Multiple congenital defects are traditionally corrected surgically, but nowadays can be treated percutaneously. There are few reports in the literature attesting to its efficacy and safety. We aimed to describe an experience with combined procedures to treat different congenital and structural defects, in a single therapeutic session.
MethodsSince 2007, different defects were treated in a single treatment session. All were selected by echocardiography. The procedures were performed using traditional techniques already described for each defect.
ResultsTen patients were treated, five males, aged 1-67 years, weighting 11-90 kilograms. The most prevalent isolated defect was patent ductus arteriosus (PDA, n = 5), followed by ostium secundum atrial septal defects (osASD, n = 4) and ventricular septal defects (VSD, n = 4). The most common combinations were VSD with PDA (n = 2) and VSD with osASD (n = 2). Two pulmonary valve stenosis were dilated with osASD and patent foramen ovale (PFO), and one aortic coarctation with PDA. Additionally, a left atrial appendage with PFO was occluded and an aortopulmonary fistula with PDA was embolized. All procedures were successful. The mean follow-up was 31 ± 28.1 months, with only two complications. There were no deaths.
ConclusionsThe small number of reported cases showed that the combined procedures were safe and effective and can be reproduced by experienced operators in specialized centers and may be considered as the first therapeutic option in these patients.
Defeitos congênitos múltiplos são tradicionalmente corrigidos cirurgicamente, mas, atualmente, podem ser tratados percutaneamente. Existem poucos relatos na literatura atestando sua eficácia e segurança. Objetivamos descrever uma experiência com a realização de procedimentos combinados para tratar diferentes defeitos, congênitos e estruturais, numa mesma sessão terapêutica.
MétodosDesde 2007, foram tratados, numa mesma sessão terapêutica, diferentes defeitos. Todos foram selecionados por ecocardiograma. Os procedimentos foram realizados segundo as técnicas tradicionais já descritas para cada defeito encontrado.
ResultadosForam tratados dez pacientes, cinco do sexo masculino, com idades de 1 a 67 anos, e pesos de 11 a 90kg. O defeito mais prevalente de forma isolada foi a persistência do canal arterial (PCA, n = 5), seguido da comunicação interatrial ostium secundum (CIA OS, n = 4) e da comunicação interventricular (CIV, n = 4). As combinações mais frequentes foram CIV com PCA (n = 2) e CIV com CIA OS (n = 2). Foram dilatadas duas estenoses valvares pulmonares, com CIA OS e com forame oval patente (FOP), e uma coarctação de aorta com PCA. Adicionalmente, foi ocluído um apêndice atrial esquerdo com FOP e foi embolizada uma fístula aortopulmonar com PCA. Todos os procedimentos foram bem-sucedidos. O tempo médio de seguimento foi de 31 ± 28,1 meses, havendo apenas duas complicações. Não houve nenhum óbito.
ConclusõesA pequena série de casos relatada mostrou que os procedimentos combinados foram seguros e eficazes, podendo ser reproduzidos por operadores experientes em centros especializados, podendo vir a se constituir como primeira opção terapêutica para esses pacientes.
Percutaneous intervention is currently the therapeutic modality of choice for most simple congenital heart diseases, as well as for some structural defects, with similar results and sometimes even advantage over conventional surgery.1,2
Multiple congenital defects are traditionally corrected through surgical techniques. Although the feasibility of percutaneous intervention for different defects in the same procedure has been described in some case reports, its efficacy and safety have not been consistently evaluated.3–6
In this article, the authors analyze their experience with combined procedures performed for treatment of different defects, both congenital and structural, and discuss technical aspects and indications. Finally, some comments are offered on the effectiveness and safety of the procedures performed.
MethodsThe medical records of all patients who underwent percutaneous procedures to treat various heart defects in a single therapeutic session were retrospectively analyzed. Cases of more than one device implanted for treating the same type of anatomical defect and cases in which an atrial septal defect and patent foramen ovale (PFO) were occluded during the same procedure were excluded from this analysis.
ProceduresAll procedures were performed under general anesthesia and orotracheal intubation after at least an 8 hour fasting. The patients underwent right and left heart catheterization by femoral puncture. Relevant angiograms were performed in each case.
Heparin was administered at doses of 100 IU/kg in children and of 5,000-10,000 IU in adults after a venous access was obtained and a transesophageal sheath was introduced. Antimicrobial prophylaxis with cefazolin (50mg/kg in children or 2g in adults) was routinely administered.
As a general rule, procedures regarded as more complex, time-consuming, or laborious were performed first, followed by those considered simpler or technically less demanding. In the case of association with valvar stenosis, these conditions were addressed first.
All occlusive procedures and all dilations were performed according to standard techniques, detailed elsewhere.2,7–11 Occlusive procedures were monitored by transesophageal echocardiography (TEE), in addition to fluoroscopy.
All patients were followed-up in the intensive care unit after the procedure and were discharged after undergoing a control transthoracic echocardiography (TTE) 24hours after the index procedure.
Follow-upPatients undergoing ostium secundum atrial septal defect (osASD), PFO, ventricular septal defect (VSD), and left atrial appendage (LAA) occlusions were instructed to use acetylsalicylic acid (3-5mg/kg/day in children or 200mg/day in adults) for 6 months, in addition to 75mg of clopidogrel bisulfate in adults for 3 months in cases with this indication. In patients whose procedure involved only stenting, antiplatelet therapy with acetylsalicylic acid was recommended for 6 months. All patients were instructed to observe the prophylactic recommendations for infective endocarditis for a period of 6 months, when necessary.
Clinical follow-up was performed at 1, 3, 6, and 12 months after the procedure, and annually thereafter. Image monitoring with TTE was performed in visits at 1, 3, and 12 months, and annually thereafter; at the 6 month visit, TEE was performed for evaluation of the procedure outcome.
In addition to clinical examinations and routine echocardiograms, stents were angiographically evaluated after 6 months.
ResultsSince 2007, 982 patients were submitted to interventional procedures; in 10 patients (1.0%), two different defects were treated in the same session. Five patients were male, with age ranging from 1 to 67 years (14 ± 24.3 years), and with weight ranging from 11 to 90kg (50 ± 26.9kg).
Table 1 shows demographic data of these ten patients included in this study, as well as combinations of the structural defects treated. Globally, 20 congenital and structural defects were treated, with patent ductus arteriosus (PDA) the most prevalent (n = 5) in isolation, followed by osASD (n = 4) and VSD (n = 4). The most common combinations were VSD with PDA (n = 2) and VSD with osASD (n = 2).
Demographics and structural defects.
| N° | ID | Gender | Age (years) | Weight (kg) | Defects |
|---|---|---|---|---|---|
| 1 | JAA | M | 57 | 90 | PVS + PFO |
| 2 | GCO | M | 1 | 11 | PDA + aortopulmonary collaterals |
| 3 | BMOA | F | 14 | 50 | Perimembranous VSD + osASD |
| 4 | LCMM | F | 35 | 59 | Multiple muscular VSDs + PDA |
| 5 | GOR | F | 29 | 56 | PDA + osASD |
| 6 | JKVSG | M | 3 | 14 | PVS + osASD |
| 7 | JMFV | M | 4 | 15 | Perimembranous VSD + PDA |
| 8 | SMGQ | F | 67 | 65 | Chronic AF with stroke + PFO |
| 9 | GGR | M | 10 | 42 | Perimembranous VSD + osASD |
| 10 | IVM | F | 7 | 24 | AoC + PDA |
ID: patient identification; M: male; PVS: pulmonary valve stenosis; PFO: patent foramen ovale; PDA: patent ductus arteriosus; F: female; VSD: ventricular septal defect; osASD: ostium secundum atrial septal defect; AF: atrial fibrillation; AoC: aortic coarctation.
Of the 20 procedures performed in these ten patients, a combination of two procedures of occlusion was performed in the majority of cases (n = 7). In two cases, balloon pulmonary valvuloplasty (BPV) was performed in combination with PFO (n = 1) and osASD (n = 1) occlusion. In another patient with aortic coarctation (AoC) and PDA, AoC was treated with balloon dilation and with the implantation of a polytetrafluoroethylene (PTFE)-coated stent, positioned so as to also occlude the ductus arteriosus origin. A variety of devices with different characteristics and from different suppliers were used to conduct these procedures (Table 2).
Procedures completed and prostheses used.
| N | ID | Procedures | Prostheses |
|---|---|---|---|
| 1 | JAA | PBV + PFO occlusion | MULTI-TRACKTM system 14/40 + AmplatzerTM PFO Occluder 25 mm |
| 2 | GCO | PDA occlusion + aortopulmonary collateral embolization | AmplatzerTM Duct Occluder 1, 6-4 mm + FlipperTM Coils 3PDA3 |
| 3 | BMOA | Perimembranous VSD occlusion + osASD occlusion | CeraTM VSD Occluder type 18 mm + CeraTM ASD Occluder 16 mm |
| 4 | LCMM | Muscle VSD occlusion + PDA occlusion | CeraTM VSD Muscle Occluder 10 and 14 mm + CeraTM VSD Muscle Occluder 16 mm |
| 5 | GOR | PDA occlusion + osASD occlusion | CeraTM PDA Occluder 8/6 mm + CeraTM ASD Occluder 18 mm |
| 6 | JKVSG | PBV + osASD occlusion | OsypkaTM Vacs II balloon 16/30 mm + PFM ASD-R 24 mm |
| 7 | JMFV | Perimembranous VSD occlusion + PDA occlusion | CeraTM VSD Occluder type 1, 7 mm + CeraTM PDA Occluder Flex 8/6 mm |
| 8 | SMGQ | AAE occlusion + PFO occlusion | AmplatzerTM Cardiac Plug 24 mm + AmplatzerTM PFO Occluder 25 mm |
| 9 | GGR | Perimembranous VSD occlusion + osASD occlusion | CeraTM VSD Occluder type 1, 14 mm + CeraTM ASD Occluder Flex 22 mm |
| 10 | IVM | AoC dilation + stenting | Power Flex Plus 12/40 mm balloon + 8ZIG28 CP-coated stent |
ID: patient identification; PBV: pulmonary balloon valvuloplasty; PFO: patent foramen ovale; PDA: patent ductus arteriosus; VSD: ventricular septal defect; osASD: ostium secundum atrial septal defect; LAA: left atrial appendage; AoC: aortic coarctation.
Pulmonary balloon valvuloplasty (PBV) was performed as first procedure in two cases. The pulmonary transvalvar gradients were 40mmHg and 60mmHg, and reduced for 8mmHg and 28mmHg, respectively, after dilation.
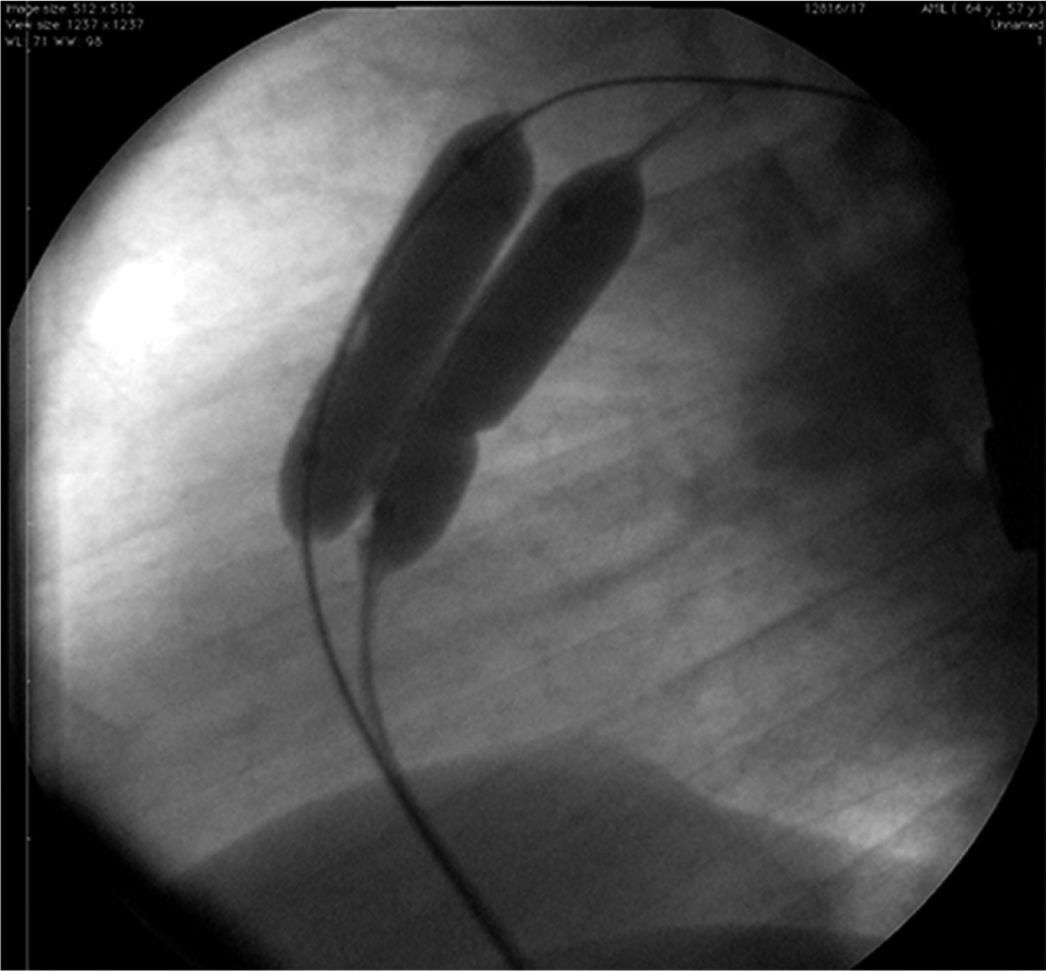
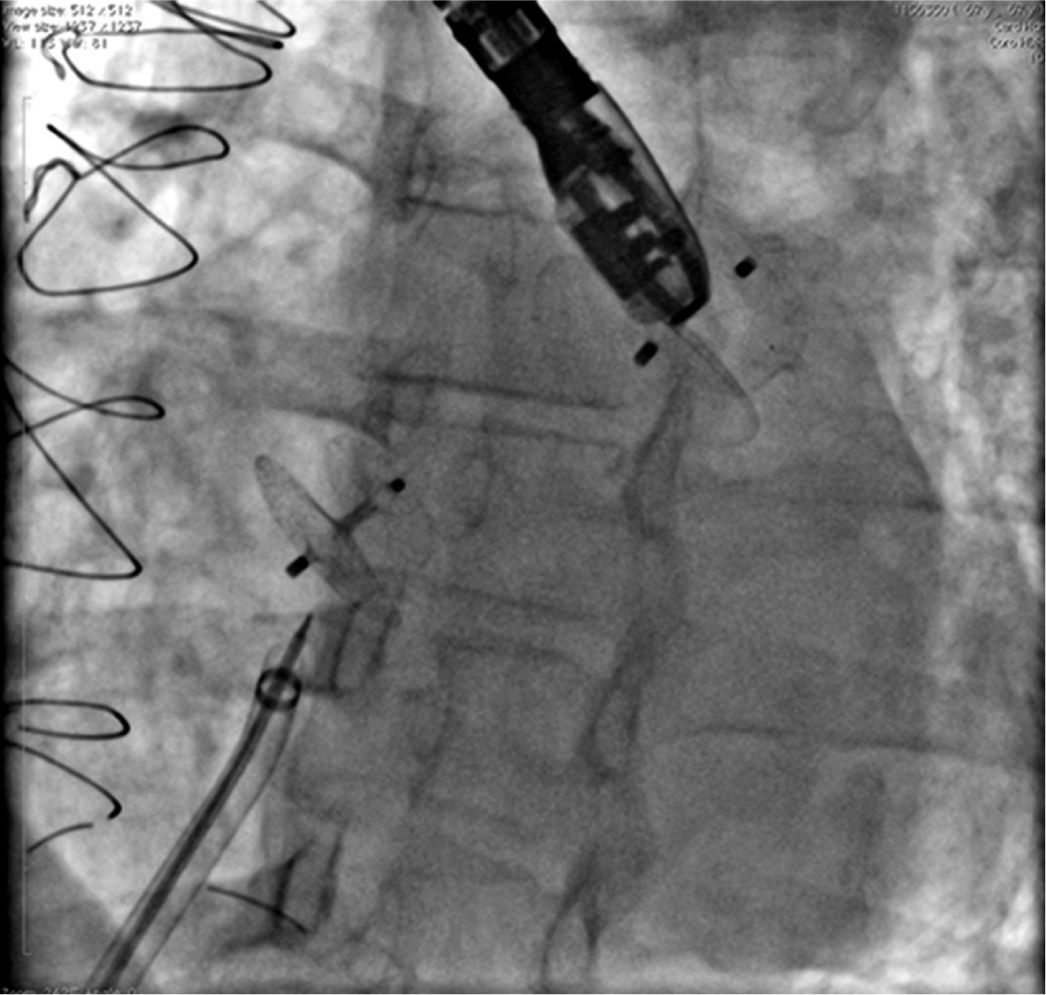
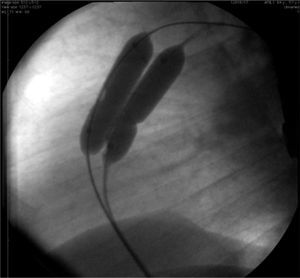
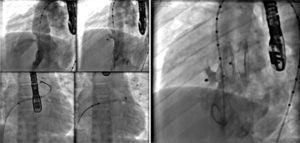
In Case 1, an adult patient, the double-balloon technique was used on a single guidewire (MULTI-TRACKTM system – Numed, Hopkinton, USA), with two 14/40mm balloons, with good results (Fig. 1). In Case 6, a 3-year-old child, a 16mm OsypkaTM Vacs II balloon was used (Osypka AG – Rheinfelden-Herten, Germany). In both cases, the valve lesion was approached first; then, septal occlusion was performed with a 25mm AmplatzerTM PFO occluder (St. Jude Medical – St. Paul, USA) (Case 1) and with a 22mm PFM ASD-R 22mm occluder (PFM AG, Köln, Germany) (Case 6). Both patients recovered uneventfully.
Patent ductus arteriosus occlusionArterial ducts were occluded as a first procedure in three cases (Cases 2, 4, and 5) through the traditional technique with the use of a 6/4mm AmplatzerTM Duct Occluder type I (St. Jude Medical – St. Paul, USA) and of a 16mm Muscular VSD CeraTM Occluder and a 6/8mm CeraTM PDA Occluder (Lifetech – Shenzhen, China). In Case 2, the occlusion of a collateral vessel was performed as a second procedure, using two FlipperTM 3PDA3 springs (Cook Medical – Bloomington, USA) released sequentially. In Case 4, two muscular VCDs were occluded (described below). In Case 5, one osASD was also occluded with an 18mm CeraTM ASD Occluder (Lifetech – Shenzhen, China). Both patients had a successful outcome, with no case of descending aorta or pulmonary left branch obstruction (Fig. 2).
Perimembranous ventricular septal defect occlusionPerimembranous VSD occlusion was performed as the first procedure in three cases. All patients had a normal pulmonary systolic pressure and some degree of increase in their left ventricular diastolic diameters by volume overload. No case exhibited aortic regurgitation before or after the procedure. In Case 3, the VSD was a consequence of a postoperative residual hole caused by surgical patch dehiscence, and the other two were birth defects (Cases 7 and 9). The defects measured, respectively, 7mm, 5mm, and 8mm at the right ventricular side. These defects were sealed with CeraTM VSD type I Occluders (8mm, 7mm, and 10mm). In the same procedure, two osASD (Cases 3 and 9) and one PDA (Case 7) were also occluded. The osASD cases were occluded as a second procedure with the use of a 16mm CeraTM ASD Occluder and a 22mm CeraTM Flex ASD Occluder. PDA was closed with a 6/8mm CeraTM PDA Flex Occluder device.
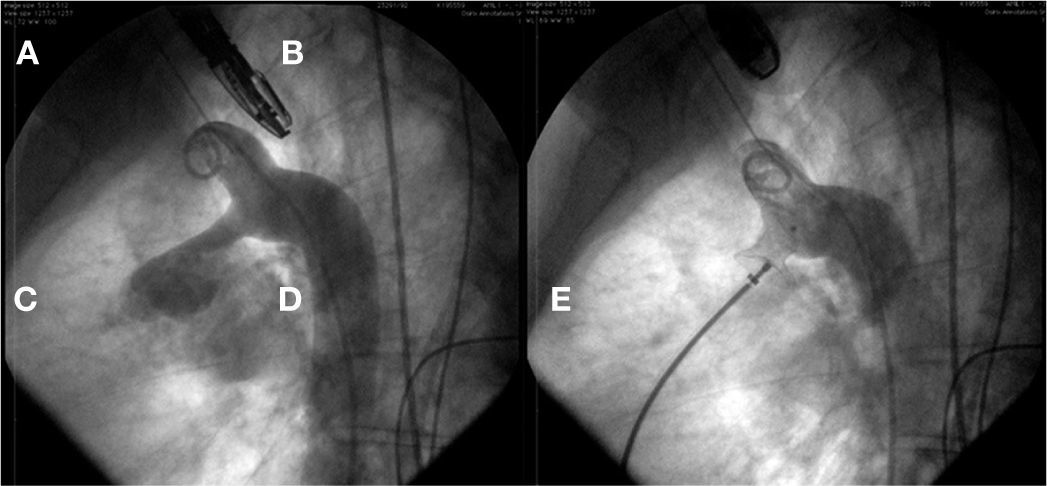
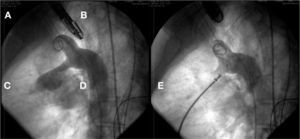
In case 9, after osASD occlusion, it was observed that the VSD prosthesis had embolized into the left pulmonary artery branch. The device was then captured with a 20mm loop catheter and removed through the 12mm long sheath used to implant the osASD prosthesis. At the end of the procedure, both defects were occluded, and the intervention was finished without further complications (Fig. 3).
In A, left ventriculography in a long-axis left anterior oblique view shows VSD below the aortic valve. In B, VSD was occluded by a type-I VSD CeraTM Occluder. In C, the presence of a CeraTM ASD Flex Occluder device can be seen occluding the ostium secundum atrial septal defect and the device in the ventricular septal defect embolized into the left pulmonary artery branch. Note that the connecting pin is captured by the loop catheter. In D, the VSD prosthesis is being removed through the long sheath. In E, ASD device in position and the second VSD prosthesis completely closing the defect can be seen.
In none of the cases treated was there interference in the tricuspid valve; in addition, there was no aortic regurgitation. After the procedure, there were no disturbances in atrioventricular conduction.
Membranous ventricular septal defect occlusionCase 4, a woman, exhibited severe pulmonary hypertension resulting from multiple muscular VSDs. During routine catheterization, a 10mm tubular PDA not diagnosed by transthoracic echocardiography was noted. This defect was occluded as a first procedure, with a 16mm muscular VSD CeraTM Occluder device. In continuation, two muscular VSDs with 8 and 11mm were occluded with 10mm and 14mm Muscular VSD CeraTM Occluder prostheses. At the end of the procedure, no significant reduction in pulmonary pressure was noted, and the woman remained in outpatient treatment, following a specific protocol for pulmonary arterial hypertension.
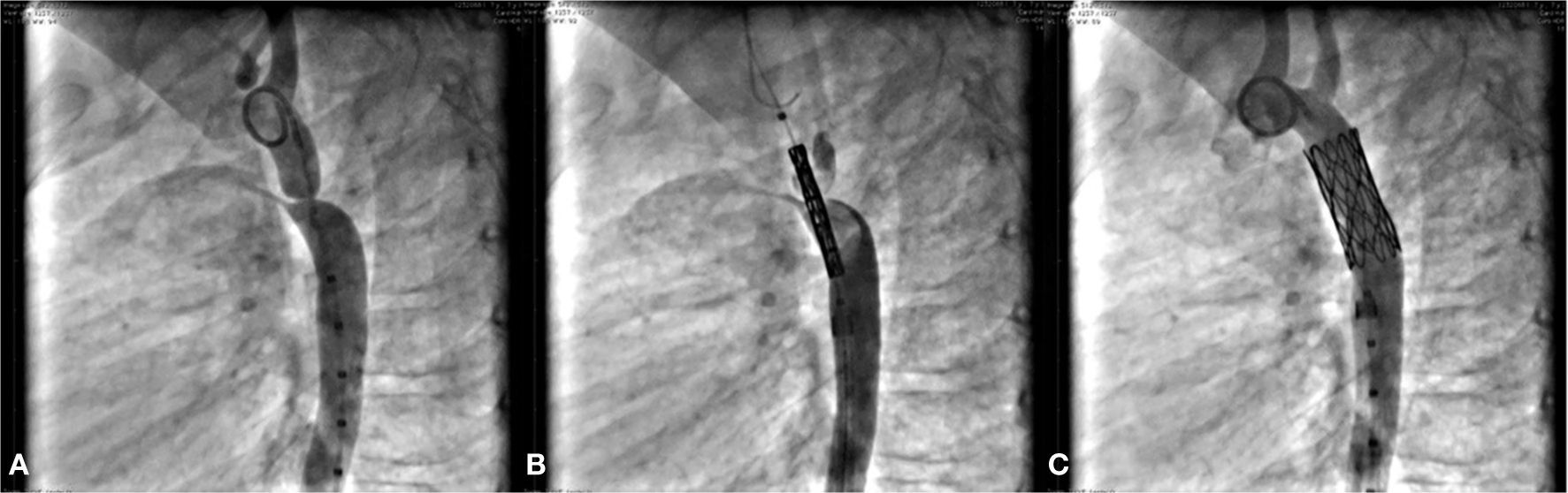
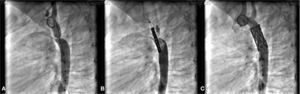
Dilation of aortic coarctationCase 10, a 7-year-old child, had AoC and a conical PDA. This patient had the AoC dilated and the PDA occluded simultaneously by stenting with a CP Coated 8ZIG28 device, mounted on a Power Flex Plus 12/40mm balloon introduced through a Mullins 10 F sheath over a rigid guidewire positioned into the left subclavian artery. The gradient through the narrowed area was 64mmHg and, after stenting, fell to 5mmHg. The procedure was successful and both lesions were treated properly without complications (Fig. 4).
In A, the aortography in the left view shows the presence of aortic coarctation. Note that there is also a conical ductus arteriosus, opacifying the pulmonary trunk. In B, in the same view, the coated stent positioned through the coarctation can be seen, already outside and above the delivery sheath. In C, the fully expanded stent, promoting coarctation dilation, and a complete occlusion of the ductus arteriosus are observed.
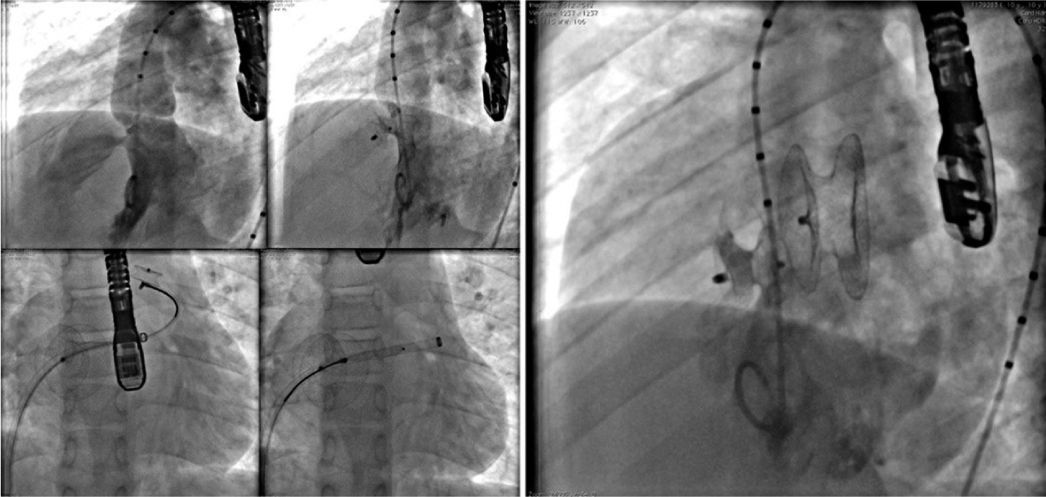
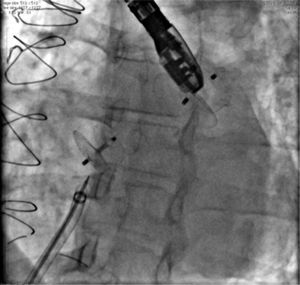
Case 8, a 67-year old woman with chronic coronary artery disease, suffered two myocardial infarctions at the ages of 32 and 33, having undergone coronary artery bypass grafting in 1983 and 2000. Since 2013, this patient had atrial fibrillation. In May 2014, the woman suffered an ischemic stroke, when PFO was diagnosed by TEE. Oral anticoagulation was introduced with warfarin and discontinued for retinal hemorrhage. The LAA was accessed through the foramen ovale, with no need for a transeptal puncture, and the defect was closed with a 24mm AmplatzerTM Cardiac Plug prosthesis. Her PFO was closed with a 25mm AmplatzerTM PFO Occluder, using the same cable and delivery sheath. Both defects were successfully closed (Fig. 5).
The picture shows the presence of the AmplatzerTM Cardiac Plug into the left atrial appendage, in the uppermost position; and, more inferiorly, the AmplatzerTM PFO Occluder device occluding the foramen ovale. The long sheath with double curvature and the delivery cable inwardly can also be seen, immediately after the release of the second prosthesis.
The mean time of follow-up was 31 ± 28.1 months (1-84 months).
There were no residual shunts in cases of aortopulmonary collaterals, PDA, osASD, PFO, perimembranous VSD, and LAA occlusions. In Case 4, a small residual shunt remained in the upper portion of the second muscular VSD prosthesis implanted.
In Case 10, no significant residual gradient in AoC was noted.
Two minor complications were noted: embolization of a muscular VSD prosthesis (Case 9), which was removed without difficulty from the left pulmonary artery branch, and a small arteriovenous fistula at the puncture site (Case 8), which was repaired by surgical suture; this complication was probably caused by an arterial injury provoked during previous coronary angiograms. There were no deaths in this small series.
DiscussionThe repair of more than one heart defect has been traditionally performed by surgery. The development of percutaneous therapeutic techniques allowed for an approach for most of the simpler cardiac defects and, in some cases, became the treatment of choice for the aforementioned defects in centers with specialized professionals.3–6,12–16
The accomplishment of combined procedures is not a simple task and demands careful planning. Some basic principles should be followed with the utmost accuracy: in a patient with a combination of heart defects, it is essential that all of them can be addressed percutaneously through established techniques, and with universally proven results. Otherwise, the patient should be referred for surgical correction, which will promote a full and proper resolution of the defects found.
Similarly, it is not good practice to treat a defect percutaneously before all possibilities of spontaneous resolution have been exhausted.
With respect to technical aspects, most authors guide their procedures based on dogmas; for instance, addressing valvar lesions first,12 i.e., approaching the most difficult or more complex defect first and then the simpler or less complicated condition,14 and considering the feasibility of access to defects. The more remote defects should be tackled first. Thus, a septal defect should not be occluded first, knowing that the left atrium must be accessed in order to treat the atrial appendage or the mitral valve subsequently.
In the procedures presented in this study, it was attempted to follow the above considerations. However, the approach sequence was reversed in two cases without jeopardizing the outcomes. In Case 4, the patient's ductus arteriosus was closed before the muscular VSDs, which caused no difficulty whatsoever for the occlusion of the ventricular septal defects. In Case 9, the perimembranous VSD was closed first, followed by osASD closure, as recommended. After the release of the osASD device, an embolization of the VSD prosthesis was noted; thus, the prosthesis was removed. Then, an equivalent prosthesis was implanted into the ventricular septum, but this new device was of a larger size than the previous prosthesis, and there was no interference or difficulty caused by the presence of a prosthesis in the atrial septum. As a general rule, the guidance listed herein is quite adequate, but there may be room for improvisations at the discretion of physician experience, provided they do not hinder nor jeopardize the full resolution of the defects in question.
The advantages of a simultaneous treatment of combined defects are quite obvious. Besides offering an alternative treatment for patients traditionally addressed surgically, with all its inherent risks and drawbacks, this alternative prevents the occurrence of multiple interventional procedures, minimizes radiological exposure, prevents the risk of repeated anesthetic procedures, reduces the number of vascular punctures, and cuts costs.
ConclusionsIn this small study, all procedures performed have proven to be safe, effective, and potentially reproducible by experienced physicians. In the present state of interventional cardiology, the completion of concomitant procedures in combined defects is a reality, which can represent the first therapeutic choice in specialized centers.
Funding sourcesNone declared.
Conflicts of interestFrancisco Chamié is a technical consultant and proctor for Boynton, a representative of Lifetech products. The other authors declare to have no conflicts of interests.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.