Transcatheter aortic valve implantation (TAVI) is a treatment option for patients with aortic valve stenosis (AS) and high or prohibitive surgical risk. We report our experience using the Medtronic CoreValve™ self-expending system.
MethodsFrom 2009 to 2013, 51 consecutive patients with severe symptomatic AS and high or prohibitive surgical risk were submitted to TAVI. Results were analyzed according to the criteria of the Valve Academic Research Consortium (VARC) –2.
ResultsMean age was 82±6 years, 49% were female, 19% were diabetic, 21% had renal failure and the logistic EuroScore was 17.4±11.4%. The success rate of the device was 84.3%. All of the patients had a significant decrease of transaortic gradients, which was maintained over time. Hospitalization time was 6 days (interquartile range: 5-8.8). In-hospital mortality at 30days as 7.8% and 9.8%, respectively. Permanent pacemaker implantation was required in 32.6% of the cases; ischemic stroke was observed in 3.9% and major vascular complications in 6% of the patients. Survival at 6 months and 1 year was 86.3% and 84.4%, respectively. NYHA functional class improved significantly after TAVI and remained low in the medium-term follow-up.
ConclusionsIn this preliminary experience, the treatment of patients with AS and high or prohibitive surgical risk with TAVI, using the CoreValve™ self-expanding system was feasible and safe and led to sustained improvement of cardiac symptoms. After overcoming the initial risks of death and stroke, the procedure guaranteed good long-term clinical outcomes.
Sobrevivência a Médio Prazoe Estado Funcional de Pacientes com Estenose Valvar Aórtica Grave Submetidos a Implante Transcateter da Válvula Aórtica
IntroduçãoO procedimento de implante transcateter da válvula aórtica (TAVI, do inglês transcatheter aortic valve implantation) representa opção de tratamento em pacientes com estenose valvar aórtica (EA) com risco cirúrgico elevado ou proibitivo. Relatamos nossa experiência usando o sistema autoexpansível Medtronic CoreValve®.
MétodosNo período de 2009 a 2013, 51 pacientes consecutivos com EA grave sintomática e risco cirúrgico alto ou proibitivo foram submetidos ao TAVI. Os resultados foram analisados de acordo com os critérios Valve Academic Research Consortium (VARC) –2.
ResultadosA média de idades dos pacientes foi 82±6 anos, 49% eram do sexo feminino, 19% diabéticos, 21% tinham insuficiência renal e o EuroSCORE logístico foi 17,4±11,4%. O sucesso do dispositivo foi alcançado em 84,3%. Todos os pacientes tiveram diminuição significativa dos gradientes transaórticos, que foi mantida ao longo do tempo. A internação hospitalar foi de 6 dias (intervalo interquartil: 5-8,8). A mortalidade intra-hospitalar e aos 30 dias foi 7,8% e 9,8%, respectivamente. Implante de marca-passo permanente foi necessário em 32,6% dos casos; acidente cerebrovascular isquêmico ocorreu em 3,9%; e complicações vasculares maiores em 6% dos pacientes. A sobrevivência aos 6 meses e em 1 ano foi 86,3% e 84,4%, respectivamente. A classe funcional NYHA melhorou significativamente após o TAVI e permaneceu baixa no seguimento de médio prazo.
ConclusõesNesta experiência preliminar, o tratamento de pacientes com EA e risco cirúrgico alto ou proibitivo com TAVI, usando o sistema autoexpansível CoreValve®, foi considerado viável e seguro, e levou à melhoria sustentável dos sintomas cardíacos. Após a superação dos riscos iniciais de morte e de acidente cerebrovascular, o procedimento garantiu um bom resultado clínico, no longo prazo.
The calcified aortic valve stenosis (AS) is a common heart problem that is particularly prevalent in the elderly population. For several decades, surgical aortic valve replacement has been the mainstay of treatment for symptomatic AS, and it is considered a Class I recommendation in international guidelines.1 In selected individuals, the surgery brings considerable improvement of symptoms and in life expectancy.1 Nonetheless, there is a considerable proportion of elderly individuals with AS to whom a surgical alternative is not offered due to the high or prohibitive risk.1
Due to the unmet need for treatment of this highrisk subgroup, minimally invasive procedures began to emerge. The transcatheter aortic valve implant (TAVI) is a percutaneous procedure which is a therapeutic option for patients with AS with no surgical possibility.2
Since the first TAVI in 2002, over 70,000 procedures were performed with a high rate of success. However, the basic demographic data and the characteristics of the procedure, as well as the clinical results, may differ in Argentina when compared with those found in the rest of the world. To date, there is only limited data regarding the feasibility and safety of TAVI in this country.3−5 Thus, this study presents an initial experience with TAVI using the Medtronic CoreValve® self-expanding system (Medtronic Inc. – Minneapolis, USA).
METHODSPatient population and diagnostic proceduresBetween March of 2009 and March of 2013, 51 consecutive patients with severe symptomatic AS and high or prohibitive surgical risk underwent TAVI at this institution.
A multidisciplinary team determined the eligibility for TAVI based on the clinical situation and transesophageal echocardiography (TEE), on selective coronary angiography, on aortography, and on iliofemoral angiography. TEE was performed in all patients, whereas multiple detectors computed tomography (MDCT) was performed in only a few, to assess the anatomy of the aortic root (including the assessment of the aortic ring) and the feasibility of a transfemoral approach.
Severe AS was defined as an area of the aortic valve <0.8cm2, besides a mean aortic valve gradient >40mmHg, or a maximal aortic jet velocity >4.0m/s.
Patients were considered at high risk of surgical complications or death based on coexisting conditions associated with the risk of death of, at least, 15% at 30days after the procedure (Society of Thoracic Surgeons score [STS]≥10 or Logistic EuroSCORE≥15).
The anatomical inclusion criteria were as follows: orifice of the aortic valve with area <0.8cm2, diameter of the aortic annulus≥20mm and≤29mm, and diameter of ascending aorta to the level of the sinotubular junction≤45mm.
Patients were excluded if there was intracavitary thrombus; extremely low (<20%) and irreversible left ventricle ejection fraction (LVEF); severe mitral or aortic regurgitation (4 +); iliofemoral arterial axis with a bore <6mm or severe tortuosity, which could prevent the delivery of the prosthesis; or low implantation of coronary ostium (<10mm).
The success of the device was defined as the absence of mortality in the procedure and a correct positioning of a single prosthesis in the proper location, and with adequate performance (no prosthesis-patient mismatch; mean aortic valve gradient <20mmHg or maximum speed <3m/s; and without moderate/severe regurgitation of the valve prosthesis).
All patients provided informed consent to be submitted to TAVI by transfemoral route, including consent for anonymous processing of their data. Information on death was obtained at the hospital or by telephone contact with the patient’s family. All events were defined according to the criteria from Valve Academic Research Consortium (VARC) −2.6
Implantation procedureAll procedures were performed under general anesthesia, using the 18F self-expanding CoreValve® (Medtronic – Minneapolis, Minnesota, United States) prosthesis. Briefly, the CoreValve® prosthesis consists of porcine pericardial tissue mounted on a self-expanding nitinol stent.
The procedures were initially performed with a 18F release catheter which was later enhanced by an AccuTrak® stabilizing membrane. Three sizes of prostheses were available (26, 29, and 31mm) for the sizes 20 and 29mm of the aortic valve annulus. Details of the device and technical aspects of the procedure were previously published.7
The transarterial access was obtained percutaneously or by surgical incision. After obtaining vascular access, a guide wire of 0.35” (a normal J wire or a straight wire, when appropriate) was positioned in the left ventricle and two pigtails were placed in the ascending aorta and left ventricle, to perform simultaneous measurements of pressure and transvalvular gradient evaluation. The intraventricular guide wire was then exchanged for a high support wire with a customized curve, and positioned at the apex. The positioning and implantation of the prosthetic valve into the aortic ring were guided by fluoroscopy and TEE. The pre-dilation was left to the discretion of the operator. The gradual release of the prosthesis was taken by retraction of the sheath. A post-dilation was performed in cases of device underexpansion. The femoral access was surgically or percutaneously closed with two Perclose ProGlide® Suture-Mediated 6F Closure Systems (Abbott Vascular – Redwood City, United States).
MedicationAll patients were medicated with acetylsalicylic acid before the procedure and continued to receive this drug indefinitely. The patients were also medicated with a loading dose of clopidogrel 300mg. The maintenance dose of clopidogrel, administered for three months, was 75mg. During the procedure, unfractionated heparin was administered according to the patient’s weight, to achieve activated clotting time≥250sec.
Follow-upClinical and echocardiographic examinations were performed before hospital discharge, at 30days, six months, and 12 months. The results were analyzed according to updated standard outcomes, defined by VARC-2 criteria.
Statistical analysisQualitative variables were expressed as percentages, and quantitative variables as mean±standard deviation, or as median (interquartile range [IQ], 25% to 75%). Categorical variables were compared with the chi-squared test. To compare the mean and maximum serial gradients, the t-paired test was used. The statistical analysis was performed using SPSS, version 21.0 (IBM – Armonk, New York, United States).
RESULTSPatient characteristicsThe baseline characteristics are summarized in Table 1. All patients had dyspnea, and most of them at rest or after minimal physical activity. The mean age was 82±6 years, 49% were female, 19% were diabetic, and 21% had chronic renal failure. The mean logistic EuroSCORE was 17.4±11.4%, and 17% of patients had comorbidities not measured in the current surgical risk scores: four patients (7.8%) had porcelain aorta, three patients (5.8%) had severe chest wall deformity or previous thorax irradiation, and one patient underwent previous liver transplantation in immunosuppressive therapy. Table 1 shows the baseline echocardiographic parameters, illustrating the severity of AS.
Patient characteristics (n=51)
| Age, years | 82±5.7 |
| Female, n (%) | 25 (49) |
| Body mass index, kg/m2 | 27±4 |
| Logistic EuroSCORE, % | 17.4±11.7 |
| STS score*, % | 10.2±9 |
| NYHA functional class **, n (%) | |
| II | 6 (11.8) |
| III | 18 (35.3) |
| IV | 27 (52.9) |
| Hypertension, n (%) | 43 (84.3) |
| Dyslipidemia, n (%) | 35 (68.6) |
| Diabetes mellitus, n (%) | 10 (19.6) |
| Smoking, n (%) | 15 (29.4) |
| Prior stroke, n (%) | 4 (7.8) |
| Atrial fibrillation, n (%) | 11(21.6) |
| Permanent pacemaker, n (%) | 6 (11.8) |
| Previous angioplasty, n (%) | 16 (31.4) |
| Previous heart surgery, n (%) | 8 (15.7) |
| Chronic obstructive pulmonary disease, n (%) | 5 (9.8) |
| Chronic renal failure, n (%) | 11 (21.6) |
| Peripheral arterial disease, n (%) | 11 (21.6) |
| Fragility, n (%) | 17 (33.3) |
| Echocardiographic findings | |
| Aortic valve area, cm2 | 0.5±0.2 |
| Mean aortic valve gradient, mmHg | 50±10 |
| Left ventricle ejection fraction, % | 55±13 |
| Aortic annulus size, cm2 | 23±2 |
Of a total of 51 patients, 82.4% underwent elective procedures and the remaining were submitted to urgent procedures. The total percutaneous access was performed in 43% of cases, and most procedures (50/51) were guided by TEE (Table 2).
Results of procedure and follow-up (n=51)
| Results | |
|---|---|
| Procedure, n (%) | |
| Elective | 42 (82.4) |
| Urgent | 9 (17.6) |
| Prosthesis labelled size in mm, n (%) | |
| 26 | 22 (43.1) |
| 29 | 26 (51.0) |
| 31 | 2 (3.9) |
| General anesthesia, n (%) | 51 (100) |
| Transesophageal echocardiography, n (%) | 50 (98.0) |
| Fully percutaneous procedure, n (%) | 22 (43.1) |
| Pre-dilation, n (%) | 28 (54.9) |
| Post-dilation, n (%) | 19 (37.3) |
| Success of the device*, n (%) | 43 (84.3) |
| Death, n (%) | |
| Intraprocedure | 2 (3.9) |
| In-hospital | 4 (7.8) |
| 30days | 5 (9.8) |
| Six months | 7 (13.7) |
| One year | 8 (15.7) |
| Aortic regurgitation, n (%) | |
| ≥ Grade 2 postprocedure | 6 (11.6) |
| ≥ Grade 2 within 1 year | 5 (9.8) |
| Stroke, n (%) | 2 (3.9) |
CoreValve® devices of 26 and 29mm were implanted in 44% and 52% of patients, respectively. The CoreValve® device of 31mm has been available since December 2012, and was used in two cases (4%). Pre-dilation with balloon was performed in 28 patients (54.9%), while 19 (37.3%) required post-dilation due to valve underexpansion immediately after its implantation.
84.3% of patients (43/51) achieved success with the device. A dramatic decrease of the maximum (83±22 to 13±5mmHg; P <0.001) and mean (50±10 to 6.4±2.6mmHg; P <0.001) transvalvular gradients was observed by echocardiography immediately after the procedure. At the end of the procedure, no patient showed severe aortic regurgitation; its occurrence was moderate in six (11.8%) and mild in 32 patients (62.7%).
Two patients died during the procedure (one due to ring rupture during the pre-dilation, and the other immediately before the pre-dilation, due to a secondary hemodynamic instability). Two patients required a second valve (one of them due to prosthesis embolization in the aortic arch and the other due to a deep annular implant associated with severe aortic regurgitation).
Results in-hospital and at 30daysThe median hospital stay was six days (IQ=5–8.8), and 32.6% of patients received a permanent pacemaker. The in-hospital mortality and at 30days for all causes were 7.8% (4/51) and 9.8% (5/51), respectively. Two patients (3.9%) had ischemic stroke. Major vascular complications occurred in three patients (5.9%).
Follow-upThe initial reduction of the observed maximum and mean gradients, after the procedure, was maintained throughout the follow-up (Figure 1).
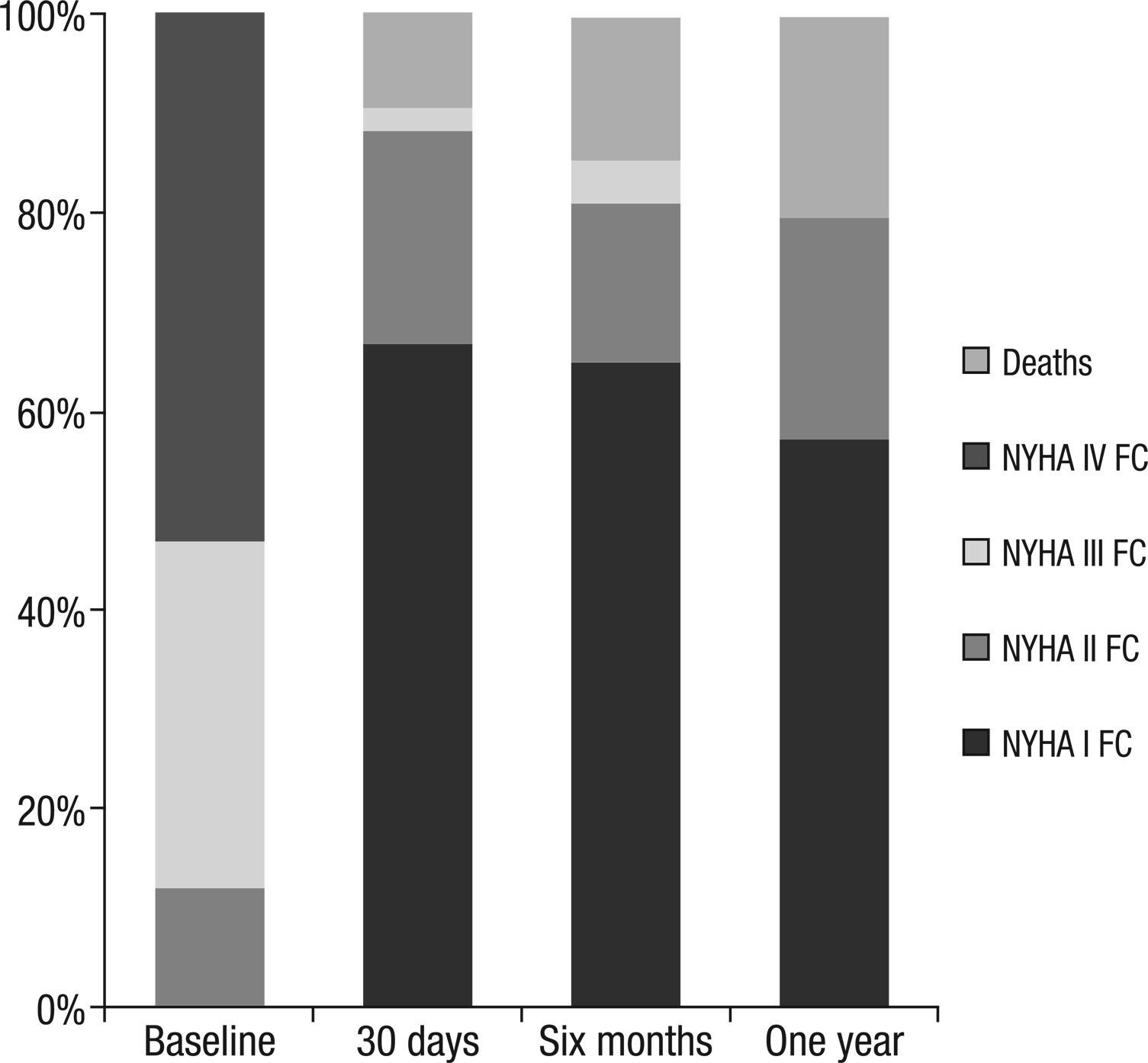
The survival at six months and one year was 86.3% and 84.4%, respectively. The NYHA functional class improved significantly after TAVI and remained low in the long-term follow-up (Figure 2). At one year, no patient had severe aortic regurgitation, whereas 5/43 patients (11.6%) had moderate aortic regurgitation.
DISCUSSIONSymptomatic AS is a heart disease associated with an impaired functional status and that portends a poor prognosis.1 For decades, older individuals with this disease did not receive an appropriate treatment, due to the presence of comorbidities and to the high surgical risk. The present study reports an initial experience with TAVI, a minimally invasive alternative procedure for the definitive treatment of patients with symptomatic AS who are at high or prohibitive surgical risk.
In the present study, the mortality at 30days was 9.8%, which is consistent with other TAVI registries that used CoreValve® systems (United Kingdom registry: 7.1%; France registry: 12.7%; France-2 registry: 9.4%; and Germany registry: 12.4%),8−10 reflecting the feasibility and safety of the procedure in the patient population in the clinical practice with AS, which usually remains without a proper treatment. The mortality rate in this study at 30days was slightly higher than that observed in a recently published Brazilian trial (6.7%). Unlike the present study, the authors performed TAVI without pre-dilation with balloon, which may have helped to improve the short-term survival of their patients.4 Subsequently, a Brazilian TAVI registry (n=112) with patients undergoing a mandatory pre-dilation, showed a similar mortality (9.4%).3 Also in line with previous registries,8,11 the survival at one year was 84.4%. The present study also reported a significant decrease in the gradient of the aortic valve, which resulted in a sustained decrease in heart symptoms after TAVI. Therefore, after an initial exposure to a risk, patients who survived had a considerable improvement in quality of life and an excellent clinical outcome in the long-term, confirming the durability of the device.
In the present study, the high surgical risk (logistic EuroSCORE: 17.4±11.4%) would have contraindicated the surgical valve replacement procedure or led to excessive mortality and morbidity consequent to the surgery. Apparently, the risk of TAVI is reasonable, given the absence of a definitive alternative treatment and the poor prognosis observed with an exclusive medical treatment. According to contemporaneous TAVI registries,12−15 the number of cardiovascular adverse events decreased with advances in device technology and the growing experience of the surgeons and of the heart team.
There is considerable evidence indicating that the paravalvular aortic regurgitation after TAVI may lead to a worse prognosis.16,17 According to previous series, the paravalvular aortic regurgitation after TAVI is present in 48 to 93% of cases,18,19 while the rate of moderate aortic regurgitation is around 14-21%.18,19 In line with these trials, 12.2% of the present patients had moderate aortic regurgitation after TAVI. Likewise, a Brazilian experience in 112 patients showed a rate of moderate/severe aortic regurgitation of 11.6% after the procedure.3 In the initial experiences with TAVI, the majority of centers conducted pre-procedural basal assessments with two-dimensional echocardiography; however, several trials have subsequently demonstrated that often the aortic annulus is an elliptical structure and that, in consequence, the two-dimensional echocardiographic evaluation is inaccurate, because it assumes that the ring is circular. More recente trials shown that a more precise assessment of the aortic annulus allows for a better selection of the prosthesis size, which translates into a smaller paravalvular leak.20,21 Consequently, current guidelines indicate that pre-assessment procedures should be performed with three-dimensional echocardiography or MDCT, to obtain an accurate measurement of the perimeter and the area of the ring.1,20,21 Moreover, a new generation of prostheses with better sealing proprieties and the ability to be repositioned and recaptured will probably be able to reduce the paravalvular leak.22−24
The implantation of a pacemaker remains a cause for concern, especially after the implantation of CoreValve® device. According to previous trials with CoreValve®, approximately one-third of patients require a permanent pacemaker.25−28 Interestingly, the need for a permanent pacemaker appears to be not detrimental to the survival of patients undergoing TAVI.27,29 The presence of a new left bundle branch block and/or a QRS duration >120ms after release of the prosthesis can be a predictor for the development of a high degree AV block; besides, this finding will help guiding the clinical decision. However, accurate clinical indications, related to the implantation of a pacemaker after TAVI, are still under development.
So far, the reported rates of ischemic stroke after TAVI have remained around 1 to 10%.30,31 In the present studies, two patients suffered an ischemic stroke, one with a fatal hemorrhagic transformation in the context of a systemic inflammatory response syndrome and of severe thrombocytopenia. The second patient also had a hemorrhagic transformation, but with mild neurological involvement.
CONCLUSIONSIn this preliminary experience from a single center, treating patients with AS and high or prohibitive surgical risk through TAVI using the self-expanding CoreValve® system was considered feasible and safe, leading to a sustainable improvement of heart symptoms. After overcoming the initial risk of death and stroke, the procedure ensured a good clinical outcome in the long term.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.