bloody diarrhea in the Central Region of Argentina. Only the first study reported the STEC incidence in the BD group, which was 9.6%19. In comparison, we found STEC in a lower proportion of cases overall, 4.1% of the samples from patients with BD; however, the incidence varied greatly among the different sites, ranging from 1.3% in La Plata and Santa Fe to 7.9% and 8.7% in Bahía Blanca and Neuquén. The latter two sites are located in the Southern region, with high rates of HUS (https://www.argentina.gob.ar/sites/default/files/biv_463_cuatrimestral.pdf). However, the higher incidence of STEC-positive BD found could also relate to the fact that these two sites are affiliated with several referral units with diagnosis capacity for STEC, where patients often seek primary medical care, and arrive at tertiary hospitals with already established HUS, and in this settings BD is less often observed.
The young age of STEC-positive BD cases resembles the pattern of prior studies from Argentina19,33. The highest proportion was found among 12–23-month-old patients. Although there was a slightly larger proportion of males in the population studied, the gender distribution in the STEC-positive BD cases was even.
In the present study, 4.2% of 714 patients with BD were prescribed antibiotics; however, none of the patients presenting with STEC infections, either BD or HUS, received antibiotic treatment. These results are in agreement with the National Consensus of the Argentine Society of Pediatrics (https://www.sap.org.ar/uploads/consensos/consenso-de-diarreas-agudas-en-la-infancia.pdf), which recommends the treatment of diarrhea with antibiotics only in the case of patients who present a systemic compromise that is not attributable to hydroelectrolytic alterations or in whom sepsis or bacteremia is suspected. The use of certain antimicrobial agents can worsen or complicate the clinical course of the disease.
Most of the STEC-positive BD cases, including all E. coli O157:H7 cases, as well as the majority of HUS cases occurred during the summer months; this is in line with previous findings about seasonality, both in Argentina and other countries6,34,40.
It is estimated that around 10–15% of patients develop HUS following STEC infection in the 2 weeks after diarrhea onset, depending on the region and the season, and it is known that early recognition of infected patients is critical to improve the overall patient outcome and to be able to respond to outbreak situations in an effective and timely manner21,24.
In the present study, four (13.8%) STEC-positive BD patients developed HUS three to five days after BD onset, during summertime. This rate is similar to those found in previous reports4,9. On the other hand, Harkins et al.12 have recently reported higher rates in outbreak scenarios. The low prescription of antibiotics for treatment of BD in Argentina might account for a lower risk of HUS development.
All STEC isolated from these cases were Stx2a-producing strains in combination with other stx subtypes and belonged to O157:H7 (3) and ONT:H2 (1) serotypes. These results agree with previous studies, in which E. coli O157:H7 harboring the stx2a/stx2c genes was prevalent among diarrhea and HUS pediatric patients in Argentina25,28,31. The risk of progression to HUS associated with Stx2-producing strains has been described in other countries. Adams et al.1, in the UK, found age (1–4 year-old children), infection with stx2-only, antibiotic exposure and BD or vomiting to be predictor factors. In the same line, Tarr et al.39 described a substantial increase in HUS risk associated with the stx2a genotype as compared to the stx1a/stx2a genotype, two of the most common genotypes in USA and Japan14,20,26. They also found that the virulence of the stx2a/stx2c genotype, predominant in other settings18,29, was similar to the stx2a-only genotype. Ardissino et al.3, have reported similar findings from Italy. The paradoxical findings of greater virulence among STEC O157 that produce Stx2 compared to those that produce Stx1 and Stx2 were also demonstrated in animal models27.
In Argentina, STEC-HUS is one of the most common etiologies of acute kidney injury in children, mortality rates can reach up to 3.6%2, and long-term renal sequelae remain, on average, around 30–50%37. For this reason, the early and accurate diagnosis of STEC infection would be beneficial for the early initiation of supportive treatment. Moreover, it is known that the isolation rate of STEC in feces declines quickly after the first symptoms41. In this study, only 40.5% of stool samples from patients with BD were collected on the same day of BD onset, highlighting the need to strengthen public awareness regarding early consultation, as well as to improve the health system response so that both pediatricians and laboratory staff make sure to order and process sample collection timely. It will also be desirable to reduce turnaround time, and in this regard, rectal swab specimens might be preferred over bulk stool cup specimens, when the latter are not immediately available7.
Of the diagnostic techniques applied, STQC allows detection of the most common Stx1 and Stx2 subtypes, and is easy to perform and interpret. Chui et al.5 reported a sensitivity and specificity of 70.0% and 99.4%, respectively, and when the enrichment culture was used, sensitivity increased to 85.0%. In our study, the STQC showed lower sensitivity compared with the results obtained using mPCR. The use of an enrichment step improved STQC performance, raising from 44.8% STEC-positive BD cases identified by the direct method to 72.4%, in line with previous reports5,38.
Among the 27 STEC-HUS patients of the second cohort, 77.8% were under 5 years of age and 51.9% were female, just like those reported for Argentina (https://www.argentina.gob.ar/sites/default/files/biv_463_cuatrimestral.pdf). With regard to origin, 63% resided in different locations of Buenos Aires, 22.2% in Mendoza, and the rest in Chubut, La Pampa, Neuquén and Río Negro provinces (3.7% each). Of the 26 HUS patients, 11 (44%) were Stx-positive by STQC, while all 26 cases were positive by mPCR. Historically in Argentina, the evidence of STEC infection in HUS patients was around 30%; however, diagnostic performance has significantly improved since 2014, reaching >60% with the incorporation of anti-O serogroup-specific antibodies O157, O145, O121 detection23. In the present study, anti-O157 antibodies were identified in 8 and anti-O145 in 6 of the serum samples available from 19 patients.
The serotypes and genotypes of the STEC strains isolated in the present study are similar to those previously reported in Argentina, which showed the prevalence of O157 (59%) followed by O145 (12.6%), as well as the predominance of Stx2 (90.3%) over Stx1 (9.7%)31. Pianciola et al.28 found that the stx2a/stx2c genotype was prevalent in human (76.1%) and bovine (55.5%) O157 strains from different regions of Argentina. Moreover, 87.6% of the strains belonged to the hypervirulent clade 8. These data may help to understand the causes of the epidemiological situation related to HUS in Argentina.
In summary, 4.1% of BD cases was associated with STEC infections, with a great variability among the different regions of Argentina. Moreover, 13.8% of these STEC-positive BD cases evolved to HUS. Considering the endemic behavior of HUS in Argentina and its impact on public health, it is important that professionals have updated information about the epidemiology of this diarrheal disease, so that an accurate diagnosis can be made in a timely manner and patients can have the best opportunity for a positive outcome.
FundingThis work was supported by funds from INMUNOVA S.A.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors thank Dr. Phillip I. Tarr, Division of Gastroenterology, Hepatology and Nutrition, Washington University School of Medicine, St. Louis, USA, for his critical revision of this manuscript. We also thank all the professionals, technicians and assistants who collaborated in this multicenter study.
Lucas Iván Lucarelli, Lara Parada Fennen, Patricia Valles, Ana María Poidomani, María Huerga, Leonardo Fioravanti, Emanuel De Rose, Eugenia Crivaro, Nerina Pallaoro, Analía Prado, Paula A. Coccia, Verónica Ferraris, Carolina Aro, Mariana Castañeira, Andrea Rodríguez Llach, Luis Marcelo Casabona.