Radial access is effective in reducing bleeding complications in percutaneous coronary interventions (PCI). In acute coronary syndromes (ACS), the crossover from low molecular weight to unfractioned heparin increases the risk of bleeding after transfemoral PCI. The aim of this study was to evaluate the incidence of bleeding in patients with ACS undergoing PCI using the radial approach according to the occurrence or not of crossover of heparin therapy.
MethodsObservational study of patients with ACS undergoing PCI using the radial access, divided into groups: A (with crossover) and B (without crossover). Bleeding events were classified according to GUSTO criteria; EASY criteria were used for bleeding events in the radial access.
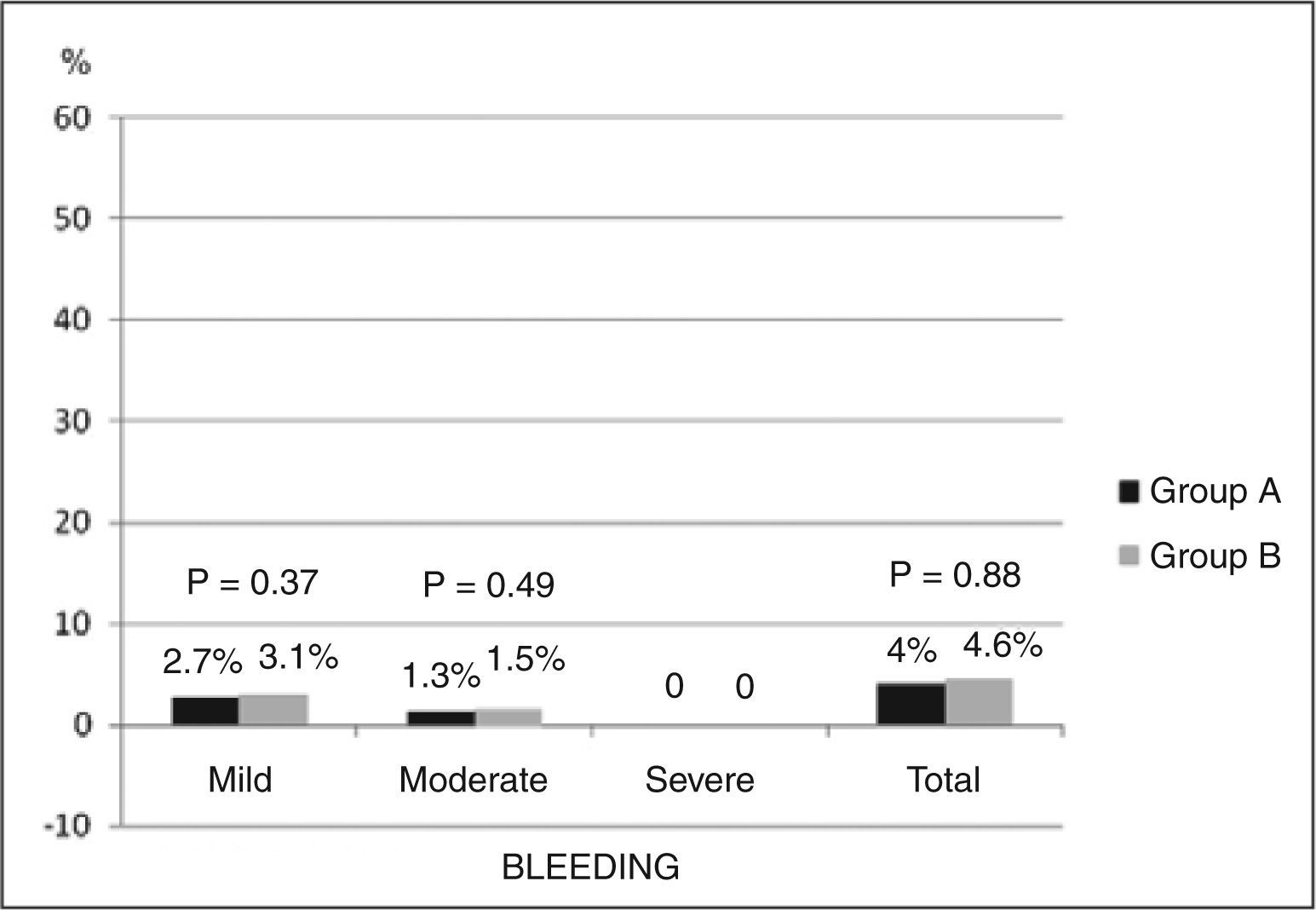
ResultsWe included 140 consecutive patients, 74 in group A and 66 in group B, with mean age of 59.7±9.4years, 72.8% were male, and 21.4% diabetic. There were six cases of bleeding complications (4.3%), and two (1.4%) were classified by GUSTO as moderate (one digestive bleeding and one femoral bleeding after intraaortic balloon pump), and four (2.9%) were classified as mild (hematomas with diameter≤5cm). In the comparison between groups, moderate bleeding occurred in 1.3% in group A and 1.5% in group B (P=0.49) and mild bleedings in 2.7% and 3.1% in groups A and B, respectively (P=0.37).
ConclusionsIn this cohort of patients with ACS undergoing PCI using the radial access, a low incidence of bleeding events was observed, and most of these bleeding complications resulted from small hematomas of the access route. Crossover of heparin therapy did not increase the risk of bleeding after transradial PCI.
Crossover da Terapia com Heparina e Risco de Sangramentona Intervenção Coronária Percutânea Transradial na Síndrome Coronária Aguda
IntroduçãoA utilização da via radial tem reconhecida eficácia em reduzir complicações hemorrágicas na intervenção coronária percutânea (ICP). Nas síndromes coronárias agudas (SCAs), o crossover (transição) entre a heparina de baixo peso molecular e a heparina não-fracionada aumenta o risco hemorrágico após ICP por via femoral. O objetivo deste estudo foi comparar a incidência de sangramentos em pacientes com SCA submeti dos a ICP por via radial de acordo com a ocorrência, ou não, do crossover da terapia com heparina.
MétodosEstudo observacional de pacientes com SCA submetidos a ICP por via radial, divididos em grupos A (no qual ocorreu crossover) e B (sem crossover). Os eventos hemorrágicos foram classificados conforme os critérios do GUSTO; para os sangramentos da via de acesso radial, foram utilizados os critérios do EASY.
ResultadosForam incluídos 140 pacientes consecutivos, 74 pacientes no grupo A e 66 pacientes no grupo B, com média de idade de 59,7±9,4 anos, 72,8% do sexo masculino e 21,4% diabéticos. Ocorreram seis casos de complicações hemorrágicas (4,3%), sendo duas (1,4%) classificadas pelo GUSTO como moderadas (uma hemorragia digestiva e um sangramento femoral após balão intra-aórtico) e quatro (2,9%), como leves (hematomas com diâmetro≤5cm). Na comparação entre os grupos, sangramentos moderados ocorreram em 1,3% no grupo A e em 1,5% no grupo B (P=0,49) e sangramentos leves, em 2,7% e em 3,1% nos grupos A e B, respectivamente (P=0,37).
ConclusõesNesta casuística de pacientes com SCA submetidos a ICP pela via radial, foi observada baixa incidência de sangramentos, sendo a maioria das complicações hemorrágicas decorrente de pequenos hematomas da via de acesso. O crossover da terapia com heparina não conferiu risco aumentado de sangramentos após ICP pela via radial.
The adverse impact of bleeding events after percutaneous coronary intervention (PCI) has been consistently demonstrated in several studies, 1-3 and such events are equivalent to acute myocardial infarction (AMI) as a predictor of mortality in the short and middle term. 4,5 Transradial PCI approach is currently one of the main strategies to reduce bleeding, 6,7 as most observed bleeding events are associated with the femoral access route. It has been shown that the benefit of the transradial approach is even more marked in patients with acute coronary syndrome (ACS), considering the wider spectrum of antithrombotic drugs and higher risk of bleeding complications in these patients. 8,9
A subanalysis of the Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors (SYNERGY) study demonstrated that the crossover of heparin therapy (crossover or transition between low-molecular-weight heparin and unfractionated heparin) is associated with greater risk of bleeding after PCI using the femoral access route. 10,11 However, the potential risk of bleeding complications associated with the crossover of heparin therapy has not been investigated in patients undergoing transradial PCI approach. Thus, the primary objective of this study was to evaluate the incidence and characteristics of bleeding complications in patients with ACS undergoing transradial PCI approach, according to the presence or absence of crossover between low-molecular-weight heparin and unfractionated heparin.
METHODSStudy design and populationThis was a retrospective analysis of observational data from patients admitted with a diagnosis of ACS with or without ST-segment elevation who underwent coronary angiography and primary or ad hoc PCI via the transradial approach at Instituto Dante Pazzanese de Cardiologia (São Paulo, SP, Brazil). Cases that required changing of the vascular access from radial to femoral or those who were in cardiogenic shock were excluded. The clinical characteristics, procedures, and hospital outcomes had been prospectively collected since 2007, and made available in the database of the Department of Invasive Cardiology of the institution. All patients signed an informed consent prior to the PCI. The study was approved by the Research Ethics Committee of the hospital.
ProceduresIn all patients who underwent the radial puncture technique to obtain access with hyperextension of the wrist and infiltration of 0.5 to 2mL of 2% lidocaine, the radial artery was punctured 1cm proximal to the styloid process of the radius using a needle with a 20/22-gauge Jelco™ polyethylene catheter and the Seldinger technique. After puncture, a 0.021-inch guide wire was introduced, followed by small skin incision with a #11 scalpel blade; then, a 6F introducer was inserted (Terumo Glidesheath, Terumo Corporation – Tokyo, Japan). A solution containing 5,000IU heparin sulfate was administered through the introducer to all patients undergoing procedures through radial approach, according to the service routine. This local heparinization is performed in all patients to prevent thrombosis of the radial artery. After the end of the procedure, the introducer was immediately removed, and hemostasis was achieved using a manual compression dressing consisting of gauze and an elastic bandage or radial compression strap (TR Band™, Terumo Corporation – Tokyo, Japan). Clinical examination of the puncture site, and radial pulse evaluation during hospitalization and at discharge were routinely performed.
After the antithrombotic drugs that were used before and during the PCI procedure were determined, two groups were defined according to the occurrence (group A) or not (group B) of crossover. Group A comprised patients who received full dose of low-molecular-weight heparin; the last dose was given 12 hours before PCI (enoxaparin 1mg/kg, subcutaneously, every 12 hours; in case of creatinine clearance<30mL/min or 0.75mg/kg, subcutaneously every 12 hours in case of age>75 years with no renal dysfunction). This group received unfractionated heparin in the catheterization lab and included not only patients who received 5,000IU of unfractionated heparin by introducer to prevent thrombosis of the radial artery, but also those who, in addition to the dose of unfractionated heparin received through the introducer, also received a complementary dose of unfractionated heparin intravenously (up to 100IU/kg or 70IU/kg in cases receiving glycoprotein IIb/ IIIa) when undergoing ICP. Group B included patients who did not receive low molecular-weight heparin within the 12 hours prior to PCI and who received only unfractionated heparin as an antithrombotic agent for coronary angiography and PCI.
The activated clotting time was not routinely monitored. All patients were taking acetylsalicylic acid (loading dose of 200mg to 300mg, followed by 100mg to 200mg daily) and clopidogrel (loading dose of 300mg to 600mg, followed by 75mg daily). None of the patients included in the study used any other antiplatelet drugs during hospitalization.
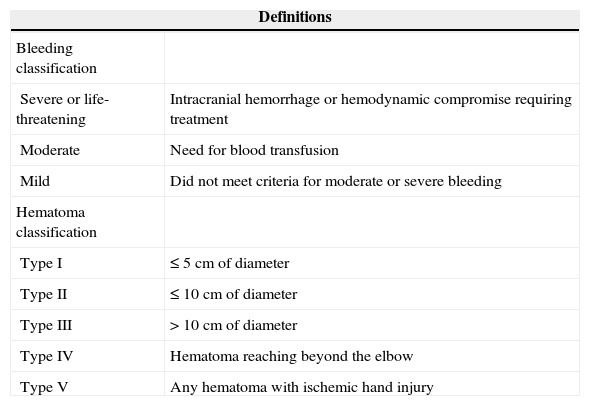
DefinitionsBleeding complications were evaluated as the primary endpoint of safety, using the criteria of the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO)12 study and those of the Early Discharge after Transradial Stenting of Coronary Arteries (EASY) classification 13 for the bleeding in the transradial access route (Table 1). All bleeding events at the radial puncture site or else-where that occurred from the beginning of PCI until discharge were considered hemorrhagic complications.
Bleeding criteria according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study and the Early Discharge after Transradial Stenting of Coronary Arteries (EASY) classification for hematoma at the puncture site
| Definitions | |
|---|---|
| Bleeding classification | |
| Severe or life-threatening | Intracranial hemorrhage or hemodynamic compromise requiring treatment |
| Moderate | Need for blood transfusion |
| Mild | Did not meet criteria for moderate or severe bleeding |
| Hematoma classification | |
| Type I | ≤ 5cm of diameter |
| Type II | ≤ 10cm of diameter |
| Type III | > 10cm of diameter |
| Type IV | Hematoma reaching beyond the elbow |
| Type V | Any hematoma with ischemic hand injury |
Chronic renal failure was considered when the plasma creatinine at hospital admission>1.4mg/dL or creatinine clearance<60mL/ minute.
Statistical AnalysisFor the descriptive data analysis, categorical variables were expressed as absolute frequencies and percentages, and were compared using the chi-squared test or Fisher’s exact test. Continuous variables were expressed as the mean and standard deviation and compared using Student’s t-test. Groups A and B were compared with regard to baseline clinical characteristics; clinical presentation; occurrence of major adverse cardiac events (MACE), i.e., composite outcome of death, AMI, and stroke; and the incidence and severity of bleeding (with the bleeding specified as originating or not originating from the puncture site). A comparative analysis was also performed in patients from group A regarding the incidence of bleeding in cases that required or did not require intravenous unfractionated heparin complement. SPSS release 13.0 was used for the statistical analyses, and P<0.05 was considered statistically significant.
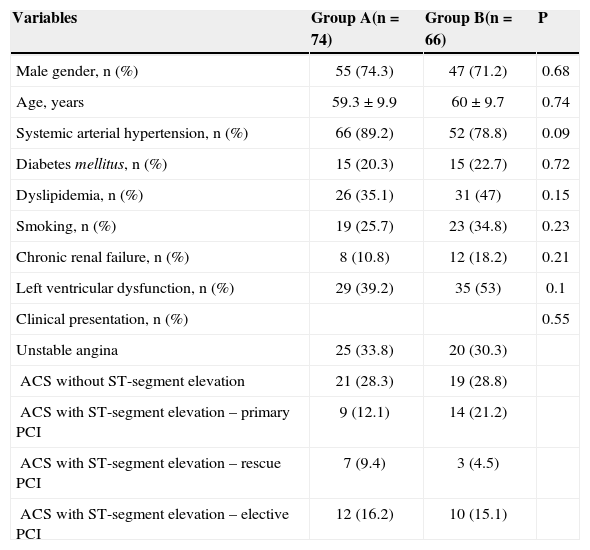
RESULTSBetween May 2008 and June 2010, 140 consecutive patients admitted with a diagnosis of ACS with or without ST-segment elevation and submitted to primary or ad hoc PCI by transradial approach were included in this analysis. Among these patients, 102 (72.8%) were males, the mean age was 59.7±9.4years, and 21.4% were diabetics. Group A consisted of 74 patients (52.9%), and group B consisted of 66 patients (47.1%). The mean length of hospitalization, which corresponded to the duration of patient follow-up, was 5.4±2.2days. The baseline characteristics and clinical presentations of patients are shown in Table 2.
Basal clinical characteristics and clinical presentation
| Variables | Group A(n=74) | Group B(n=66) | P |
|---|---|---|---|
| Male gender, n (%) | 55 (74.3) | 47 (71.2) | 0.68 |
| Age, years | 59.3±9.9 | 60±9.7 | 0.74 |
| Systemic arterial hypertension, n (%) | 66 (89.2) | 52 (78.8) | 0.09 |
| Diabetes mellitus, n (%) | 15 (20.3) | 15 (22.7) | 0.72 |
| Dyslipidemia, n (%) | 26 (35.1) | 31 (47) | 0.15 |
| Smoking, n (%) | 19 (25.7) | 23 (34.8) | 0.23 |
| Chronic renal failure, n (%) | 8 (10.8) | 12 (18.2) | 0.21 |
| Left ventricular dysfunction, n (%) | 29 (39.2) | 35 (53) | 0.1 |
| Clinical presentation, n (%) | 0.55 | ||
| Unstable angina | 25 (33.8) | 20 (30.3) | |
| ACS without ST-segment elevation | 21 (28.3) | 19 (28.8) | |
| ACS with ST-segment elevation – primary PCI | 9 (12.1) | 14 (21.2) | |
| ACS with ST-segment elevation – rescue PCI | 7 (9.4) | 3 (4.5) | |
| ACS with ST-segment elevation – elective PCI | 12 (16.2) | 10 (15.1) |
PCI, percutaneous coronary intervention; ACS, acute coronary syndrome.
Among the patients in group A, 32 (43.2%) received only unfractionated heparin through the introducer, without a supplemental dose, and the remaining 42 received an intravenous supplemental dose of unfractionated heparin. Glycoprotein IIb/IIIa inhibitors were administered to 37 patients (26.4%); 20 in group A and 17 in group B (27% vs. 25.7%, respectively; P=0.86).
Procedural success (residual stenosis diameter>30% in the final Thrombolysis in Myocardial Infarction [TIMI] flow 3) was achieved in 136 patients (Group A, 97.3%; Group B, 96.9%; P=0.38).
The incidence of bleeding complications in the study population was 4.3% (six events). Two of these events (1.4%) were classified as moderate by the GUSTO criteria (one upper gastrointestinal hemorrhage and bleeding in the femoral puncture site after use of an intra-aortic balloon; these cases received concomitant administration of glycoprotein IIb/IIIa). The four additional bleeding events (2.9%) occurred at the radial puncture site and were defined as mild bleeding according to GUSTO or type I according to the EASY study classification (hematomas with diameter<5cm). There were no differences between groups A and B regarding the occurrence of bleeding (Figure 1). In group A, there was no difference in the overall incidence of bleeding among patients who received only unfractionated heparin through the radial introducer and those who required a supplemental intravenous dose of unfractionated heparin (3.1% and 4.7%, respectively; P=0.72). No other complications related to the vascular puncture site, such as arteriovenous fistula, pseudoaneurysm, symptomatic radial artery thrombosis, or local infection were observed.
The rate of in-hospital MACE was similar between groups A and B (2.7% vs. 1.5%; P=0.39), and the incidence of isolated adverse outcomes was also similar between groups A and B (death, 1.4% vs. 1.5%; P=0.50; AMI, 1.4% vs. 0%; P=0.52); no strokes were recorded.
DISCUSSIONIn the authors’ experience, the routine use of transradial access in PCI is feasible, as demonstrated by the high rate of procedural success and safety of this access route. The low incidence of bleeding events due to the use of transradial access makes it an attractive strategy for subgroups prone to this complication. This study demonstrated a low occurrence of bleeding at the puncture site after transradial PCI in patients hospitalized because of ACS. It was also observed that these events consisted only of small hematomas that did not require any medical intervention and spontaneously resolved, thus requiring no additional costs, procedures, or prolonged hospitalization. These patients received antiplatelet and antithrombotic medications, according to the routine of the institution, and the use of unfractionated heparin in the catheterization laboratory (whether at full dose or not) did not affect the occurrence of bleeding, regardless of the recent use of enoxaparin at hospital admission.
The discussion regarding the superiority of particular antithrombin agents has long concerned the scope of PCIs, but different rates of periprocedural MACE have never been demonstrated when comparing the use of unfractionated heparin with that of low-molecular-weight heparin. Regarding vascular bleeding complications, conflicting results have been published in the literature comparing these two agents, with most studies reporting the reliability of dose-effect association, without the need for laboratory control, as the main advantage for low-molecular-weight heparin compared to unfractionated heparin.
However, crossover heparin therapy has been considered inappropriate in ACS since the publication of the SYNERGY trial, due to the higher frequency of bleeding complications without any benefits in terms of efficacy. In this study, there was no difference between the unfractionated heparin and low-molecular-weight heparin groups regarding the rate of death and AMI or regarding the composite endpoint of death or nonfatal AMI (14% vs. 14.5%). The rates of acute occlusion, failure, and emergency surgery were also similar. Although there was no difference in the rate of major bleeding determined by the GUSTO criteria (2.7% vs. 2.2%; P=0.08), patients in the enoxaparin group had higher rates of major bleeding according to the TIMI criteria (9.1% vs. 7.6%; P=0.008). 10 Among the patients included in the SYNERGY trial, 47% (n=4,687) underwent PCI, and antithrombotic therapy was administered before randomization in 78% (n=7,778). Despite the higher incidence of bleeding and need for transfusion in patients undergoing crossover, association or causality could not be established, as these crossovers occurred after randomization. However, the crossover of heparin therapy has been discouraged by the guidelines. 14
A recent meta-analysis compared the radial and femoral accesses in AMI and demonstrated a decrease of>70% in the risk of major bleeding for the transradial approach relative to the femoral approach (0.77% vs. 2.61%; odds ratio [OR] 0.30; 95% confidence interval [95% CI]: 0.16 to 0.55; P=0.0001). The reduction in the composite endpoint of death, AMI, and stroke was also significant (3.65% vs. 6.55%; OR=0.56; 95% CI, 0.39 to 0.79; P=0.01), and the analysis of the death outcome also showed benefits when using the radial access (OR 0.54; 95% CI, 0.33 to 0.86; P=0.01). A significant benefit was demonstrated when using the radial approach in this context, through the reduction of both post-procedural bleeding and ischemic complications.15 Notably, several studies have observed that bleeding complications are associated to an increased risk of MACE.16,17
The recent radial vs. femoral access for coronary intervention (RIVAL) study is the largest clinical investigation to date comparing the radial and femoral routes for coronary angiography and PCI. The RIVAL study was a randomized, multicenter, international study that included 7,021 patients and used outcomes such as death, AMI, stroke, and major bleeding unrelated to CABG surgery in 30 days. The primary outcome (combined events) was similar in both radial and femoral groups (3.7% vs. 4%; P=0.50), but there was significant benefit for radial access in centers with a higher volume of procedures performed through this approach (relative risk [RR], 0.49; 95% CI, 0.28 to 0.87; P=0.015)] and for patients with ACS with ST-segment elevation (RR, 0.60; 95% CI, 0.38 to 0.94; P=0.026). Although similar rates of major bleeding unrelated to CABG in 30 days (0.7% vs. 0.9%; P=0.23) have been reported, the radial group had a lower incidence of both local hematomas (RR, 0.40, 95% CI, 0.28 to 0.57; P=0.0001) and pseudoaneurysm requiring occlusion (RR, 0.30; 95% CI, 0.13 to 0.71; P=0.006). 18
The authors believe that the greatest benefit of using the transradial access for PCI in ACS cases is the greater freedom in the use of antithrombotic drugs. Although this study did not have sufficient power to support the hypothesis, it is thought that it is possible to decrease the risk of bleeding complications by using the radial approach when performing crossover heparin therapy.
Study limitationsAlthough it provides important information on heparin management when using the transradial access in the daily routine of PCIs in Brazil, this study has limitations. The main limitation is the small sample size, which makes it impossible to confirm the equality of the bleeding rates between the groups. Furthermore, the study has a retrospective design, which may allow the selection of less complex patients for crossover heparin therapy by the interventional physician. Patients who used low-molecular-weight heparin<12 hours after the crossover were not evaluated. Variables that may influence the bleeding risk, such as the number of treated vessels and time of procedure, were not compared. The GUSTO bleeding criteria were employed because this classification is easy and convenient to use, although it does not represent the ideal or most reliable model.
CONCLUSIONSIn this study of patients with ACS with or without ST-segment elevation and treated through the radial approach, crossover heparin therapy was safe, with a low incidence of bleeding associated with the puncture site, and no increase in the incidence of other types of bleeding. There are no validated algorithms for crossover heparin therapy in ACS, but the crossover can be implemented without fear of bleeding at the radial puncture site when this is the route of choice.
CONFLICT OF INTERESTThe authors declare no conflicts of interest.