In 2005 a universal vaccination program against hepatitis A was introduced in Argentina. Nevertheless, there are still some unvaccinated marginal population groups. There are no data about the seroprevalence of hepatitis E in the northern region of Argentina mainly because of lack of awareness of this emergent pathogen. We aimed to determine the seroprevalence of hepatitis A, and hepatitis E in an indigenous population in northern Argentina. One hundred and twenty six (126) donor serum samples collected near San Salvador de Jujuy were analyzed for anti-HAV IgG and HEV IgG and IgM, alkaline phosphatase and transaminase values. Volunteers were interviewed about their living conditions, animal farming, consumption of tap water or river water, and level of education. Seroprevalence of specific anti-HAV antibodies was high (80.2%, 95% confidence interval, 72.1–86.7%) in children under 5 years of age, indicating early infection in life. Seroprevalence of anti-HEV antibodies was 5.6% (95% CI: 2.3–11.2%), being slightly higher than the values found in healthy patients from other regions of the country. Although we could not characterize the genotype of the circulating HEV strain, the clear epidemiological difference between seroprevalence of HAV and HEV in a community with poor sanitary conditions suggest that the circulating HEV strains spread through a different transmission route than HAV. Furthermore a significant correlation between anti-HEV IgG and swine farming was found (p<0.05), which supports a zoonotic transmission path. We reassessed the epidemiological pattern of HAV infection and reported evidence of HEV infection for the first-time in a community belonging to the Guarani ethnic group, highlighting the need to include hepatitis E testing in routine diagnostics in the region.
En 2005 se inició un programa de vacunación universal contra la hepatitis A en Argentina, pero todavía existen algunas poblaciones marginales no vacunadas. Además, los datos sobre la circulación de hepatitis E en el noroeste argentino son escasos. El objetivo de este trabajo fue determinar la seroprevalencia de la hepatitis A y la hepatitis E en una población autóctona del norte de Argentina. Se colectaron y analizaron 126 muestras de suero en habitantes de las yungas jujeñas; se determinaron transaminasas, fosfatasa alcalina y anticuerpos contra los virus de hepatitis A (HAV) (IgG) y hepatitis E (HEV) (IgG e IgM). Se obtuvieron los consentimientos informados y los voluntarios fueron entrevistados para identificar posibles factores de riesgo, como las condiciones de vida y la cría de animales, entre otros. La seroprevalencia de anticuerpos específicos anti-HAV fue alta (80,2%; intervalo de confianza [IC] 95%: 72,1-86,7%) en niños menores de 5 años, lo que indica infección temprana. La seroprevalencia de anticuerpos anti-HEV fue del 5,6% (IC 95%: 2,3-11,2%), ligeramente más alta que en otras regiones del país en pacientes sanos. Aunque no se caracterizó el genotipo circulante del HEV, la clara diferencia epidemiológica entre la seroprevalencia de ambos virus en una comunidad con malas condiciones sanitarias sugiere que la cepa circulante de HEV se transmite por una vía diferente que la del HAV. Además, encontramos una significativa correlación entre la cría de cerdo y la presencia de anticuerpos IgG anti-HEV (p<0,05), lo que sugiere una vía de transmisión zoonótica. Reevaluamos el patrón epidemiológico de infección por el HAV y aportamos por primera vez una evidencia de infección por el HEV en una comunidad que pertenece a la etnia guaraní, por lo que destacamos la necesidad de incluir la detección de hepatitis E en la región.
Hepatitis A virus (HAV) is a naked RNA virus of the genus Hepatovirus, family Picornaviridae48,52, transmitted by the fecal–oral route, mainly by consuming water or food contaminated with infected feces but also by close contact between individuals living in the same home or children in kindergarten, and by sexual contact especially of men having sex with men17,52.
Sanitary conditions, housing density and age of the population correlate with HAV infection rates. Argentina was considered a high endemicity area, though sanitary and hygienic conditions vary along the country11,17. For instance, the poverty index in 2016 for the northern provinces was higher than in the central region, reaching 21.5%, according to INDEC21.
Due to a hepatitis A outbreak between 2003 and 2004, which resulted in high rates of hepatic failure, a universal anti-HAV vaccination program was introduced by the Ministry of Health of Argentina in 20059. The vaccination schedule consisted of a single-dose for 12-month-old children, which proved to be effective and conferred up to 9 years of protection34,51,55. Despite the success of the program, which reduced the incidence of hepatitis A not only in children but in all age groups by herd immunity, certain communities such as the Guaraní in the Yungas of Jujuy had still not been vaccinated until this study was conducted9,51.
Hepatitis E virus (HEV) is an emergent virus in Argentina classified in the family Hepeviridae within the genus Orthohepevirus A26,48. HEV has eight genotypes but only four of them infect humans. Genotype 1 (HEV-1) is mainly found in Africa and Southeast Asia, HEV-2 particularly in Africa and Mexico; HEV-3 is widely distributed whereas HEV-4 is limited to Asia56.
HEV infection is a major cause of human viral disease with clinical and pathological features of acute hepatitis31. It is primarily transmitted via the fecal–oral route due to contaminated water and food, and is often responsible for epidemic outbreaks22,25. This infection represents an important public health concern in developing countries, being endemic for several tropical and subtropical regions28. In contrast, in industrialized countries, including many European countries, USA and Japan, acute hepatitis E occurs sporadically and its transmission pathways are still not fully understood29,44. The infection affects primarily young adults and is generally mild, except for women in late pregnancy with a reported higher mortality of up to 20% depending on the genotype29,44.
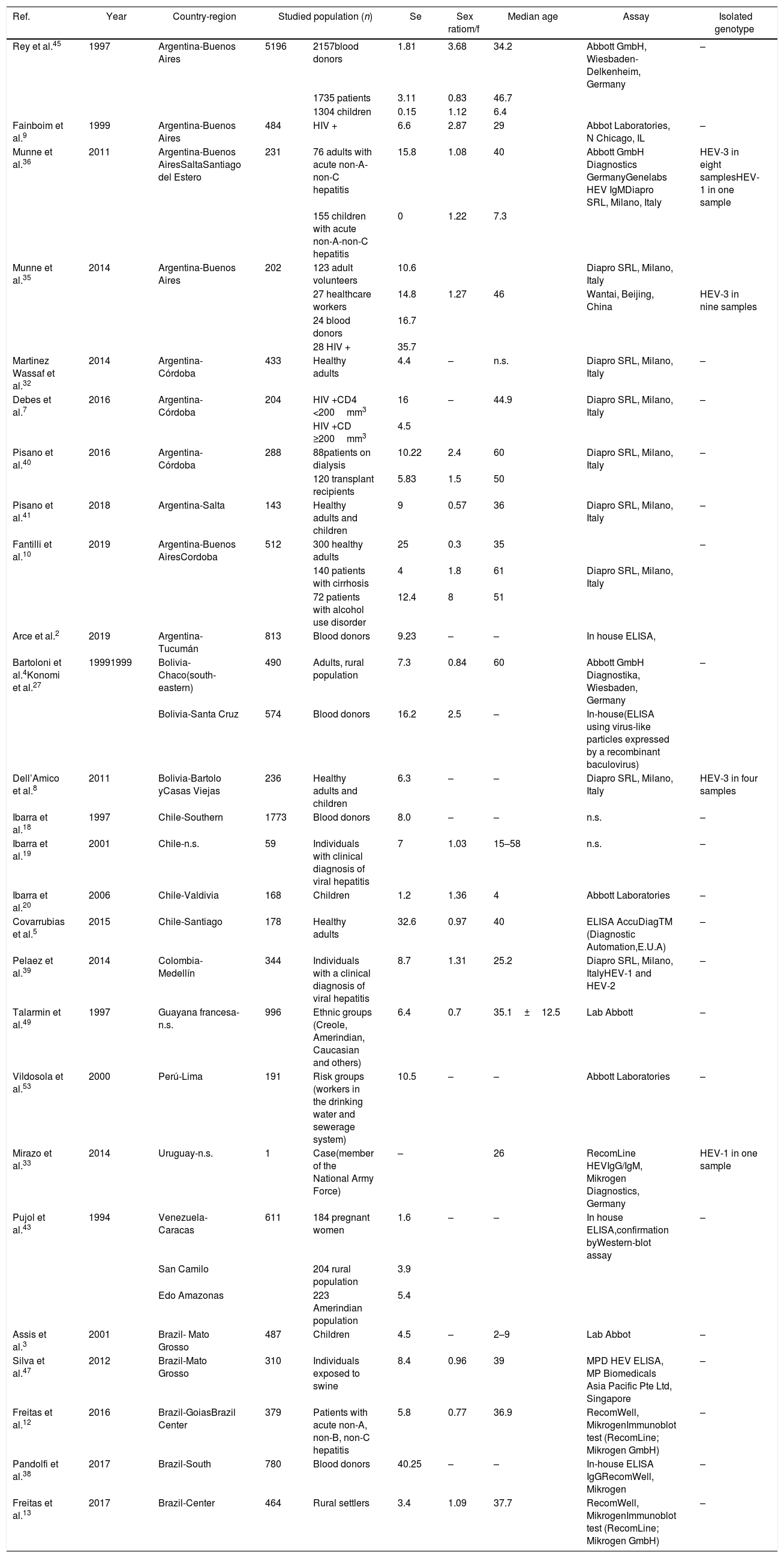
Hepatitis caused by HEV-1 is mostly a waterborne disease associated with large epidemics due to contamination of water and water supplies, and poor sanitation conditions22. Swine and human strains of HEV are genetically closely related and, in some cases, indistinguishable30. In contrast, hepatitis E caused by HEV-3 is a zoonotic disease, swine being a major host, and maybe other animal species are reservoirs and therefore potential sources of transmission. Human to human transmission is very rare for HEV-3 infections. There are scarce epidemiologic data of the seroprevalence of HEV in South America as reflected in Table 1, which summarizes published HEV results for the continent. Although both HEV-1 and HEV-3 were isolated from acute patients in South America (Table 1), HEV-1 isolates were related to travelers, except for the case of a patient from Uruguay with no travel history. In the rest of Latin-American countries where surveys were conducted and published, only HEV-3 was isolated (Table 1).
HEV seroprevalences reported in South America between 1994 and 2017.
| Ref. | Year | Country-region | Studied population (n) | Se | Sex ratiom/f | Median age | Assay | Isolated genotype | |
|---|---|---|---|---|---|---|---|---|---|
| Rey et al.45 | 1997 | Argentina-Buenos Aires | 5196 | 2157blood donors | 1.81 | 3.68 | 34.2 | Abbott GmbH, Wiesbaden-Delkenheim, Germany | – |
| 1735 patients | 3.11 | 0.83 | 46.7 | ||||||
| 1304 children | 0.15 | 1.12 | 6.4 | ||||||
| Fainboim et al.9 | 1999 | Argentina-Buenos Aires | 484 | HIV + | 6.6 | 2.87 | 29 | Abbot Laboratories, N Chicago, IL | – |
| Munne et al.36 | 2011 | Argentina-Buenos AiresSaltaSantiago del Estero | 231 | 76 adults with acute non-A-non-C hepatitis | 15.8 | 1.08 | 40 | Abbott GmbH Diagnostics GermanyGenelabs HEV IgMDiapro SRL, Milano, Italy | HEV-3 in eight samplesHEV-1 in one sample |
| 155 children with acute non-A-non-C hepatitis | 0 | 1.22 | 7.3 | ||||||
| Munne et al.35 | 2014 | Argentina-Buenos Aires | 202 | 123 adult volunteers | 10.6 | Diapro SRL, Milano, Italy | |||
| 27 healthcare workers | 14.8 | 1.27 | 46 | Wantai, Beijing, China | HEV-3 in nine samples | ||||
| 24 blood donors | 16.7 | ||||||||
| 28 HIV + | 35.7 | ||||||||
| Martinez Wassaf et al.32 | 2014 | Argentina-Córdoba | 433 | Healthy adults | 4.4 | – | n.s. | Diapro SRL, Milano, Italy | – |
| Debes et al.7 | 2016 | Argentina-Córdoba | 204 | HIV +CD4 <200mm3 | 16 | – | 44.9 | Diapro SRL, Milano, Italy | – |
| HIV +CD ≥200mm3 | 4.5 | ||||||||
| Pisano et al.40 | 2016 | Argentina-Córdoba | 288 | 88patients on dialysis | 10.22 | 2.4 | 60 | Diapro SRL, Milano, Italy | – |
| 120 transplant recipients | 5.83 | 1.5 | 50 | ||||||
| Pisano et al.41 | 2018 | Argentina-Salta | 143 | Healthy adults and children | 9 | 0.57 | 36 | Diapro SRL, Milano, Italy | – |
| Fantilli et al.10 | 2019 | Argentina-Buenos AiresCordoba | 512 | 300 healthy adults | 25 | 0.3 | 35 | – | |
| 140 patients with cirrhosis | 4 | 1.8 | 61 | Diapro SRL, Milano, Italy | |||||
| 72 patients with alcohol use disorder | 12.4 | 8 | 51 | ||||||
| Arce et al.2 | 2019 | Argentina-Tucumán | 813 | Blood donors | 9.23 | – | – | In house ELISA, | |
| Bartoloni et al.4Konomi et al.27 | 19991999 | Bolivia-Chaco(south-eastern) | 490 | Adults, rural population | 7.3 | 0.84 | 60 | Abbott GmbH Diagnostika, Wiesbaden, Germany | – |
| Bolivia-Santa Cruz | 574 | Blood donors | 16.2 | 2.5 | – | In-house(ELISA using virus-like particles expressed by a recombinant baculovirus) | |||
| Dell’Amico et al.8 | 2011 | Bolivia-Bartolo yCasas Viejas | 236 | Healthy adults and children | 6.3 | – | – | Diapro SRL, Milano, Italy | HEV-3 in four samples |
| Ibarra et al.18 | 1997 | Chile-Southern | 1773 | Blood donors | 8.0 | – | – | n.s. | – |
| Ibarra et al.19 | 2001 | Chile-n.s. | 59 | Individuals with clinical diagnosis of viral hepatitis | 7 | 1.03 | 15–58 | n.s. | – |
| Ibarra et al.20 | 2006 | Chile-Valdivia | 168 | Children | 1.2 | 1.36 | 4 | Abbott Laboratories | – |
| Covarrubias et al.5 | 2015 | Chile-Santiago | 178 | Healthy adults | 32.6 | 0.97 | 40 | ELISA AccuDiagTM (Diagnostic Automation,E.U.A) | – |
| Pelaez et al.39 | 2014 | Colombia-Medellín | 344 | Individuals with a clinical diagnosis of viral hepatitis | 8.7 | 1.31 | 25.2 | Diapro SRL, Milano, ItalyHEV-1 and HEV-2 | – |
| Talarmin et al.49 | 1997 | Guayana francesa-n.s. | 996 | Ethnic groups (Creole, Amerindian, Caucasian and others) | 6.4 | 0.7 | 35.1±12.5 | Lab Abbott | – |
| Vildosola et al.53 | 2000 | Perú-Lima | 191 | Risk groups (workers in the drinking water and sewerage system) | 10.5 | – | – | Abbott Laboratories | – |
| Mirazo et al.33 | 2014 | Uruguay-n.s. | 1 | Case(member of the National Army Force) | – | 26 | RecomLine HEVIgG/IgM, Mikrogen Diagnostics, Germany | HEV-1 in one sample | |
| Pujol et al.43 | 1994 | Venezuela-Caracas | 611 | 184 pregnant women | 1.6 | – | – | In house ELISA,confirmation byWestern-blot assay | – |
| San Camilo | 204 rural population | 3.9 | |||||||
| Edo Amazonas | 223 Amerindian population | 5.4 | |||||||
| Assis et al.3 | 2001 | Brazil- Mato Grosso | 487 | Children | 4.5 | – | 2–9 | Lab Abbot | – |
| Silva et al.47 | 2012 | Brazil-Mato Grosso | 310 | Individuals exposed to swine | 8.4 | 0.96 | 39 | MPD HEV ELISA, MP Biomedicals Asia Pacific Pte Ltd, Singapore | – |
| Freitas et al.12 | 2016 | Brazil-GoiasBrazil Center | 379 | Patients with acute non-A, non-B, non-C hepatitis | 5.8 | 0.77 | 36.9 | RecomWell, MikrogenImmunoblot test (RecomLine; Mikrogen GmbH) | – |
| Pandolfi et al.38 | 2017 | Brazil-South | 780 | Blood donors | 40.25 | – | – | In-house ELISA IgGRecomWell, Mikrogen | – |
| Freitas et al.13 | 2017 | Brazil-Center | 464 | Rural settlers | 3.4 | 1.09 | 37.7 | RecomWell, MikrogenImmunoblot test (RecomLine; Mikrogen GmbH) | – |
Se: seroprevalence; m/f: male/female; n.s.: not specified.
Hepatitis E virus has been isolated in humans46 and swine37 in Argentina; however, non-A non-B hepatitis epidemics have not been described yet37, which is consistent with HEV-3 circulation. As hepatitis E is still believed to be rare, diagnoses are not made routinely. Thus, data about HEV circulation may help increase the awareness of the disease and ultimately improve diagnostics and management of patients with unidentified high transaminases. Otherwise, the presumptive diagnosis of such patients is idiopathic hepatotoxicity.
Detection of HEV RNA in 15.8% of patients with acute non-A non-B hepatitis evidenced circulation of the virus in central Argentina36 (Table 1). Further, the virus was detected in rivers and sewage samples in 2013 in the central region of Argentina during an environmental surveillance study32. Munné et al.,45 showed that in blood donor banks in Buenos Aires the prevalence of anti-HEV antibodies was surprisingly low (less than 2%). Further, in a seroprevalence study of anti-HEV antibodies conducted in a population of HIV-positive patients in central Argentina, the seroprevalence of anti-HEV IgG was 6.6%, significantly higher than in control groups of non-HIV patients9 (1.8%, Table 1). The situation in the rest of South America changes from region to region. Thus, Brazil shows the highest indexes reaching 40.25% HEV seroprevalence in South Brazil using an in-house ELISA38. This finding is somehow surprising when compared to other regions were seroprevalence is 3.4–8.4%. The next highest index (32.6%) was found in Santiago de Chile, whereas other studies conducted outside the Chilean capital showed seroprevalence values from 1.2 to 8% (Table 1). According to a recent review by Pisano et al.42 and the actualized data in Table 1, HEV is widely disseminated all over South America, as assessed in humans, animals, environmental samples, and food, suggesting multifactorial ways of transmission.
Here, we reassess the epidemiological pattern of HAV infection and report evidence of HEV infection for the first time in a community belonging to the Guarani ethnic group living in the subtropical Yungas in the province of Jujuy, northern Argentina.
Materials and methodsStudy populationThe studied community belongs to the Guaraní ethnic group, which lives in the brooks of river Lavallén in a subtropical region called ‘Las Yungas’ located in the limit of the departments of Santa Bárbara and San Pedro in the province of Jujuy, northern Argentina. This community consists of about 320 inhabitants, who live in precarious conditions in clay cottages without tap water or sanitation and with free running farm animals. The population has similar socio-economic and cultural backgrounds. In general, participants have resided in the community for most of their lives. Farming is the major source of income; some farmers rear pigs, and other animals such as chickens for their own consumption and for sale to supplement their family incomes.
Written and informed consent was obtained from each participant or their guardians and the information regarding the protocol and informed consent was presented at the appropriate literacy level. All 126 consenting participants completed a structured questionnaire assessing epidemiological and socio-demographic characteristics, and a risk factor profile for the infection such as drinking water source, sanitary conditions, sewage disposal methods, pets, history of acute hepatitis in the subjects and the family in the previous six months was obtained. Around 50% of the population (n=126) aged between 1 and 65 years old participated in the study. Children were arranged into five-year-old groups, whereas adults older than 20 were grouped by decades.
The protocols were approved by the Ethics Committee of the Paterson Hospital, San Pedro de Jujuy, Argentina (Ref. 158-C-10).
Sample collectionBlood samples from each participant were collected in plain tubes. Samples were centrifuged, and aliquots of sera were kept frozen at −20°C and −80°C until analysis.
Liver function testsSerum samples were analyzed for alkaline phosphatase (ALP), aspartate amino transferase (AST) and alanine amino transferase (ALT) using commercial colorimetric assays (BioSystems S.A., Barcelona, Spain), following the manufacturer's instructions.
Anti-HEV IgG and IgM detectionAntibodies to hepatitis E virus were assayed using commercial kits (recomWell HEV IgG and IgM, Mikrogen, Germany) with a specificity of 98.8% and a sensitivity of 98.6%, according to the manufacturer's instructions. Samples tested positive for anti-IgG (96.6% sensitivity and 97.1% specificity and anti-IgM (sensitivity of 93.3% and 96.9% specificity) in ELISA were further analyzed by a commercial immunoblot assay (RecomLine HEV, Mikrogen, Germany), following the manufacturer's instructions.
Anti-HAV immunoglobulin detectionIgG antibodies to HAV were assayed using commercial kits (immunoassay, Architect System, Abbott) according to the manufacturer's instructions.
HEV RNA detectionSerum samples of anti-HEV Ig (IgG and/or IgM) positive patients (n=17) were examined by a routine diagnostic real-time RT-PCR adapted from Jothikumar et al.23 for the presence of HEV RNA.
Statistical analysisFrequencies and percentages of HEV antibody-positive donors were calculated and compared by demographic variables using the Fisher's exact test p<0.05 with the GraphPad Prism 6 Software. HEV seroprevalence with a 95% confidence level was calculated with the EpiTool software.
ResultsThe study group included 126 individuals, representing 39.37% of the community. The general characteristics of the population are shown in Table 2. Socio-economic and education levels were low as compared to urban areas of Argentina. The study panel was composed of 79 females and 47 males with a sex ratio (m/f) of 0.59 and the mean age was 20.87.
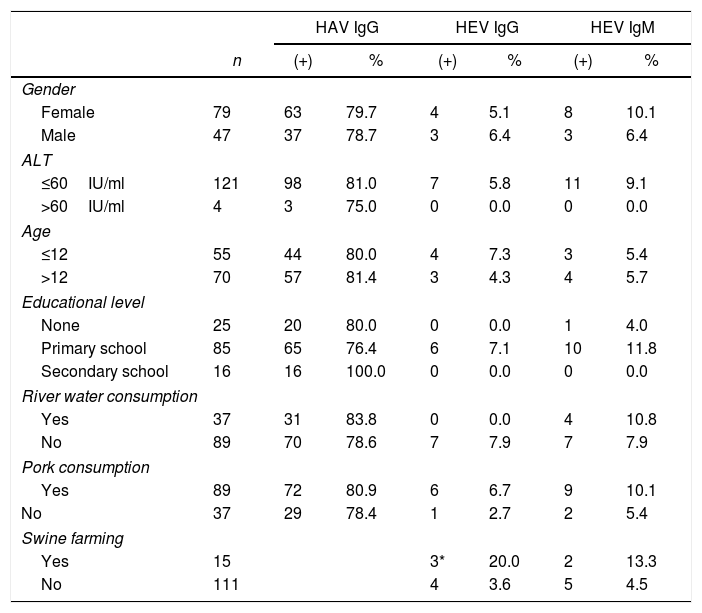
Prevalence of anti-HAV and anti-HEV antibodies as determined by ELISA according to sex, liver enzyme levels, age, educational level, consumption of river water and pork.
| HAV IgG | HEV IgG | HEV IgM | |||||
|---|---|---|---|---|---|---|---|
| n | (+) | % | (+) | % | (+) | % | |
| Gender | |||||||
| Female | 79 | 63 | 79.7 | 4 | 5.1 | 8 | 10.1 |
| Male | 47 | 37 | 78.7 | 3 | 6.4 | 3 | 6.4 |
| ALT | |||||||
| ≤60IU/ml | 121 | 98 | 81.0 | 7 | 5.8 | 11 | 9.1 |
| >60IU/ml | 4 | 3 | 75.0 | 0 | 0.0 | 0 | 0.0 |
| Age | |||||||
| ≤12 | 55 | 44 | 80.0 | 4 | 7.3 | 3 | 5.4 |
| >12 | 70 | 57 | 81.4 | 3 | 4.3 | 4 | 5.7 |
| Educational level | |||||||
| None | 25 | 20 | 80.0 | 0 | 0.0 | 1 | 4.0 |
| Primary school | 85 | 65 | 76.4 | 6 | 7.1 | 10 | 11.8 |
| Secondary school | 16 | 16 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| River water consumption | |||||||
| Yes | 37 | 31 | 83.8 | 0 | 0.0 | 4 | 10.8 |
| No | 89 | 70 | 78.6 | 7 | 7.9 | 7 | 7.9 |
| Pork consumption | |||||||
| Yes | 89 | 72 | 80.9 | 6 | 6.7 | 9 | 10.1 |
| No | 37 | 29 | 78.4 | 1 | 2.7 | 2 | 5.4 |
| Swine farming | |||||||
| Yes | 15 | 3* | 20.0 | 2 | 13.3 | ||
| No | 111 | 4 | 3.6 | 5 | 4.5 | ||
Eighty percent (80%) of the sera were positive for anti-HAV antibodies. There was no association with age or sex (Tables 2 and 3) but the pattern reflects infection early in life, similar to developing countries without vaccination programs. In previous studies, HAV seropositivity was associated with low socioeconomic status. Here, all the inhabitants had low incomes and lived under similar conditions; thus, we could not analyze the socioeconomic condition as a variable.
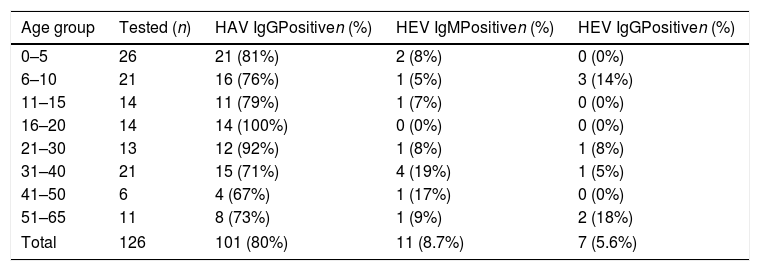
Age-specific prevalence of HEV and HAV antibodies as determined by ELISA in an Amerindian community in North Argentina.
| Age group | Tested (n) | HAV IgGPositiven (%) | HEV IgMPositiven (%) | HEV IgGPositiven (%) |
|---|---|---|---|---|
| 0–5 | 26 | 21 (81%) | 2 (8%) | 0 (0%) |
| 6–10 | 21 | 16 (76%) | 1 (5%) | 3 (14%) |
| 11–15 | 14 | 11 (79%) | 1 (7%) | 0 (0%) |
| 16–20 | 14 | 14 (100%) | 0 (0%) | 0 (0%) |
| 21–30 | 13 | 12 (92%) | 1 (8%) | 1 (8%) |
| 31–40 | 21 | 15 (71%) | 4 (19%) | 1 (5%) |
| 41–50 | 6 | 4 (67%) | 1 (17%) | 0 (0%) |
| 51–65 | 11 | 8 (73%) | 1 (9%) | 2 (18%) |
| Total | 126 | 101 (80%) | 11 (8.7%) | 7 (5.6%) |
The mean age of HEV-positive individuals in this community was 28 years old. When we grouped the subjects into three categories according to age, i.e. 0–20, 21–40 and older than 41, to evaluate if there was an increase in seroprevalence related to age, we found seroprevalences of 5.3%,14.7% and 11.7%, respectively. Despite not finding significant differences among the groups (p>0.05, Chi-square test), the highest seroprevalence was found in the group of adults (21–40-year old), which is similar to other epidemiological studies on hepatitis E in different regions of the globe, where young adults are the main affected group1. According to the Fisher's exact test, there were no significant differences in HEV seropositivity related to gender, occupation, education level, water source or consumption of pork meat (Table 2). Interestingly, there was a positive association of serum that tested positive for HEV immunoglobulins and people who farmed pigs at home (p<0.036). It is noteworthy that people farming porcine have free running animals in their backyards and live close to the riverbanks. In some cases, the physical separation from the porcine livestock is precarious or inexistent.
None of the subjects had clinical signs of hepatitis; therefore, the presence of anti-HEV IgG and anti-HEV IgM antibodies was used as an epidemiological tool to measure previous exposure to the virus and acute hepatitis, respectively.
Only four volunteers had ALT values out of the normal range (>40IU/ml), three of them tested positive for anti-HAV (IgG) and one of them was also positive for anti-HBc (data not shown).
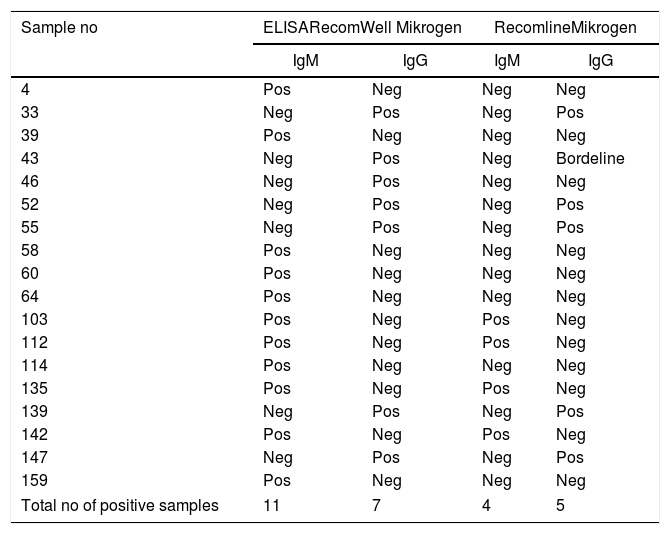
Overall, 11 sera tested positive for anti-HEV antibodies of the IgM type, whereas 7 were positive for anti-HEV class IgG according to ELISA testing (RecomWell). Nevertheless, when these sera were retested using an HEV immunoblot assay (RecomLine), only 4 sera were confirmed positive for anti-HEV IgM and five for anti-HEV IgG (Table 4). None of the samples was positive for HEV-RNA.
Comparison of two different assay types for detection of anti-HEV antibodies.
| Sample no | ELISARecomWell Mikrogen | RecomlineMikrogen | ||
|---|---|---|---|---|
| IgM | IgG | IgM | IgG | |
| 4 | Pos | Neg | Neg | Neg |
| 33 | Neg | Pos | Neg | Pos |
| 39 | Pos | Neg | Neg | Neg |
| 43 | Neg | Pos | Neg | Bordeline |
| 46 | Neg | Pos | Neg | Neg |
| 52 | Neg | Pos | Neg | Pos |
| 55 | Neg | Pos | Neg | Pos |
| 58 | Pos | Neg | Neg | Neg |
| 60 | Pos | Neg | Neg | Neg |
| 64 | Pos | Neg | Neg | Neg |
| 103 | Pos | Neg | Pos | Neg |
| 112 | Pos | Neg | Pos | Neg |
| 114 | Pos | Neg | Neg | Neg |
| 135 | Pos | Neg | Pos | Neg |
| 139 | Neg | Pos | Neg | Pos |
| 142 | Pos | Neg | Pos | Neg |
| 147 | Neg | Pos | Neg | Pos |
| 159 | Pos | Neg | Neg | Neg |
| Total no of positive samples | 11 | 7 | 4 | 5 |
In this study, we investigated the presence of specific HAV and HEV antibodies in a close community of Jujuy, Argentina, to evaluate if seroprevalence patterns differed from those in the rest of the region. The people of this community live in clay houses without running water or sewage. They keep free running animals in their yards (chicken, dogs, pigs, and cats). Although the size of the studied population is small, these data give information about HAV and HEV vulnerability of the “Guaraní” ethnic population, such as those of other communities living under similar conditions in northwestern Argentina.
Early exposure to HAV is reflected by the high percentage of children under 5 years of age who tested positive for HAV IgG (81%). There were no differences between female and male exposure. The seroprevalence of HAV observed in this study, within a homogeneous population (sharing low income and low educational status) was close to a previous report for central Argentina, where low income populations showed a total HAV seroprevalence of 81.9% whereas the middle/high-income population presented a much lower (66.2%) seroprevalence23. Furthermore, this report showed that in low income groups the seroprevalence did not increase with age in contrast to our study57.
Hepatitis A infection is mainly transmitted by contaminated water, food and contact with infected individuals. It should be mentioned that the sanitary conditions of this community were precarious and that many people did not have access to tap water. In areas of high endemicity, where individuals are infected early in life, the severity of the disease as well as the outbreak risk are low. Until 2005, prior to compulsory HAV vaccination, Argentina was considered, in general, a country with moderate endemicity, but including specific regions with high endemicity (such as Tucumán province with 81.4% in contrast to Buenos Aires with 29.4% in 1997)14,15. After introducing the HAV one-dose schedule vaccine to the national immunization calendar, the infection rates declined considerably (80-fold) from 113.3 in 100000 inhabitants to 1.4 in 100000 inhabitants in 2011. Although HAV cases were expected in non-vaccinated groups, the overall high HAV seroprevalence found in this isolated community that did not receive the HAV vaccine, resembles the high endemicity values found prior to the introduction of vaccination in other northern areas of the country (such as Tucumán). This finding reinforces the need of continuing with HAV compulsory vaccination and epidemiological surveillance in all regions of Argentina.
Based on the data reviewed in Table 1, there is evidence of moderate HEV circulation in South America with seroprevalences ranging from 1.8 to 9.8%. A recent report in South Brazil suggests that HEV may be endemic in that region with a seroprevalence of 40.25%38. Our group conducted a recent seroprevalence study in blood donors in Tucumán, which is a province also located in northern Argentina, using an in-house ELISA, and found a seroprevalence of 9.23% (95% confidence interval, 7.33–11.43%)2. In this study, we found an IgG seroprevalence of 5.6% and of 8.7% for IgM in the close Guarani community with data obtained with a commercial ELISA. Jaundice outbreaks have not been reported in the Yungas region, and there are no registers of jaundice in pregnant women of this community. HEV-3, which is the only autochthonous genotype detected in Argentina so far (Table 1), does not produce outbreaks, it usually has a subclinical course, and a zoonotic transmission path.
We confirmed the presence of anti-HEV IgM antibodies by immunoblot in the samples of four out of eleven subjects, who did not have any signs or symptoms of acute hepatitis or elevated transaminases. Overall, the results obtained with ELISA and the immunoblot assay correlate well, considering that ELISA has a higher sensitivity whereas immunoblot is more specific. The presence of specific IgM anti-HEV antibodies without IgG may be due to the timing of sampling as described by others54 or to tests lacking specificity for populations with a different infection background than the Europeans, considering that the tests were developed in Germany. The absence of detectable RNA may be related to the timing of sampling as well as to the difficulties in sample conservation due to the location of the community. These HEV specific IgM positive samples in the absence of signs and symptoms and viral RNA, pose a diagnostic challenge and highlight the need to continue improving specificity and sensitivity of the available diagnostic methods. In a disease context, it would have been possible to draw a second sample to clear up borderline cases.
Vitral et al.54 suggested that HEV-specific IgM antibodies confirmed by immunoblotting in a setting of healthy individuals give a hint about HEV circulation in the actual population. HEV-3 transmission is zoonotic but it is also related to poor sanitary conditions. In this study, the whole community lived under poor sanitary conditions and although the source of HEV infection was not identified, the only environmental factor that was significantly associated to anti-HEV IgG seropositivity was swine farming. Thus, the mode of transmission might be zoonotic by the contact with pigs although environmental studies including testing in river waters and in swine should be conducted to confirm this finding. These results agree with similar findings in rural communities of Portugal50.
The epidemiological patterns of infection of two major hepatotropic viruses (HAV and HEV) found in this population were different in terms of frequency, age of infection, and infection routes. HAV triggers a lifelong immunity after infection reflected in the presence of lifelong anti-HAV IgG antibodies. For HEV, specific IgG have been detected for at least 12 years but secondary infections have been documented suggesting that antibody titers decrease with time6. Therefore, HEV incidence may even be higher as assumed by seroprevalence data.
The increasing awareness of HEV as an etiologic agent of chronic hepatitis especially in immunocompromised individuals (AIDS, cancer, transplant patients or organ transplant, among others) as well as extrahepatic disorders (such as neurological or hematological conditions) caused predominantly by strains of zoonotic origin evidences that HEV is an undervalued public concern16,24. In South America, there were two case reports of chronic hepatitis E in solid organ transplant recipients40.
HEV is circulating in Argentina, even in populations far from large urban centers. It is about time to include HEV in the diagnostic algorithm of acute and chronic hepatitis, as well as in cases of unexplained high transaminases.
Conflict of interestThe authors declare that there are no conflicts of interest.
We thank Mr. Felix Jozsa for his manuscript proofreading service and Ms. Sevinc Akin for her excellent technical support. We acknowledge the financial support from competitive grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and Fondo para la Investigación Científica y Tecnológica (FONCyT) (PICT2012-2169 and PICT Start Up 2017-4652). MGVP and LA are members of CONICET.