The diagnosis of ischemic cardiomyopathy is frequently difficult. Coronary angiography (CA) is limited because it is invasive and the evaluation is exclusively anatomic. Cardiac magnetic resonance imaging (MRI) with late gadolinium enhancement (LGE) measures patterns of myocardial fibrosis caused by ischemia. However, LGE does not detect ischemia that does not result in fibrosis. Thus, a thorough clinical evaluation by a cardiologist seems to be the most effective option for diagnosis. The aim of this study was to evaluate CA and LGE as complementary methods for the diagnosis of ischemic cardiomyopathy in patients with systolic heart failure of unknown etiology.
MethodsPatients with systolic heart failure, left ventricle ejection fraction < 45% and unknown etiology after initial non-invasive evaluation were submitted to CA and MRI with LGE to define the etiology of the disease. Patient evaluation by two cardiologists was the gold standard for the diagnosis of ischemic cardiomyopathy.
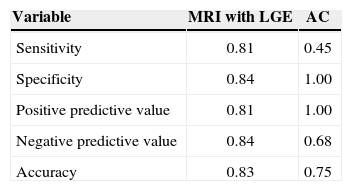
ResultsTwenty-four patients were included. The sensitivity to detect ischemic cardiomyopathy was 0.45 for CA vs. 0.81 for LGE. The specificity was 1.0 for CA vs. 0.84 for LGE. The positive predictive value was 1.0 vs. 0.81 and the negative predictive value was 0.68 vs. 0.84 for CA and LGE, respectively. LGE accuracy was superior to CA accuracy (0.83 vs. 0.75).
ConclusionsLGE was more sensitive than CA to evaluate the etiology of ventricular dysfunction, whereas CA was more specific. The diagnosis of ischemic cardiomyopathy using each one of the methods separately presented limitations.
Utilidade Clínica da Angiografia Coronária e da RessonânciaNuclear Magnética no Diagnóstico da Cardiomiopatia Isquêmica
IntroduçãoO diagnóstico da cardiomiopatia isquêmica é frequentemente difícil. A angiografia coronária (AC) é limitada, por ser invasiva e de avaliação exclusivamente anatômica. A ressonância nuclear magnética cardíaca (RNM) com realce tardio pelo gadolínio (RTG) pode mensurar padrões de fibrose miocárdica ocasionados pela isquemia. Porém, o RTG pode não detectar isquemia que não resultou em fibrose. Assim, uma avaliação clínica meticulosa pelo cardiologista parece ser a maneira mais eficaz para definir o diagnóstico. O objetivo deste estudo foi avaliar a AC e o RTG como métodos complementares para o diagnóstico de cardiomiopatia isquêmica em pacientes com insuficiência cardíaca sistólica sem etiologia definida.
MétodosPacientes com insuficiência cardíaca sistólica, fração de ejeção do ventrículo esquerdo < 45% e etiologia indefinida após avaliação não invasiva inicial foram submetidos à AC e à RNM com RTG para definição etiológica. A análise dos casos por dois cardiologistas foi o padrão-ouro para o diagnóstico de cardiomiopatia isquêmica.
ResultadosForam incluídos 24 pacientes. A sensibilidade para detecção de cardiomiopatia isquêmica foi de 0,45 para AC vs. 0,81 do RTG. A especificidade da AC foi de 1,0 vs. 0,84 do RTG. O valor preditivo positivo foi de 1,0 vs. 0,81, e o valor preditivo negativo foi 0,68 vs. 0,84 para AC e do RTG, respectivamente. A acurácia do RTG foi superior a da AC (0,83 vs. 0,75).
ConclusõesO RTG foi mais sensível do que a AC na avaliação etiológica da disfunção ventricular, enquanto a AC foi mais específica. A definição de cardiomiopatia isquêmica utilizando cada um dos métodos em separado apresentou limitações.
Ischemic heart disease is responsible for approximately two-thirds of systolic heart failure cases in the United States,1 and is also currently the main cause of systolic heart failure in Brazil.2,3 Although the mortality associated with ischemic heart disease has decreased in recent decades due to pharmacological advances and interventional treatment, it still remains the leading cause of death in developed countries.4 Differentiation of cardiomyopathies, from the etiological point of view, can be decisive for decision-making in clinical practice due to a number of reasons. First, patients with ischemic cardiomyopathy have the worst prognosis when compared to other cardiomyopathies.5 Additionally, ventricular dysfunction of ischemic cause may require specific treatment, both through coronary artery bypass grafting (CABG) procedures and pharmacological treatment for secondary prevention, with statins and acetylsalicylic acid. Patients with systolic heart failure are considered to have ischemic etiology when they show angiographic evidence of prior coronary artery disease (CAD), percutaneous coronary intervention or CABG, or history of myocardial infarction.
Coronary angiography (CA) is the procedure of choice for the detection of coronary artery stenosis in patients with systolic heart failure with undefined etiology. 6 However, it has the limitation of being an invasive method, with potential complications and exclusively anatomical evaluation. Recently, cardiac magnetic resonance imaging (MRI) with late gadolinium enhancement (LGE) technique has become a compelling method for the identification of ischemic cardiomyopathy, by demonstrating standard patterns of myocardial fibrosis. LGE can clearly delineate subendocardial infarction and evaluate the transmural extent of the infarction area.7 However, despite aggregating a great deal of information, LGE evaluation also has the limitation of not detecting ischemia that did not result in fibrosis. Thus, the global clinical assessment by the cardiologist, with access to all methods, appears to be the most effective way to detect the biases of each of these methods, and to define the diagnosis of ischemic cardiomyopathy. Therefore, the role of MRI with LGE and CA as complementary methods is yet to be established for the etiologic diagnosis of ischemic cardiomyopathy in patients with systolic heart failure of unknown cause.
The aim of this study was to evaluate the usefulness of MRI with LGE and CA for the diagnosis of ischaemic cardiomyopathy in patients with systolic heart failure heart of unknown etiology.
METHODSThis is a cross-sectional, single-center study performed at a tertiary care institution of high complexity cardiology. The research was based on the analysis of databases and medical chart review.
Study PopulationThe present study analized data from patients treated in the outpatient clinic for treatment of heart failure of Instituto do Coração do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, between 1 January 2009 and 31 July 2012. Patients who underwent CA and MRI with LGE in this period to evaluate the etiology of dilated cardiomyopathy of unknown cause were included. The inclusion criteria were: presence of systolic heart failure, characterized by left ventricular ejection fraction (LVEF) < 45%, documented by echocardiography in the period up to one year after the procedure; onset of heart failure symptoms for more than one month, and age≥18 years. Patients with prior history of CAD, positive serology for Chagas disease, valvular heart disease, or heart transplantation were excluded from the analysis.
ProceduresThe indication for CA was defined by the criteria of the attending physicians and follows the recommendations of current guidelines for chronic heart failure,2 which recommend the method for symptomatic patients due to angina or persistent heart failure (e.g., New York Heart Association [NYHA] functional class II, III or IV, despite optimized drug treatment) or asymptomatic patients with the presence of two or more risk factors for CAD (age > 45 years for men and 55 for women; hypercholesterolemia; smoking, systemic arterial hypertension, chronic renal disease, or diabetes mellitus). After the CA, patients were classified as having ischemic or non-ischemic etiology. As for the angiographic criteria for ischemic cardiomyopathy, previously published definitions were used, considering as ischemic etiology patients with obstructive lesions (≥75 %) in left main coronary artery or proximal anterior descending branch, or in two or more epicardial vessels.8
All patients included in the study had undergone MRI with LGE. The LGE pictures were acquired during respiratory pauses and coupled to the electrocardiogram, with the following guidelines: two chambers, long axis, four chambers, long axis, left ventricular outflow tract, and short-axis images with scanning of the entire left ventricle. Gadolinium was used as contrast at a dose of 0.2 mmoL/kg, and image acquisition was performed 10 minutes after the infusion.
Global analysis of cases by two clinical cardiologists, including all data inclinical history and laboratory tests available in medical records, was defined as the gold standard for the diagnosis of ischemic cardiomyopathy.
Data collection and statistical analysisDemographic data, as well as information on the presence of traditional risk factors for CAD were collected. Continuous variables were expressed as means and standard deviations, and categorical variables were described as percentages. To evaluate the diagnostic performance of each method, measures of sensitivity, specificity, and predictive values and accuracy were calculated, considering the global assessment performed by the cardiologists as the gold standard.
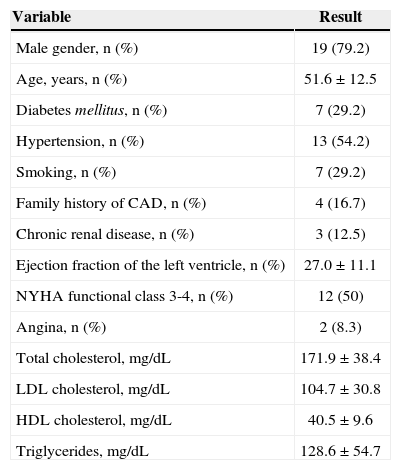
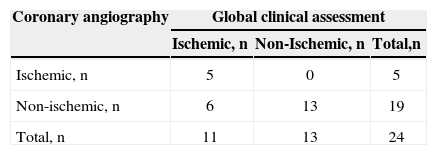
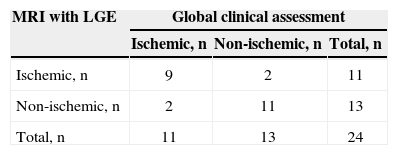
RESULTSA total of 1,955 consecutive patients were evaluated. After applying the study eligibility criteria, 24 patients were included in the analysis, of whom 19 (79.2%) were males; mean age was 51.6±12.5years and LVEF was 27%±11.1%. The demographic data of this population are shown in Table 1. According to the global clinical assessment, 11 (45.8%) patients were classified as ischemic cardiomyopathy and 13 (54.2 %) as non-ischaemic cardiomyopathy. At the CA, 5 (20.8%) patients had coronary obstructions consistent with ischaemic cardiomyopathy and 19 (79.2%) had no major lesions (Table 2). LGE detected subendocardial or transmural pattern compatible with ischaemic cardiomyopathy in 11 (45.8%) patients, while 13 (54.2%) did not have LGE or showed an enhancement pattern different from CAD (e.g. mesocardic fibrosis), as shown in Table 3.
Basal characteristics of the population
| Variable | Result |
|---|---|
| Male gender, n (%) | 19 (79.2) |
| Age, years, n (%) | 51.6±12.5 |
| Diabetes mellitus, n (%) | 7 (29.2) |
| Hypertension, n (%) | 13 (54.2) |
| Smoking, n (%) | 7 (29.2) |
| Family history of CAD, n (%) | 4 (16.7) |
| Chronic renal disease, n (%) | 3 (12.5) |
| Ejection fraction of the left ventricle, n (%) | 27.0±11.1 |
| NYHA functional class 3-4, n (%) | 12 (50) |
| Angina, n (%) | 2 (8.3) |
| Total cholesterol, mg/dL | 171.9±38.4 |
| LDL cholesterol, mg/dL | 104.7±30.8 |
| HDL cholesterol, mg/dL | 40.5±9.6 |
| Triglycerides, mg/dL | 128.6±54.7 |
CAD=coronary artery disease; NYHA=New York Heart Association; LDL=low-density lipoprotein; HDL=high-density lipoprotein.
Diagnostic performance of cardiac magnetic resonance imaging with late gadolinium enhancement (MRI with LGE)
| MRI with LGE | Global clinical assessment | ||
|---|---|---|---|
| Ischemic, n | Non-ischemic, n | Total, n | |
| Ischemic, n | 9 | 2 | 11 |
| Non-ischemic, n | 2 | 11 | 13 |
| Total, n | 11 | 13 | 24 |
MRI with LGE=magnetic resonance imaging with late gadolinium enhancement.
The variables of the diagnostic performance assessment for the methods used to define ischemic cardiomyopathy are described in Table 4.
Diagnostic capacity of the methods to detect ischemic cardiomyopathy
| Variable | MRI with LGE | AC |
|---|---|---|
| Sensitivity | 0.81 | 0.45 |
| Specificity | 0.84 | 1.00 |
| Positive predictive value | 0.81 | 1.00 |
| Negative predictive value | 0.84 | 0.68 |
| Accuracy | 0.83 | 0.75 |
MRI with LGE=magnetic resonance imaging with lategadolinium enhancement, CA=coronary angiography.
There was agreement between the methods in 16 (66.6%) patients, whereas in eight (33.4%), CA and LGE were discordant for the detection of ischemic cardiomyopathy.
DISCUSSIONAccording to the current heart failure guidelines, the CA should be considered in the etiologic assessment of patients with systolic heart failure in the presence of risk factors for CAD, symptoms of refractory heart failure, or angina.6 In this population, the performance of the CA, according to these criteria, confirmed the ischemic etiology of cardiomyopathy in five (20.8 %) patients, but only in four did the MRI show the delayed enhancement pattern compatible with ischaemic cardiomyopathy. This finding can be explained by the possibility that the ventricular dysfunction was due to myocardial hibernation. That is, the loss of contractile function can be due to cardiac muscle hibernation caused by severe coronary obstruction, without cell death and formation of necrotic tissue. Similar findings were published in a series of 291 patients with biventricular dysfunction and no history of acute myocardial infarction submitted to CA and endomyocardial biopsy due to progressive systolic heart failure symptoms.9 Seven (2.4%) had significant obstructive lesions at the CA, but the endomyocardial biopsy, in all cases, showed histological alterations with conclusive criteria for myocarditis, demonstrating, once again, that the presence of severe coronary obstruction does not necessarily have a causal relationship with ventricular dysfunction.
Conversely, among the remaining 19 (79.1%) patients in whom CA did not show alterations consistent with ischemic cardiomyopathy, 7 (29.1%) showed transmural or subendocardial LGE pattern when myocardial necrosis was due to ischemia of the heart muscle. This finding corroborates the physiopathological rationale that the infarcted area identified by MRI may have been consequent to a transient coronary flow obstruction in a time period prior to CA. Brener et al.10 has demonstrated that approximately 18% of patients who had an acute myocardial infarction experienced spontaneous recanalization of the artery responsible for the event, with TIMI 3 flow, when submitted to cardiac catheterization. This can be explained, among other possibilities, by thrombolysis through action of the endogenous fibrinolytic system. Thus, this aspect emphasizes the limitations of CA when used with the objective of evaluating the ischemic component of systolic heart failure. Similar findings to those of the present population have been reported in the literature in studies with MRI in patients with dilated cardiomyopathy and CA without obstructive lesions, in which 13% had LGE patterns indistinguishable from those seen in CAD.11
The prognostic value of MRI in patients with an established diagnosis of ischemic ventricular dysfunction has been previously demonstrated. In patients with ischemic cardiomyopathy and substantial reduction in LVEF, the length of the LGE is associated with increased mortality, and the need for cardiac transplantation.4 Representing a non-invasive method with a low rate of adverse events, it may be of interest to indicate MRI instead of CA, in the initial diagnostic approach of systolic heart failure. That is because, although the risk of major complications is low when CA is performed with diagnostic purpose in unselected patients from experienced centers,12 patients with cardiomyopathy or heart failure symptoms have increased complication likelihood, which increases the risk of adverse events by 3.3 – and 2.2-fold, respectively.13 In the present study, the rate of complications related to the procedure was not evaluated. Another possible indication of MRI could be in subgroups of patients in which the lower diagnostic power of CA is known, as demonstrated by the publication of Melo et al.14 This study of 107 patients undergoing CA to rule out ischemic heart disease as the etiology of left ventricular dysfunction showed that the CA had a good diagnostic result for ischemic cardiomyopathy only when indicated in symptomatic patients with angina or refractory heart failure symptoms. When performed in asymptomatic patients with two or more risk factors for CAD, there was no diagnosis of IC (ischemic cardiomyopathy).14
However, the potential benefit of LGE as an isolated test was not observed in this study. As gadolinium has the capacity to occupy the myocardial extracellular space, with increased concentration in areas that suffered myocardial necrosis,15 it was not possible to identify patients with severe coronary artery lesions at the CA that did not undergo cell apoptosis (hibernating myocardium). Therefore, although the ischemic etiology has been identified in patients with normal CA, the LGE did not detect some patients considered to have ischemic cardiomyopathy, according to the CA criteria used as the default method in most clinical studies.
Thus, considering that the two methods are not likely to detect ischemic cardiomyopathy as the cause of ventricular dysfunction due to the afore mentioned reasons, it is possible that the association between the two tests is a strategy with greater capacity to identify individuals with ischemic cardiomyopathy. Moreover, this study considered the clinical assessment by two cardiologists as the gold standard for the diagnosis of ischemic cardiomyopathy. This allowed the biases of each method to be identified when classifying individuals regarding the presence or absence of ischemic ventricular dysfunction. To illustrate this fact, in one case of this study, a male patient with severe left ventricular dysfunction at the expense of diffuse hypokinesis at the MRI showed no lesions at the CA, and LGE with transmural fibrosis in the apical and antero apical segments. This case would be classified as ischemic cardiomyopathy by the LGE, and as a non-ischemic case according to the CA. However, considering that the patient had no clinical history consistent with myocardial infarction and that the presence of fibrosis, albeit transmural, in a small and distal segment of the ventricle would not justify the diffuse contractile dysfunction, the global clinical evaluation defined this case as non-ischemic heart disease.
Although the findings of this study are quite compatible with previous publications in this area, this method of measuring the gold standard for ischemic cardiomyopathy, throughthe clinical independent assessment of two cardiologists, makes this a pioneering analysis among diagnostic studies of ischemic ventricular dysfunction.
Study limitationsThis study has some limitations. This is a retrospective review subject to inherent biases of this modality, such as obtaining data on epidemiology and the indication of the CA. The study was carried out in one center and with a convenience sample, referred from a tertiary hospital with a limited number of patients included in the analysis.
CONCLUSIONSIn this study, cardiac magnetic resonance imaging with late gadolinium enhancement was more sensitive than coronary angiography in the etiological assessment of left ventricular dysfunction of unknown cause. The coronary angiography, in turn, was more specific. The definition of ischemic cardiomyopathy using each one of the methods separately showed limitations. These two assessments showed that they were complementary in the etiological diagnosis of heart failure.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.