Very few data exist on coronary obstruction following transcatheter aortic valve implantation (TAVI) for degenerative bioprosthetic valves (valve-in-valve [ViV]). The present trial evaluated, through a systematic review of the literature, the clinical characteristics, management and clinical outcomes of patients with coronary obstruction after ViV-TAVI.
MethodsStudies published between 2002 and 2013 evaluating coronary obstruction as a complication of ViV-TAVI were identified using a systematic electronic search. Data on the clinical and procedural characteristics, management of the complication, and clinical outcomes were analyzed.
ResultsA total of four publications describing seven patients were identified. Most patients (71%) were women, with mean age of 82±5 years, and STS-PROM score of 9.4±2.6%. Mean left coronary artery (LCA) ostium height and aortic root width were 8.8±1.5mm and 28.0±5.0mm, respectively. Most patients had stented bioprosthetic valves with externally mounted leaflets or stentless aortic bioprosthesis, and the LCA was involved in all patients. Percutaneous coronary intervention (PCI) was attempted in all patients and was successful in four (57%). In-hospital mortality was 42.9% (three cases), all of them after failed PCI.
ConclusionsCoronary obstruction following ViV-TAVI occurred more frequently in women with stented bioprosthetic valves with externally mounted leaflets or with stentless bioprosthesis. The LCA was involved in all cases and PCI was successful in 60% of them. Continued efforts may help identify the factors associated with this complication so that appropriate prevention measures may be implemented.
Obstrução Coronária Após Implante de Válvula Aórtica porCateter para o Tratamento de Bioprótese Valvular Cirúrgica com Disfunção: Revisão Sistemática da Literatura
IntroduçãoHá poucos dados na literatura que avaliam a obstrução coronária após implante transcateter de válvula aórtica (transcatheter aortic valve implantation [TAVI]) para o tratamento de disfunção de bioprótese aórtica (valve-in-valve – ViV). O presente estudo avaliou, por meio de revisão sistemática da literatura, as características clínicas, o manejo e os desfechos clínicos de pacientes com obstrução coronária após TAVI-ViV.
MétodosEstudos publicados entre 2002 e 2013 avaliando a obstrução coronária como complicação de TAVI-ViV foram identificados por meio de busca eletrônica sistemática. Foram avaliados dados basais sobre as características clínicas e do procedimento, manejo da complicação e desfechos clínicos.
ResultadosForam identificadas, no total, quatro publicações descrevendo sete pacientes. A maioria dos pacientes era do sexo feminino (71%), sendo a média de idade de 82±5 anos, com STS-PROM de 9,4±2,6%. As médias da altura do tronco da artéria coronária esquerda (TCE) e o diâmetro médio da raiz aórtica foram de 8,8±1,5mm e 28,0±5,0mm, respectivamente. A maioria dos pacientes apresentava biopróteses com suporte e folhetos montados externamente, ou eram próteses sem suporte (stentless) e o TCE foi envolvido em todos os casos. A intervenção coronária percutânea (ICP) foi tenta da em todos os pacientes, tendo sucesso em quatro deles (57%). A mortalidade intra-hospitalar foi 42,9% (três casos), todos após ICP sem sucesso.
ConclusõesA obstrução coronária após TAVI-ViV ocorreu mais frequentemente em mulheres com bioprótese com suporte e folheto montado externamente ou com bioprótese sem suporte. O TCE foi envolvido em todos os casos e a ICP foi realizada com sucesso em 60% deles. Esforços contínuos poderão auxiliar na detecção dos fatores associados a essa complicação, no intuito de se implementarem medidas apropriadas para sua prevenção.
The transcatheter aortic valve implantation (TAVI) has been established as the standard for the treatment of patients with severe symptomatic aortic stenosis considered inoperable, and as an alternative to those considered at high risk for aortic valve replacement surgery.1,2 However, TAVI has also been associated with very rare complications, but many of them fatal, such as coronary obstruction, which is typically related to the displacement of the calcified native leaflets of the valve toward coronary ostia.
Recently, a systematic review of the literature on symptomatic coronary obstruction as a complication of TAVI was conducted, which included a total of 24 cases, all of them described in isolation as case reports or as small series of cases.3 Such review demonstrated that coronary obstruction after TAVI occurred more often in women and in patients receiving expansible valve by balloon, and the left main coronary artery (LMCA) was the most often involved vessel. However, that study excluded procedures in patients with previous surgical bioprosthesis (valve-in-valve [ViV]). Moreover, a recent multicenter registry, which included over 6,500 patients, suggested that this complication could be up to four times more frequent in patients with surgical prostheses, similarly to those reported in the ViV global registry.4
However, no study to date has evaluated specifically this complication in a ViV population. Thus, the present study aimed to evaluate baseline characteristics, management, and clinical outcomes of coronary obstruction as a complication of TAVI in patients with previous surgical aortic bioprosthesis through a systematic review.
METHODSAll relevant articles in English on TAVI and coronary obstruction in patients with previous surgical bioprosthesis published between December 2002 and September 2013 were systematically searched at BioMedCentral (http://www.biomedcentral.com), Google Scholar (http://www.scholar.google.com), and PubMed (http://www.pubmed.gov). The following key terms were used: “aortic stenosis”, “trans catheter valve implantation”, “transcatheter aortic valve replacement”, “transcatheter heart valve”, “heart valve prosthesis implantation”, “coronary stenosis”, “coronary occlusion, coronary obstruction”, “prior surgical bioprosthesis” and “valve-in-valve”. Other studies were evaluated through manual research of secondary sources, including references of primary articles (backward snowballing) and contact with international experts.
The quotations were selected based on title/abstract by two independent reviewers (HBR and LNF), and retrieved as complete manuscripts when considered potentially relevant. Disagreements were resolved by consensus, aiming to gather all reports and relevant case series on coronary obstruction after TAVI-ViV. The published articles which included only the incidence of the complication were excluded from this analysis.
Data gathered included the basal clinical characteristics, beyond echocardiographic and tomography (CT) features. The CT variables included data from LMCA height in relation to the aortic annulus and aortic root and annulus diameters. Procedural data, such as type and size of transcatheter valve used, type of approach, clinical picture, and management of coronary obstruction were also evaluated. Finally, data on in-hospital mortality were also recovered.
Categorical variables were reported as n (%) and continuous variables as mean±standard deviation. All analyzes were performed using SAS statistical package, version 9.3 (SAS Institute Inc., Cary, NC, United).
RESULTSA total of four publications were identified, describing a total of seven patients who had coronary obstruction related to TAVI-ViV.5−8 All studies were case reports, except for one multicenter trial that included three cases of coronary obstruction. The main baseline clinical characteristics were available for all patients. CT data on the height of the LMCA ostium, aortic annulus, and aortic root measures were reported in four, four, and three patients, respectively. Clinical and procedural data about clinical presentation, diagnosis, and management of the coronary occlusion were available for all patients. All studies reported data on in-hospital outcomes.
The main clinical, echocardiographic, CT, and procedural characteristics to which the patients were submitted are listed in Tables 1 (individual data) and 2 (means). The mean age of the study population was 82±5 years, and most were female (71.4%). Data from CT revealed a mean height of LMCA of 8.8±1.5mm and a mean aortic root diameter of 28.0±5.0mm. The Edwards Balloon Expandable Valve® (Edwards Lifesciences, Irvine, CA) was used in five patients, and a self-expanding valve, CoreValve® (Medtronic, Minneapolis, MN), was used in two patients.
Baseline clinical and procedural characteristics
| Study | Age (years) | Gender | Bioprosthesis | Surgical bioprosthesis name | Bioprosthesis size (mm) | Previous CABG | STS score (%) | Mean aortic gradient (mmHg) | Aortic annulus (mm) | Aortic root (mm) | LMCA height (mm) | Access | TAVI type | TAVI size (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fiorina et al.5 | 82 | M | Supportless (stentless) | CryoLife-O'Brien® | 25 | No | − | − | − | 33 | 10 | TF | CoreValve® | 29 |
| Gurvitch et al.6 | 86 | F | Supported | Mitroflow® | 21 | Yes | 12,9 | 87 | 18 | − | − | TA | Sapien® | 23 |
| Gurvitch et al.6 | 78 | F | Supported | Mitroflow® | 21 | Yes | − | 44 | − | − | − | TF | CoreValve® | 26 |
| Chakravarty et al.7 | 79 | F | Supportless (stentless) | St. Jude | 23 | No | 6,1 | − | − | − | 7,4 | TF | Sapien® | 23 |
| Ribeiro et al.8 | 80 | F | Supportless (stentless) | Toronto | 24 | No | 8,1 | 22 | 20 | 23, 2 | 9,7 | TF | Sapien® | 23 |
| Ribeiro et al.8 | 91 | F | Supported | SPV® | 21 | Yes | 10,3 | 43 | 18 | − | − | TF | Sapien® | 23 |
| Ribeiro et al.8 | 80 | M | Supportless (stentless) | St. Jude | 23 | No | 9,7 | 15 | 21 | 28 | 8,2 | TF | Sapien XT® | 23 |
CABG=coronary artery bypass graft surgery; STS=Society of Thoracic Surgeons; LMCA=left main coronary artery; M=male; TF=transfemoral; F=female; TA=transapical.
Basal clinical, echocardiographic, CT, and procedural characteristics of the population studied
| Clinical variables | (n=7) |
|---|---|
| Age, years | 82.3±4.6 |
| Female, n (%) | 5 (71.4) |
| NYHA III-IV class, n (%) | 6 (85.7) |
| Previous CABG surgery, n (%) | 4 (57.1) |
| STS-PROM score, % | 9.4±2.6 |
| Echocardiography and computed tomography data | |
| Mean aortic gradient, mmHg | 42.2±28.1 |
| Aortic valve area, cm2 | 0.43±0.09 |
| Aortic annulus, mm | 19.1±1.8 |
| Height of the left main coronary artery, mm | 8.8±1.5 |
| Aortic root diameter, mm | 28.0±5.0 |
| Data from the procedure | |
| Approach, n (%) | |
| Transfemoral | 6 (85.7) |
| Transapical | 1 (14.3) |
| Valve type, n (%) | |
| Sapien® and Sapien XT® | 5 (71.4) |
| CoreValve® | 2 (28.6) |
CT=computed tomography; CABG=coronary artery bypass graft surgery; NYHA=New York Heart Association.
The main data on the clinical presentation and management adopted in relation to coronary obstruction are listed in Table 3 (individual data) and Table 4 (means). The LMCA was involved in all patients (in one of them, together with right coronary artery); all patients presented significant and sustained hypotension, and most of them (57.1%) had electrocardiographic changes of ST-segment. The diagnosis of coronary obstruction was established by coronary angiography for all patients, and in all of them the obstruction was related to the displacement of bioprosthesis leaflets.
Clinical picture and conduct
| Study | Clinical picture | Treatment | Successful ATC | Stent type | Hemodynamic support needed | Length of stay (days) | In-hospital mortality | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coronary obstruction | Significant hypotension | ST-segment changes | Ventricular arrhythmias or CPR | CPI | CABG | ||||||
| Fiorina et al.5 | LMCA | Yes | No | No | Yes | No | Yes | CS | Yes | - | No |
| Gurvitch et al.6 | Both | Yes | Yes | No | Yes | Yes | No | - | Yes | 1 | Yes |
| Gurvitch et al.6 | LMCA | Yes | Yes | Yes | Yes | No | No | - | Yes | 0 | Yes |
| Chakravarty et al.7 | LMCA | Yes | Yes | No | Yes | No | Yes | DES | No | - | No |
| Ribeiro et al.8 | LMCA | Yes | No | No | Yes | No | Yes | DES | No | 3 | No |
| Ribeiro et al.8 | LMCA | Yes | No | Yes | Yes | No | No | - | Yes | 3 | Yes |
| Ribeiro et al.8 | LMCA | Yes | Yes | No | Yes | No | Yes | DES | No | 4 | |
CPR=cardiopulmonary resuscitation; PCI=percutaneous coronary intervention; CABG=coronary artery bypass grafting; LMCA=left main coronary artery; CS=conventional stent; DEA=drug-eluting stent.
Clinical picture and conduct (n=7)
| Picture/conduct | |
|---|---|
| Coronary occlusion, n (%) | |
| Left main coronary artery | 6 (85.7) |
| Both coronary arteries | 1 (14.3) |
| Clinical picture, n (%) | |
| Severe persistent hypotension | 7 (100) |
| ST-segment changes | 4 (57.1) |
| ST-segment elevation | 1 (14.3) |
| Treatment, n (%) | |
| Attempted percutaneous coronary intervention | 7 (100) |
| Successful | 4 (57.1) |
| Unsuccessful | 3 (42.9) |
| Inability to cross the lesion with the guide wire | 1 (14.3) |
| Inability to advance the stent | 1 (14.3) |
| Stent implanted but without flow | 1 (14.3) |
| Complications, n (%) | |
| Need for hemodynamic support | 4 (57.1) |
| Conversion to CABG | 1 (14.3) |
| In-hospital death | 3 (42.9) |
CABG=coronary artery bypass graft.
Percutaneous coronary intervention (PCI) was attempted in all patients, and was successful in four of them (57.1%). The three cases of failure of PCI occurred by: inability to cross the obstruction with the coronary guide wire, requiring emergency CABG; failure to restore the coronary blood flow, despite the successful implantation of the stent, which led to a persistent cardiogenic shock and death; and inability to advance the stent. Hemodynamic support was needed in four patients (57.1%) and the in-hospital mortality was 42.9%. All patients undergoing a successful PCI survived and were discharged from the hospital; no case showed stent thrombosis or neorevascularization.
DISCUSSIONThe present systematic review assessing symptomatic coronary obstruction following TAVI for treatment of surgical bioprosthetic valve dysfunction observed that the complication occurred more often in women and in patients previously treated with supportless prostheses, or in those supported and with leaflets externally mounted. In such cases, the mean height of the LMCA ostium was~8mm and the mean diameter of the aortic root was~28mm. The clinical picture included more often a significant and sustained hypotension, besides changes in ST segment; a PCI was attempted in all cases, and was successful in approximately 60% of them.
However, mechanical hemodynamic support and conversion for CABG were still required in 57% and 14% of patients, respectively. There were no cases of acute stent thrombosis or of neorevascularization, and the rate of in-hospital mortality was 42.9% (there were no deaths in patients undergoing successful PCI).
Coronary obstruction following TAVI has been a concern since the first experimental studies,9,10 having been described in 2006 in the first experiment in humans for the treatment of native aortic valve stenosis.11The first case of coronary obstruction as a complication of TAVI-ViV procedure was reported in 2011.6 Since then, the reported incidence for coronary obstruction in different studies has been generally < 1%.
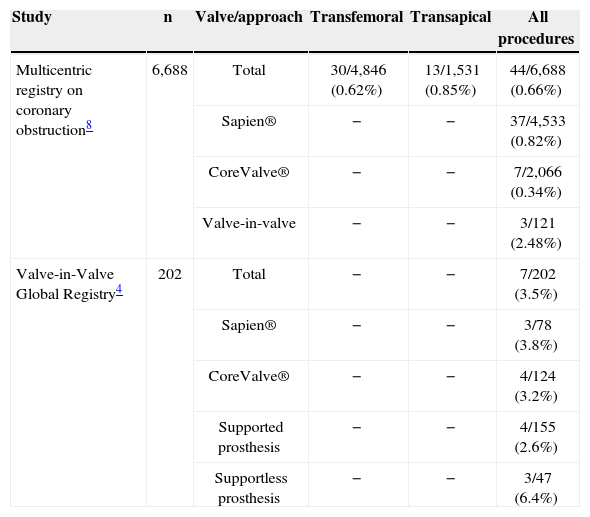
However, in a large multicenter registry recently published on coronary obstruction, with over 6,500 patients, the incidence in native aortic valves was 0.66%, much lower than the 2.48% of the patients submitted to previous surgical prostheses.8 This incidence is similar to that reported of 3.5%, described in a recent registry conducted worldwide considering only TAVI-ViV (Valve-in-Valve Global Registry), including 202 patients,4 and corroborates the presence of previous surgical bioprosthesis as a potential risk factor for this complication. The rates of coronary obstruction in recent studies evaluating this complication, both in native aortic valves and in surgical bioprostheses, are summarized in Table 5.
Data from large registries of transcatheter aortic valve implantation assessing coronary obstruction in the native aortic valve and in patients with previous surgical bioprosthesis.
| Study | n | Valve/approach | Transfemoral | Transapical | All procedures |
|---|---|---|---|---|---|
| Multicentric registry on coronary obstruction8 | 6,688 | Total | 30/4,846 (0.62%) | 13/1,531 (0.85%) | 44/6,688 (0.66%) |
| Sapien® | − | − | 37/4,533 (0.82%) | ||
| CoreValve® | − | − | 7/2,066 (0.34%) | ||
| Valve-in-valve | − | − | 3/121 (2.48%) | ||
| Valve-in-Valve Global Registry4 | 202 | Total | − | − | 7/202 (3.5%) |
| Sapien® | − | − | 3/78 (3.8%) | ||
| CoreValve® | − | − | 4/124 (3.2%) | ||
| Supported prosthesis | − | − | 4/155 (2.6%) | ||
| Supportless prosthesis | − | − | 3/47 (6.4%) |
The mechanism associated with coronary obstruction after TAVI-ViV has been the displacement of the bioprosthesis leaflet towards the coronary ostium in all patients; no cases of coronary obstruction related to the metal frame of transcatheter valves, nor to their leaflets, have been reported.3,8 Furthermore, according to the TAVI-ViV Global Registry,4 the coronary obstruction following TAVI-ViV was more frequent in supported bioprosthesis leaflets and in externally mounted leaflets (especially the Mitroflow® prosthesis) and also with supportless bioprostheses. In the case of the Mitroflow® prosthesis, the relatively long (~13mm) leaflets externally mounted on the supports (with stent), rather than internally as in most other supported prostheses, may be associated with a higher rate of coronary obstruction.6 In addition, the supportless bioprostheses (stentless) may also be associated with an increased risk of this complication. These bioprostheses are generally implanted in a supra-annular position, resulting in a shortening of the position of the coronary ostia in relation to the valve leaflets, which, together with the rodless stents, may facilitate an interaction of the prosthesis with the aortic wall and the coronary ostium (Figure 1).
Additionally, and in agreement with previous studies on TAVI in native aortic valves,3,8 the presence of coronary ostia located in a lower position, and of a narrow Valsalva sinus, were also identified as potential risk factors for this complication in cases of ViV. In this sense, among patients with available data of CT in this trial, the mean height of LMCA was 8.8mm, similar to that found in a recent multicenter registry (10.6mm) and much lower than that of 13.4mm observed in control patients without coronary occlusion (P < 0.001).8 This height is also significantly lower (~5mm) than that reported in previous CT studies in patients with and without aortic stenosis (systematically > 13mm).12−14
Although the height of the coronary ostium is an important risk factor associated with coronary obstruction after TAVI, it was demonstrated that some patients had this complication despite of an LMCA height > 12mm, indicating that other factors (such as a narrow aortic root) that left little room to accommodate the aortic leaflets also were potential risk factors for coronary obstruction after TAVI.3,8 In fact, in the present study, the mean diameter of the aortic root was 28.0mm, comparable to that observed in patients with coronary obstruction in native aortic valves (28.0mm vs. 31.9mm in controls.; P < 0.001).8 The clinical characteristics of patients who had coronary obstruction following TAVI-ViV revealed a mean age (82years) and risk profile (mean STS-PROM score=9.4%) similar to those reported in most previous studies on TAVI. Conversely, up to 71% of patients who had this complication were women, a little higher value compared to the prevalence of~50% of women in most studies on TAVI.3 It has been previously demonstrated that women generally have lower coronary ostium heights and a smaller diameter of aortic roots when compared to men, which could partly explain the higher incidence of complications in females.14,15 Moreover, it has been shown that the position of LMCA ostium is lower, when compared to the right coronary artery, which could also explain why this complication involves most often the LMCA.12−14,16,17
Regarding the characteristics of the procedure, most of the reported cases of patients who had coronary obstruction following TAVI in native aortic valves received a balloon expandable prosthesis (Table 5). In the present study, despite having found more cases with the balloon expandable valves, the authors had no domain over the entire population at risk to take definitive conclusions if this complication is also more frequent with balloon expandable valves in the TAVI-ViV context. Furthermore, in a recent TAVI-ViV Global Registry, the risk of coronary obstruction was similar with both types of valves (3.2% of cases with self-expandable prosthesis versus 3.8% for balloon-expandable prosthesis). Future studies will have to assess whether there is an interaction between the type of valve and the risk of coronary obstruction in the context of TAVI-ViV.
Clinical picture and conduct in coronary obstruction after TAVIAll patients had significant hypotension that persisted after the valve implantation, and approximately 50% of them exhibited ST segment changes. This clinical finding can be explained by the fact that LMCA was the most commonly involved vessel.3,8 Likewise, in the presence of significant and sustained hypotension after implantation of the valve, especially in the presence of an electrocardiographic change, aortographic and/ or echocardiographic studies should be immediately performed to verify the presence of coronary obstruction or of a new abnormality of segmental contraction, respectively.
In the present study, PCI was attempted in all patients, but this procedure was not successful in approximately half of them. Importantly, the failure of PCI was associated with in-hospital mortality in all cases, which led to a high mortality rate (around 50%). This high mortality rate is similar to that reported in a recent multicenter registry on coronary obstruction (41%) and also reported in TAVI – ViV global registry (57.1%).4,8 These very high mortality rates reinforce the fact that this procedure should be performed in highly experienced centers with the necessary surgical resources, in order to quickly restore the coronary flow, either by PCI or even coronary artery bypass graft surgery, in case of PCI failure. Additionally, as the cannulation of the coronary artery could be not an easy task due to the presence of the transcatheter prosthesis metal structure, or even of the valve complex, especially in patients with a low coronary ostium and a narrow sinus of Valsalva, the coronary protection with a wire guide (Figure 2) or even the preventive positioning of a coronary stent could avoid the deleterious effect of this complication.7
– (A) Coronary protection with guide wire to facilitate access and stent placement in case of coronary obstruction after aortic valve transcatheter implantation for the treatment of bioprosthesis dysfunction. (B) Interaction between the guide wire and the transcatheter heart valve.
This trial has the limitations inherent to a systematic review, which collects only the information described in publications, in such a way that relevant information that may be omitted in these publications could add important data about this complication for this specific subgroup. Furthermore, imaging data (especially CT) were not available in all cases reported. Finally, all articles retrieved were case reports or small series of cases, preventing a comparison with all the population under risk submitted to TAVI.
CONCLUSIONSCoronary obstruction remains a rare complication of TAVI, but this is a potentially fatal event and appears be more frequent in the context of ViV procedures. The baseline characteristics of the reported cases suggest that this complication occurs more often in women previously submitted to the implantation of a supportless aortic prosthesis (stentless), or of supported prostheses with externally mounted leaflets. The inherent characteristics of such prostheses, for instance, long leaflets, a shallow suture annulus, a supra-annular position, and lack of support for the rods, along with anatomical features like low-position ostia and a shallow sinus of Valsalva, may facilitate the interaction of the prosthesis with the aortic wall and the coronary ostium. Regarding the clinical picture, the occurrence of a significant and sustained hypotension and of ST segment changes immediately after valve implantation requires the exclusion of this complication. Percutaneous coronary intervention was possible in~60% of patients, with a high mortality rate, reinforcing the fact that these procedures should be performed only in highly specialized centers and that additional strategic measures, such as the protection of a guide wire, could be adopted in the presence of risky clinical or anatomic parameters. Further prospective studies, including consecutive series of patients undergoing ViV transcatheter implantation of aortic valve with this complication, are needed to better assess the predictors and the most appropriate clinical conduct for this important complication.
FINANCIAL SUPPORTHBR obtained a grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for doctorate research. LNF received a research grant from the Fundación Mutua Madrileña (Spain).
CONFLICT OF INTERESTSDr. Robert DeLarochellière is a consultant for St. Jude Medical®. Dr. Éric Dumont is a consultant for Edwards Lifesciences. Dr. Josep Rodés-Cabau is a consultant for Edwards Lifesciences and St. Jude Medical®. The other co-authors declare to have no conflicts of interest.