Leptospirosis is a neglected zoonosis that is widely distributed in the world. Although it is endemic in Argentina, prevalence remains unknown. The aims of the study were: (i) to determine the prevalence of leptospirosis in humans from a rural community in Tandil Argentina, (ii) to identify infecting Leptospira spp. serogroups, (iii) to identify factors associated with the infection, (iv) to estimate the population attributable fraction (PAF) of the risk factors and (v) to determine the spatial patterns of disease presentation and related risk factors. Blood samples from 202 participants were collected. A survey was conducted to obtain clinical and epidemiological data. Serological testing was performed by the microscopic agglutination test (MAT). Univariate and multivariate methods were applied to evaluate associations. Spatial clusters were investigated for seroprevalence and risk factors. Antibodies were found in 32.2% of participants (95% CI: 25.8–39.1). The most prevalent serogroup was Hebdomadis followed by Sejroe; Icterohaemorrhagiae; Tarassovi and Canicola. Living at lower altitudes (OR: 13.04; 95% CI: 2.60–65.32); not having access to water supply network (OR: 2.95; 95% CI: 1.30–6.69); living close to flooded streets (OR: 2.94; 95% CI: 1.14–7.69) and practicing water sports (OR: 3.12; 95% CI: 1.12–8.33) were associated with seropositivity. Factors related with housing characteristics, services and infrastructure had the higher PAF (from 17% to 81%). A spatial cluster with higher rates of positivity and of the main risk factors was determined. This work contributes useful data for specific preventive measures that should be implemented for the control of the disease.
La leptospirosis es una enfermedad desatendida, ampliamente distribuida a nivel mundial. Aunque es endémica en Argentina, su prevalencia es desconocida. Los objetivos de este estudio fueron los siguientes: (i) determinar la prevalencia de leptospirosis humana en comunidades rurales del partido de Tandil (Argentina), (ii) identificar serogrupos infectantes de Leptospira spp., (iii) identificar factores de riesgo asociados, (iv) estimar la fracción atribuible poblacional (FAP) de los factores de riesgo y (v) determinar los patrones espaciales de la enfermedad y de los factores de riesgo. Se tomaron muestras de sangre a 202 personas, y se registró información clínica y epidemiológica. El diagnóstico se realizó por microaglutinación (MAT). Para evaluar asociaciones, se utilizaron métodos univariados y multivariados. Se estudiaron clusters espaciales de la seroprevalencia y de los factores de riesgo. El 32,2% de los participantes (IC 95%: 25,8-39,1) presentaron anticuerpos. Los serogrupos más prevalentes fueron Hebdomadis, Sejroe, Icterohaemorrhagiae, Tarassovi y Canicola. Vivir a menores altitudes (OR: 13,04; IC 95%: 2,60-65,32) y cerca de calles inundables (OR: 2,94; IC 95%: 1,14-7,69), la falta de acceso a agua de red (OR: 2,95; IC 95%: 1,30-6,69) y la práctica de deportes acuáticos (OR: 3,12; IC 95%: 1,12-8,33) estuvieron asociados con la seropositividad. Factores relacionados con las características de las viviendas, los servicios y la infraestructura tuvieron mayor proporción de FAP (17 al 81%). Se encontró un área de mayor riesgo de presentación de individuos seropositivos y de los principales factores de riesgo. Este trabajo provee información útil para generar medidas preventivas específicas que podrían ser aplicadas para controlar esta enfermedad.
Leptospirosis is a globally important zoonotic disease that affects humans and animals20,22,30. In humans, its clinical manifestations include from subclinical infection, self-limited anicteric febrile illness with or without meningitis to pulmonary hemorrhage syndrome and a severe and potentially fatal illness known as Weil's syndrome, characterized by hemorrhage, renal failure, and jaundice, associated with high mortality7,12,20,30,31. Due to its nonspecific symptoms that mimic better-known diseases, leptospirosis has been frequently under-diagnosed and underreported25,54, especially within rural populations where access to health facilities and adequate diagnostic tests are limited and awareness of the disease is low. This situation in addition to scarce studies focused in rural communities particularly in developing countries, contributes to the neglected disease status12. According to Schneider et al.42 the risk for contracting leptospirosis is eight times higher in rural populations compared to urban populations due to inadequate sewage disposal and water treatment, environmental factors and agriculture practices12.
Leptospirosis is caused by spirochetes belonging to the genus Leptospira which is currently divided into 64 species and more than 300 serovars. Species are classified into 2 clades: “Saprophytes” containing species isolated from the natural environment and not responsible for infections and “Pathogens” containing all the species responsible for infections in humans and/or animals, plus environmental species for which the virulence status has not been proven. The two clades are further subdivided into two subclades each. Clades P and S and subclade P1 (formerly described as the pathogen group), P2 (formerly described as the intermediate group), S1 and S2 (formerly described as the saprophyte group)19,48,52.
In humans, the development of severe outcomes likely depends on three factors: epidemiological conditions, host susceptibility, and pathogen virulence20. Although humans are not reservoirs for any leptospiral serovar, they could present incidental infections30. Each serogroup is generally adapted to one or more animal hosts; dogs, for example, are common reservoir hosts for serogroup Canicola, pigs for Bratislava, Pomona and Tarassovi, cattle for Hardjo, Pomona and Grippotyphosa, and certain rodent species for serogroup Icterohaemorrhagiae, Ballum and Copenhageni54. Leptospirosis can have a major economic impact on livestock industries. The current knowledge base is heavily biased toward the developed agricultural economies. The disease situation in the developing economies presents a major challenge as humans and animals frequently live in close association1. In rural contexts, a variety of Leptospira serovars are maintained and circulated from a number of mammals, including domestic animals like pigs, cattle, dogs, sheep, and goats. In contrast, in typical urban and peri-urban leptospirosis, rodents (mainly Rattus norvegicus) carrying serogroup Icterohaemorrhagiae are the highly dominant reservoir18. Commonly, animals that are natural hosts for certain serogroups show no or very limited clinical signs. However, incidental animal hosts infected with different serogroups can lead to severe disease. Human infections occur through direct contact with urine, fluids or tissues from infected animals such as rodents, livestock, and domesticated pets, and exposure to contaminated objects or the environment through soil or water20,31,54. This disease is indeed a paradigm for the One Health concept as it is a zoonosis but it is also present in the environment particularly in water. Leptospires can be maintained in wet environments for weeks30,37. Some occupational activities related to handling animal tissues: sewage workers, butchers, agricultural manual laborers, veterinarians, or hunters have an increased risk of infection2,18,35. Other risk factors include contact with water or mud during work or recreational activities, mainly if the water is contaminated with animal excreta, including rodent urine20. The risk of infection increases in the presence of skin cuts or abrasions since leptospires rapidly invade the bloodstream after penetrating the skin or mucous membranes30,41. Natural disasters associated with flooding have been related to an increase in the occurrence of leptospirosis in humans40,51.
The microscopic agglutination test (MAT) is considered the gold standard test to confirm cases of Leptospirosis through the detection of serovar-specific antibodies representing different serogroups. This test provides information about the presumptive infecting serogroups circulating in species at the population level9,54.
In Argentina, leptospirosis was first recognized and reported in humans in 19154. However, there is limited data on the current disease burden of leptospirosis in the country. Furthermore, the factors influencing transmission of leptospirosis are not well understood.11 Although in Argentina, leptospirosis is a mandatory reportable disease, the information about incidence and prevalence of leptospirosis is scarce particularly in rural communities from the region. Poor availability of diagnostic laboratory infrastructure contributes to misdiagnosis12,50.
The use of the geographic information system (GIS) in determining the pattern and distribution of communicable diseases allows the development of spatial planning policies in the field of land use, sewage, rainwater or waste management. Once the local epidemiology and transmission risks are identified, it is possible to greatly mitigate risk by taking steps to reduce exposure and implement protective measures20,37.
The purpose of this study was: (i) to determine the prevalence of leptospirosis in humans from a rural community located in Tandil (Argentina), (ii) to identify the presumptive infecting Leptospira spp. serogroups, (iii) to identify factors associated with the infection, (iv) to estimate the population attributable fraction (PAF) of the risk factors and (v) to determine spatial patterns of the presentation of the disease and related risk factors.
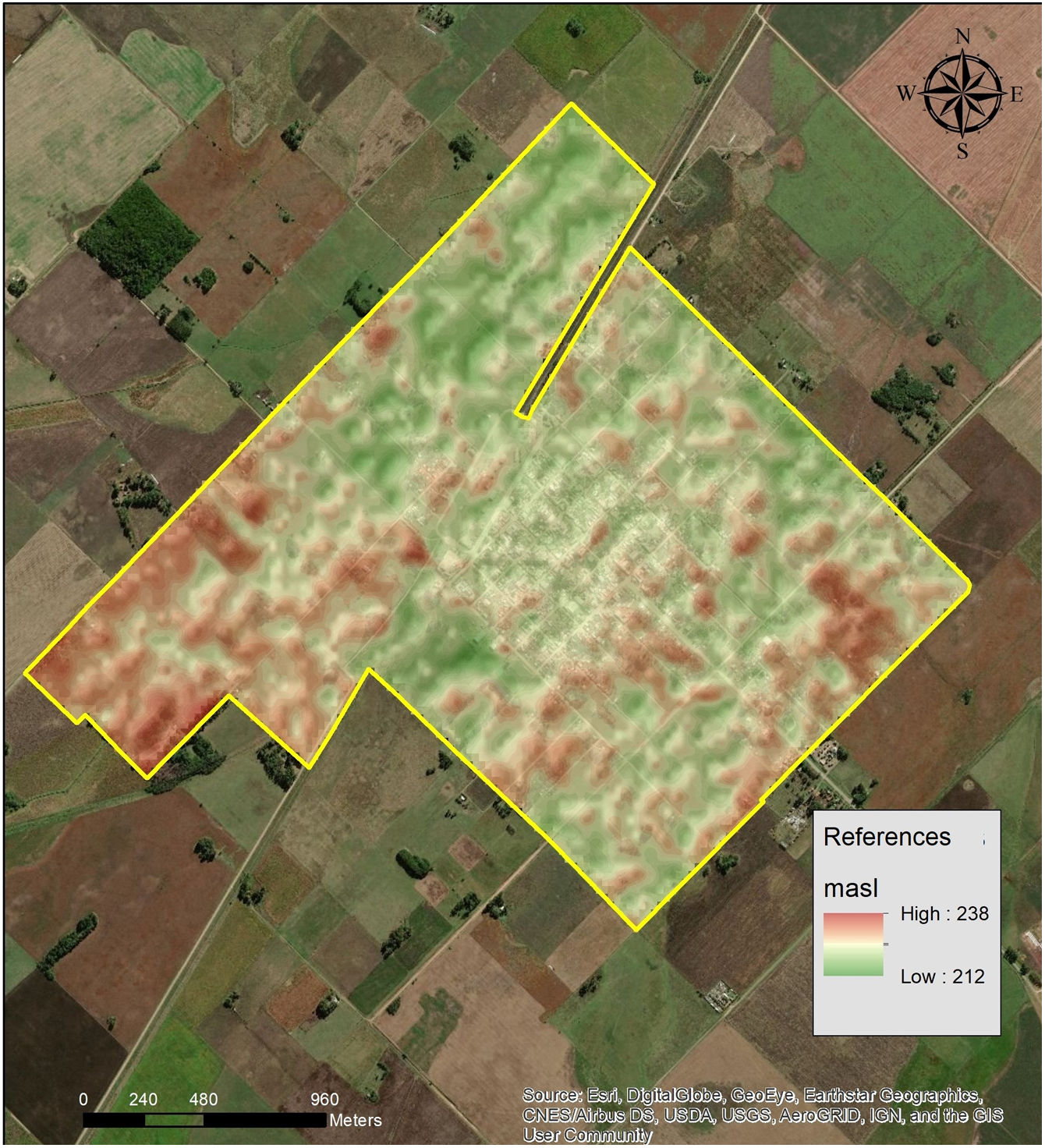
Materials and methodsStudy area and populationThe present cross-sectional study was carried out in María Ignacia Vela (coordinates 37°24′5.386″S; 59°30′21.391″W). The study area covers 5.6km2. This is a rural community located approximately 360km from Buenos Aires city in Tandil. It is the second largest town in the county, with a total population of 1948 inhabitants23. María Ignacia Vela is situated in central southeast of Buenos Aires Province. It has fertile lowlands formed by the Tandilia hill system, interrupted and surrounded by a sedimentary plain of fluvial origin. The region has oceanic temperate and humid climate. The average annual rainfall is around 800mm and the average annual temperature is 13.3°C. The middle altitude is 222 meters above sea level (masl). The whole altitude range in the study area is shown in Fig. 1. The rural area of María Ignacia Vela possesses a production system with mixed agricultural – livestock activities, based on a backyard breeding system with production of meat, milk and other commercial and farm activities for subsistence33.
In the current study, a random spatial sampling method was used to define the population of interest through the construction of a spatial sample frame using parcel-level data obtained from a cadastral database, determining households from Maria Ignacia Vela as sample units, ensuring a representative sample choosing one inhabitant for each household selected. The modeling and calculation method was based on the confidence level, margin of error and acceptance level previously described by McGrew32 and was carried out by using Data Reviewer Extension in ArcGis 10.1 software16 assuming the following parameters: a probability of the outcome of 0.5, a confidence level of 95%, a margin of error of 6% and 1% of failure threshold for 1655 total parcels. The minimum sample estimated was 189 households.
Participants were briefed regarding the nature of the study before the sample collection. After written individual or parental consent (for those aged<18 years), a venous blood sample (10ml) was collected from all the volunteers who accepted to participate in the study. Immunosuppressed patients and those previously treated with antibiotics were excluded.
Data collectionAfter the blood samples were collected, the participants were interviewed by a trained interviewer using a semi-structured questionnaire to obtain information on clinical and epidemiological data. Information from these questionnaires included: sociodemographic, housing characteristics, habits, education, occupation, animal and environmental exposure. Moreover, information was obtained regarding the presence of clinical signs and symptoms related to leptospirosis during the 30 days prior to the survey. Data about knowledge of leptospirosis disease and prevention measures was also collected. All the participants’ residences were geo-referenced using Global Positioning Systems (GPS).
Sample analysisAll blood samples were allowed to clot for 30min at room temperature. The clotted blood samples were centrifuged at 3500×g for 10minutes at 25°C to separate the serum, and then stored at −20°C for later use. Serum samples were shipped to the Leptospirosis Laboratory, Department of Rural Zoonosis (Ministry of Health of Buenos Aires Province) for the detection of Leptospira spp. using the MAT.
The MAT was performed in accordance with the Subcommittee on the Taxonomy of Leptospira spp.24 using a panel of live antigen suspensions of locally circulating reference strains of L. interrogans serogroup Canicola serovar Canicola strain H. Utrecht IV, serogroup Hebdomadis serovar Hebdomadis strain Hebdomadis, serogroup Icterohaemorrhagiae serovar Copenhageni strain M20, serogroup Pomona serovar Pomona strain Pomona, serogroup Pyrogenes serovar Pyrogenes strain Salinem, serogroup Sejroe serovar Wolfii strain 3705 and serogroup Sejroe serovar Hardjo strain Hardjo prajitno; L. borgpetersenii serogroup Ballum serovar Castellonis strain Castellon 3 and serogroup Tarassovi serovar Tarassovi strain Perepelitsin and L. kirschneri serogroup Grippotyphosa serovar Grippotyphosa strain Castellon 3, developed at 28–30°C in Ellinghausen–McCullough–Johnson–Harris (EMJH) medium and with no more than 15 days of growth. Serial serum dilutions were performed with phosphate-buffered saline (PBS, pH 7.2) starting from 1:50 dilution. The plates were incubated at 37°C for 90min. After incubation, the serum–antigen mixtures were checked for agglutination under dark field microscope. Tests were interpreted as positive when agglutination at ≥1:50 of at least 50% leptospires for any serogroup was observed. The highest serum dilution exhibiting greater than 50% agglutination or less than or equal to 50% free leptospires–as compared to the negative control – was considered the endpoint titer of quantitative MAT8,20,54.
Data entry and statistical analysisIndividual information and laboratory results were entered into an Epi-info 3.5.4 database13. Continuous variables were described using means and Standard Errors (SEs) while categorical variables were expressed as percentages (%). The variables such as age of participants, levels of altitude of the domiciles or distance of the population under study to landfills, were analyzed as categorical (four categories were created) and as quantitative. Prevalence of anti-Leptospira spp. antibodies and 95% Confidence Interval (CI) were estimated.
The association between outcome (positivity) and the variables under analysis was assessed by a univariate analysis using a chi-squared test. If the expected value of one or more cells was less than 5, Fisher's exact test was used. For quantitative variables two-sided Student's t Test was used. Odds ratios (OR) and 95% CI were also estimated and calculated for each variable. The null hypothesis was that there were no differences between groups. All the statistical tests were carried out at α=0.05. Factors having significant p<0.05 were selected and included in a multivariate logistic regression model. The estimation method was maximum likelihood with a convergence criterion of 0.01 for a maximum of 10 interactions. The significance level was p<0.05. The strength of association between each co-variable and seroprevalence was calculated and expressed as an estimated value by the adjusted OR and their respective 95% CI. Sstatistical analyses were performed in the InfoStat programme14.
The PAF and their respective 95% CI were calculated for all risk factors selected after the logistic regression analysis. The population attributable fraction (PAF) denotes the proportion of cases that could be prevented if exposure could be hypothetically totally removed from the population.
In the spatial analysis, potential clusters were investigated in the study area with space scan statistics using SaTScan software, v.9.3. Bernoulli model was applied for calculating local rates inside, where positive cases are designated as ones and negative cases are designated by zeros. Under the null-hypothesis, the expected number of cases at each location was proportional to the population size or population-time at-risk at that location. The scan procedure was used to detect clusters with high rates of MAT positive cases or high rates of the risk factor presence. The likelihood function was maximized over all windows, identifying the window that constituted the most-likely cluster, the cluster that was least likely to have occurred by chance. The likelihood ratio for this window was the maximum likelihood ratio test statistic. Its distribution under the null-hypothesis and its corresponding p-value was obtained by repeating the same analytic exercise on a large number of randomly selected replications of the data set generated under the null hypothesis. The underlying distribution was obtained by running 999 Monte Carlo simulations based on the random labeling hypothesis. The level of significance for all analyses was set at <0.05. The window sized up to 50% of the population at risk29,6,53.
Ethical considerationsThe project was reviewed and approved by the Ethics Committee of the National Institute of Epidemiology “Dr. Juan H. Jara,” Mar del Plata, Argentina. Prior to enrolment, the researchers read an information sheet to the participants describing the study, answered any questions and asked for written consent to participate. The participants received no compensation for their participation and were free to withdraw from the study at any time. Anonymity was ensured through the use of an identification code.
ResultsStudy populationA total of 202 participants were included in the study, 134 of whom were females (66.3%). The age of the individuals ranged from 5 to 91 years, with a mean age of 44.27 years (SE=1.31 years).
Seroprevalence, MAT titer and serogroup distributionThe overall seropositivity of anti-Leptospira spp. antibodies was 32.2% (95% CI: 25.8–39.1%) present in 65 participants. Hebdomadis was the most prevalent serogroup (24.8%, 95% CI: 19–31.3), followed by Sejroe (5.4%, 95% CI: 2.7–9.5), Icterohaemorrhagiae (3.5, 95% CI: 1.4–7), Tarassovi (3%, 95% CI: 1.1–6.4) and Canicola (0.5%, 95% CI: 0–2.7). The agglutinating antibody titers ranged from 1:50 to 1:200. Nine cases of cross-reactions occurred: six in relation to serogroups Hebdomadis/Hardjo, one Hebdomadis/Tarassovi, one Icterohaemorrhagiae/Tarassovi and one Canicola/Icterohaemorrhagiae/Tarassovi.
Univariate analysisSocio-demographic characteristics, housing conditions, occupational, animal and environmental exposures and other qualitative data analyzed are listed in Tables 1 and 2. In Table 3 the assessment of quantitative variables such as age of participants, levels of altitude of households in the study area and distance of the population to landfills are shown. With regard to the housing conditions, the material of the walls was associated with the infection (p=0.00037), being at higher risk of infection than the ones made of cement. Furthermore, having a water supply network was found to be a protective factor (p=0.0007) (OR: 0.35; 95% CI: 0.19–0.66). People living in flooded streets and at lower altitudes were more likely to be seropositive. Univariate analysis also revealed that water sports practice was a risk factor for the infection (OR: 2.82; 95% CI: 1.23–6.48). Inhabitants in contact with fowls or horses were at higher risk of infection (p=0.008; OR: 2.6; 95% CI: 1.26–5.34) and (p=0.024; OR: 2.68; 95% CI: 1.34–5.36), respectively. Considering the signs and symptoms presented by participants during the 30 days prior to the survey, none of them were statistically associated with the infection; however, the individuals who presented with cough and intense headache, exhibited higher seropositivity compared to the ones that did not have these symptoms.
Socio-demographic characteristics and occupations of respondents from Maria Ignacia Vela. Frequencies and association with Leptospira spp. infection.
| Variables | Frequency n (%) | Frequency seropositivity (%) | p value | OR | 95% CI |
|---|---|---|---|---|---|
| Gender | 0.08 | ||||
| Female | 134 (66.3) | 44/134 (32.8) | 1.09 | 0.58–2.05 | |
| Male | 68 (33.7) | 21/68 (30.9) | 0.91 | 0.49–1.72 | |
| Age | 0.28 | ||||
| 5–31 years | 52 (25.7) | 18 (34.6) | 1.91 | 0.77–4.71 | |
| 32–42 years | 50 (24.8) | 20 (40) | 2.40 | 0.98–5.91 | |
| 43–58 years | 54 (26.7) | 17 (31.5) | 1.65 | 0.67–4.09 | |
| 59–91 years | 46 (22.8) | 10 (21.7) | 1 | – | |
| Breadwinner educational level | 0.20 | ||||
| Primary incomplete | 47 (26.1) | 11 (23.4) | 0.53 | 0.24–1.19 | |
| Primary complete/Secondary incomplete | 85 (47.2) | 31 (36.5) | 1 | – | |
| Secondary complete or higher | 48 (26.7) | 19 (39.6) | 1.14 | 0.55–2.36 | |
| Occupation or exposure activities | |||||
| Farm work | 0.42 | ||||
| Yes | 76 (47.5) | 30 (39.5) | 1.3 | 0.7–2.5 | |
| No | 84 (52.5) | 28 (33.3) | 0.77 | 0.4–1.46 | |
| Veterinarian activities | 0.25 | ||||
| Yes | 28 (15.1) | 12 (42.9) | 1.6 | 0.7–3.6 | |
| No | 157 (84.9) | 50 (31.8) | 0.62 | 0.28–1.4 | |
| Hunter | 0.53a | ||||
| Yes | 11 (7.4) | 5 (45.5) | 1.46 | 0.43–5.05 | |
| No | 138 (92.6) | 50 (36.2) | 0.68 | 0.21–2.23 | |
| Healthcare professional | 0.72a | ||||
| Yes | 9 (5.9) | 4 (44.4) | 1.48 | 0.38–5.79 | |
| No | 143 (94.1) | 50 (35) | 0.67 | 0.18–2.45 | |
| Hospital ward maid | 0.04a | ||||
| Yes | 5 (2.9) | 4 (80) | 8.6 | 0.94–78.8 | |
| No | 167 (97.1) | 53 (31.7) | 0.12 | 0.02–0.76 | |
| Slaughterer | 0.25a | ||||
| Yes | 3 (1.7) | 2 (66.7) | 4.17 | 0.37–47.01 | |
| No | 176 (98.3) | 57 (32.4) | 0.24 | 0.03–1.86 | |
Household characteristics, environmental exposure and contact with animals of respondents. Frequencies and association with Leptospira spp. infection.
| Variables | Frequency (%) | Frequency of seropositivity (%) | p value | OR | 95% CI |
|---|---|---|---|---|---|
| Housing conditions | |||||
| Roof material | 0.73a | ||||
| Metal sheet | 159 (87.8) | 56 (35.2) | 1.63 | 0.17–16.05 | |
| Concrete slab | 12 (6.6) | 5 (41.7) | 2.14 | 0.17–27.10 | |
| Tile | 6 (3.3) | 1 (16.7) | 0.6 | 0.03–13.58 | |
| Wood | 4 (2.2) | 1 (25) | 1 | – | |
| Floor material | 0.58a | ||||
| Tiles | 122 (67.8) | 38 (31.1) | 0.54 | 0.15–1.89 | |
| Cement | 40 (22.2) | 15 (40.5) | 0.80 | 0.21–3.18 | |
| Wood | 11 (6.1) | 5 (45.5) | 1 | – | |
| Ground | 7 (3.9) | 3 (42.9) | 0.90 | 0.13–6.11 | |
| Wall material | 0.00037a | ||||
| Bricks | 115 (63.5) | 33 (28.7) | 1 | – | |
| Cement | 61 (33.7) | 28 (45.9) | 2.11 | 1.11–4.02 | |
| Wood | 2 (1.1) | 2 (100) | – | – | |
| Mud | 2 (1.1) | 0 (0) | – | – | |
| Metal Sheet | 1 (0.6) | 0 (0) | – | – | |
| Water supply network | 0.0007 | ||||
| Yes | 120 (61.5) | 28 (23.3) | 0.35 | 0.19–0.66 | |
| No | 75 (38.5) | 35 (46.7) | 2.88 | 1.55–5.32 | |
| Human excreta disposal | 0.55a | ||||
| Septic tank | 196 (99) | 64 (32.7) | 0.48 | 0.03–7.88 | |
| Latrine | 2 (1) | 1 (50) | 2.06 | 1.13–33.51 | |
| Electricity supply network | 0.17a | ||||
| Yes | 191 (97.4) | 65 (34) | – | – | |
| No | 5 (2.6) | 0 (0) | – | – | |
| Gas network | 0.12 | ||||
| Yes | 113 (57.9) | 32 (28.3) | 0.62 | 0.34–1.13 | |
| No | 82 (42.1) | 32 (39) | 1.62 | 0.89–2.95 | |
| Household exposure | |||||
| Near wastelands | 0.24 | ||||
| Yes | 134 (73.2) | 48 (35.8) | 1.56 | 0.75–3.19 | |
| No | 49 (26.8) | 13 (26.5) | 0.65 | 0.32–1.32 | |
| Near corrals | 0.51 | ||||
| Yes | 128 (69.9) | 46 (35.9) | 1.25 | 0.64–2.46 | |
| No | 55 (30.1) | 17 (30.9) | 0.8 | 0.41–1.56 | |
| Peridomestic rodents | 0.39 | ||||
| Yes | 122 (69.7) | 45 (36.9) | 1.35 | 0.68–2.7 | |
| No | 53 (30.3) | 16 (30.2) | 0.74 | 0.37–1.47 | |
| Near natural water courses | 0.08 | ||||
| Yes | 81 (44.3) | 33 (40.7) | 1.73 | 0.93–3.21 | |
| No | 102 (55.7) | 29 (28.4) | 0.58 | 0.31–1.07 | |
| Near livestock productions | 0.047 | ||||
| Yes | 48 (27.9) | 22 (45.8) | 1.99 | 1.002–3.95 | |
| No | 124 (72.1) | 37 (29.8) | 0.5 | 0.25–1.99 | |
| Near flooded streets | 0.0006 | ||||
| Yes | 34 (19.2) | 20 (58.8) | 3.68 | 1.71–7.98 | |
| No | 143 (80.8) | 40 (28) | 0.27 | 0.13–0.58 | |
| Distance of household to landfill (m) | 0.11 | ||||
| (A) 414.27–1128.05 | 50 (25.5) | 23 (46) | 2.29 | 0.98–5.34 | |
| (B) 1128.88–1374.17 | 48 (24.5) | 12 (25) | 0.90 | 0.36–2.23 | |
| (C) 1375.28–1645.35 | 50 (25.5) | 17 (34) | 1.39 | 0.58–3.29 | |
| (D) 1649.25–2518.96 | 48 (24.5) | 13 (27.1) | 1 | – | |
| Altitude of household (masl) | 0.0001 | ||||
| (A) 218.59–221.71 | 51 (26) | 23 (45.1) | 12.32 | 3.38–44.87 | |
| (B) 221.78–222.59 | 48 (24.5) | 22 (45.8) | 12.69 | 3.46–46.54 | |
| (C) 222.60–223.53 | 49 (25) | 17 (34.17) | 7.97 | 2.15–29.49 | |
| (D) 223.57–225.35 | 48 (24.5) | 3 (6.3) | 1 | – | |
| Environmental exposure | |||||
| Water sports practice | 0.012 | ||||
| Yes | 28 (17.3) | 16 (57.1) | 2.82 | 1.23–6.48 | |
| No | 134 (82.7) | 43 (32.1) | 0.35 | 0.16–0.8 | |
| Occupational or recreational water exposure in the last 3 months | 0.10 | ||||
| Yes | 22 (14.4) | 12 (54.5) | 2.14 | 0.86–5.34 | |
| No | 131 (85.6) | 47 (35.9) | 0.47 | 0.19–1.14 | |
| Flooding exposure | 0.25 | ||||
| Yes | 18 (12.3) | 9 (50) | 1.78 | 0.66–4.81 | |
| No | 128 (87.7) | 46 (35.9) | 0.56 | 0.21–1.48 | |
| Contact with domestic animals | |||||
| Canine | 0.04 | ||||
| Yes | 154 (86.5) | 58 (37.7) | 3.02 | 0.98–9.28 | |
| No | 24 (13.5) | 4 (16.7) | 0.33 | 0.11–0.97 | |
| Felines | 0.14 | ||||
| Yes | 90 (53.3) | 36 (40) | 1.62 | 0.85–3.19 | |
| No | 79 (46.7) | 23 (29.1) | 0.62 | 0.33–1.17 | |
| Cattle | 0.71 | ||||
| Yes | 75 (43.6) | 25 (33.3) | 0.89 | 0.47–1.67 | |
| No | 97 (56.4) | 35 (36.1) | 1.13 | 0.6–2.12 | |
| Fowl | 0.008 | ||||
| Yes | 42 (27.3) | 21 (50) | 2.6 | 1.26–5.34 | |
| No | 126 (72.7) | 35 (27.8) | 0.38 | 0.19–0.78 | |
| Swine | 0.26 | ||||
| Yes | 46 (26.9) | 19 (41.3) | 1.5 | 0.74–3 | |
| No | 125 (73.1) | 40 (32) | 0.67 | 0.34–1.33 | |
| Horses | 0.024 | ||||
| Yes | 40 (23.4) | 20 (50) | 2.68 | 1.34–5.36 | |
| No | 131 (76.6) | 40 (30.5) | 0.44 | 0.21–0.9 | |
| Sheep | 0.28 | ||||
| Yes | 35 (21.2) | 15 (42.9) | 1.52 | 0.71–3.25 | |
| No | 130 (78.8) | 43 (33.1) | 0.66 | 0.31–1.4 | |
Quantitative variables and association with MAT positivity.
| Variable | Median and SE MAT positive | Median and SE MAT negative | p value |
|---|---|---|---|
| Age | 41.54+/−2.31 | 45.57+/−1.59 | 0.1526 |
| Distance of household to landfills (m) | 1285.98+/−42.89 | 1396.13+/−30.21 | 0.0371 |
| Altitude of household (masl) | 221.86+/−0.18 | 222.79+/−0.13 | 0.0146 |
Only 4.5% of the participants were able to describe preventive measures about leptospirosis. Although it was not statistically associated, seroprevalence was higher (40.5%) in the group that was not able to mention preventive measures.
Logistic regression analysisAfter the multivariate logistic regression analysis, four factors were found to be associated with leptospirosis seroprevalence: (i) living at lower altitudes (<223.53masl); (ii) water sports practice; (iii) not having access to drinking water supply network (piped into dwelling) and (iv) living close to flooded streets (Table 4).
Multivariate logistic regression analysis of the variables statistically associated (p<0.05) with Leptospira spp. infection.
| Parameters | Est. | S.E | OR | Wald LI (95%) | Wald LS (95%) | Wald Chi2 | p-value |
|---|---|---|---|---|---|---|---|
| Intercept | −1.05 | 0.96 | 0.35 | 0.05 | 2.30 | 1.20 | 0.2741 |
| Altitude A (218.59–221.71masl) | 2.57 | 0.82 | 13.04 | 2.60 | 65.32 | 9.75 | 0.0018 |
| Altitude B (221.78–222.59masl) | 2.23 | 0.84 | 9.30 | 1.80 | 48.03 | 7.09 | 0.0078 |
| Altitude C (222.60–223.53masl) | 2.12 | 0.85 | 8.32 | 1.59 | 43.70 | 6.27 | 0.0123 |
| Altitude D (223.57–225.35masl) | 0 | 0 | 1 | – | – | – | – |
| Water sports practice | −1.13 | 0.51 | 3.2 | 1.13 | 8.33 | 4.82 | 0.0281 |
| Water supply network (not having) | 1.08 | 0.42 | 2.95 | 1.30 | 6.69 | 6.70 | 0.0096 |
| Flooded streets near the domicile | −1.08 | 0.49 | 2.94 | 1.13 | 7.69 | 4.95 | 0.0262 |
Est: estimate, SE: standard error, OR: odds ratio.
The Population Attributable Fraction (PAF) was calculated using all the risk factors selected in the logistic regression model. Overall, the PAF was higher in altitude level of households less than 223.57masl (PAF: 0.81, 95% CI: 0.60–0.91). With regard to household conditions and structure, not having water supply network had a PAF of 0.28 (95% CI: 0.12–0.41) followed by living near flooded streets (PAF: 0.17, 95% CI: 0.07–0.27). In addition, environmental exposure such as water sports practice had a PAF of 0.12 (95% CI: 0.02–0.21).
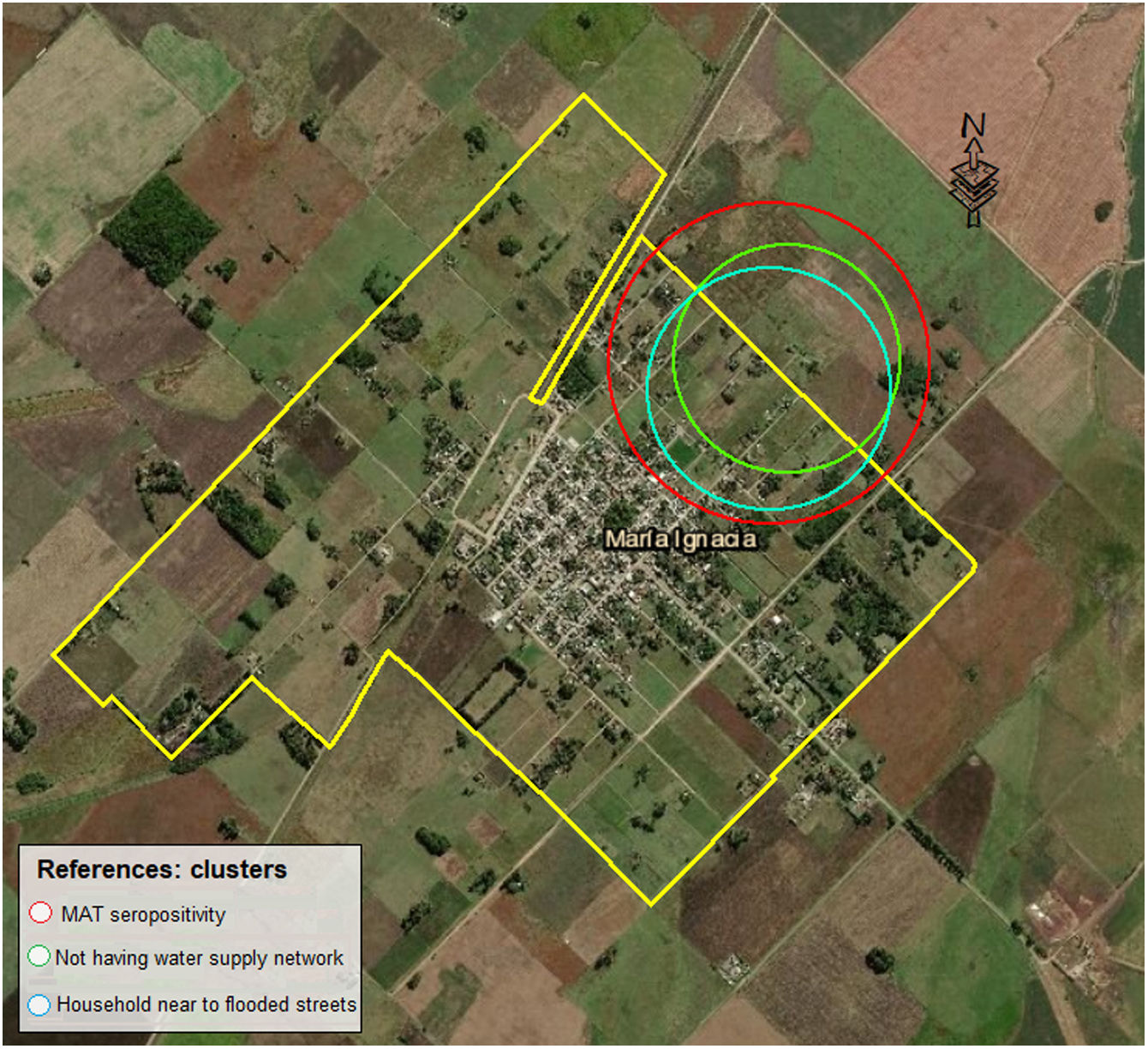
Spatial analysisA spatial cluster with high rates of MAT positive cases was detected (coordinates of centroid: 37.395733 S, 59.499346; radius: 0.67km.). This distribution indicates a trend pattern to the north–northeast in the study area. Population inside the cluster was 43 of whom 26 (60.5%) were seropositive (p value 0.014). The increased risk of disease within the cluster was 2.47. In the same area, clusters with high rates of risk factors based on “living near flooded streets” and “not having water supply network” overlapped with the MAT cluster (Fig. 2).
DiscussionThere is scarce information about the epidemiology of leptospirosis in rural communities from developing countries. It is established that environmental conditions and occupational habits of the individuals put them at risk of acquiring leptospirosis disease, which varies from community to community26,31,37.
In our study, seroprevalence of 32.2% against ten Leptospira spp. serogroups was found in inhabitants from Maria Ignacia Vela. Although, there were not reported leptospirosis cases in this community, maybe due to underdiagnosis, caused by the lack of access to health facilities, the low degree of suspicion or the lack of availability of a diagnostic test, common in rural populations12,54. Taking into account the results of the study we can affirm that leptospirosis infection is prevalent in the community and may be accompanied with mild, flu-like symptoms or a more specific symptom, but can be ignored as leptospirosis.
The results obtained in the study are similar to those reported by Sohail et al.46 in the hot and dry region from Pakistan (27.5%; 95% CI: 19.75–36.40). In rural areas in Lao PDR, a seroprevalence of 23.9% was found28. Previous studies from rural areas in developing countries estimated seroprevalences ranging from 23.9% to 37.4%28,47. In Latin America, seroprevalences were found between 6% and 67%36,43. Except for this study, there were limited studies regarding human seroprevalence of leptospirosis in the rural communities of Argentina. In a previous study performed in Olavarría (Buenos Aires province; Argentina) the overall prevalence of anti-Leptospira spp. antibodies was 7.00%, being higher in rural areas (19.66%) than in urban areas (3.64%)39.
The number of cross-reactions detected in about half of the respondents (53.2%) agrees with other studies. It reflects the possibility of co-infection with multiple serogroups in endemic areas, due to exposure to multiple serogroups or the repeated infections to different serogroups. It could be also due to the cross-reactions occurring among the serogroups used as antigens in the MAT and due to the presence of several common antigens between different leptospires17,27,30,45. The seropositivity of serogroups Hebdomadis and Icterohaemorrhagie can be attributed to the abundance of rodents in the study area (36.9% of positive individuals reported the presence of rodents near their domiciles). Further, seropositivity to serogroup Canicola, could be attributed to canine contact (37.7% of positive individuals were in contact with canines) since they are the main reservoir hosts7,38,54. The high positivity to Sejroe and Tarassovi serogroups could be attributed to the high contact rate with cattle (33.3% of positive individuals), which are widespread in the area of this study. Interactions between humans and cattle can lead to the interspecies transmission of serovar Hardjo. Moreover, cattle are incidental hosts for other serogroups, such as Tarassovi51.
Three of the serogroups detected (Hebdomadis, Icterohaemorrhagiae and Canicola) are also coincident with a previous study performed in a suburban neighborhood in Tandil city, where R. norvegicus were trapped in the banks of a stream and examined serologically by MAT. In that study, 22 of them (52.3%) reacted with Leptospira serogroups Ballum, Canicola, Grippotyphosa, Icterohaemorrhagiae and Hebdomadis44. Taking into account the results of epidemiological research, human leptospirosis infections are usually a reflection of the serogroups maintained by the animal population in the region, highlighting the need for the development of effective serovar-specific vaccines for domestic animals to prevent leptospirosis in humans15,37.
No differences were observed between Leptospira infection and gender, revealing that people living and working in this rural area would be equally exposed.
In addition, leptospirosis seropositive cases were associated with a combination of negative social and environmental conditions, as previously found by Barcellos and Sabroza5. In our study, statistical analyses such as univariate, multivariate and the PAF allowed to consider that not having drinking water supply networks (piped into dwelling) explained 28% of all the MAT positive cases. Only 61.5% of individuals studied had access to network water, which is low considering that in all the country 82.6% of people has access to network water and 78% in Buenos Aires Province. Access to water and sanitation is a human right and a key component of primary prevention to ensure better health23. Infrastructure should be invested to provide a sustainable supply of drinking water for the rural population. People who do not have access to the water supply network usually use well water as their primary drinking water source. Well water was implicated in leptospirosis cases before10 and leptospires were previously isolated from samples obtained from this kind of water source3,34. Another important factor detected was living near flooded streets, which explained 17% of all the MAT positive cases. As previously mentioned, the infective agent can survive several days in floodwaters and mud and the people may have been exposed to leptospires. Knowledge of altitude levels with the highest risk (<223.53masl) allows to develop spatial planning policies in the field of land use for urban development. From a global perspective, human leptospirosis is strongly linked to poverty wherever poor housing standards and local infrastructure result in exposure to rodent reservoirs. Housing construction that prevents rodents from invading residential living spaces greatly reduces the risks20.
Recreational activities have been identified as risk factors for leptospirosis infection15,21. In our study, the variable water sports practice was detected as a risk activity. Public swimming pools in Maria Ignacia Vela are filled with well water. Humans may contract the infection indirectly via contact with water and/or environment contaminated with urine from Leptospira spp. reservoirs, and the risk is higher if the contact is with well water, as we have mentioned before.
The spatial identification of places with higher risk of infection in Maria Ignacia Vela is coincident with the lowest areas in the same territory, as shown in Fig. 1. This finding allows to define priority places to implement preventive measures in specific areas. The data obtained in this study would be useful for making applicable decisions based on accurate and reliable information, for prevention of leptospirosis in the best interests of public health. Likewise, taking into account the small percentage of population who knows how to prevent the disease, preventive campaigns are necessary.
ConclusionThe knowledge generated from this study through the combination of epidemiological and geographical tools provides critical information and contributes useful data for adopting measures that should be implemented for the control of leptospirosis.
ImpactsThis research provides information about epidemiologic and spatial analysis of leptospirosis to be applied in the human population from rural areas.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This work was supported by the Secretary of Science, Art and Technology of the National University from the Center of the Province of Buenos Aires.
J.A. Silva is a holder of a fellowship from the Commission of Scientific Investigations from the Province of Buenos Aires.
J.A Silva is a graduate student in the Environment and Health Applied Sciences Doctoral Program (DCAAS) at UNICEN, Argentina.
We sincerely thank the staff of Integrated Public Health System from Tandil county and the Hospital “Rodriguez Larreta” from Maria Ignacia Vela.