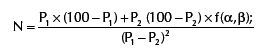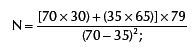Primary percutaneous coronary intervention is currently the preferred method to treat patients with STsegment elevation acute myocardial infarction. The no-reflow phenomenon, which is the inability to reperfuse a region of the myocardium after restoration of patency of a previously occluded epicardial coronary artery, is observed in a considerable proportion of these patients. The benefit of IIb/IIIa glycoprotein inhibitors, blocking the final common pathway of platelet aggregation, has been suggested in studies of acute coronary syndromes, but their actual efficacy in the context of no-reflow in patients treated with primary percutaneous coronary intervention remains unclear.
MethodsThe aim of this multicenter, double-blinded, placebo controlled study is to assess the impact of the early administration of the low molecular weight glycoprotein IIb/ IIIa inhibitor tirofiban on the incidence of no-reflow using angiographic and electrocardiographic methods to determine: (1) the epicardial coronary flow, using the TIMI score, and the microcirculatory flow, using the MBG score of opacification and myocardial flow; (2) the resolution of the ST segment elevation, as the final index of the success of reperfusion.
ConclusionsIf the decrease in no-reflow incidence at 90 minutes and 24 hours after primary percutaneous coronary intervention is confirmed, this pilot study should guide the implementation of a larger study to investigate the possible impact of the systematic inhibition of the final common pathway of platelet aggregation on the mortality of ST-segment elevation acute myocardial infarction patients.
A Inibição da Via Final Comum da Agregação Plaquetária Reduz o Fenômeno de Não Reperfusão Durantea Intervenção Coronária Percutânea Primária? Tirofiban no Infarto Agudo do miocárdio e a não Reperfusão (TIARA)
IntroduçãoA intervenção coronária percutânea primária é hoje o método preferencial de reperfusão na abordagem de pacientes com infarto agudo do miocárdio com supradesnivelamento do segmento ST. Em boa parte desses casos, ocorre o fenômeno de não reperfusão, que é a incapacidade de se reperfundir uma região do miocárdio após o restabelecimento da patência de uma artéria coronária epicárdica previamente ocluída. O benefício de inibidores da glicoproteína IIb/IIIa, bloqueando a via final comum da agregação plaquetária, tem sido sugerido em estudos de síndromes coronárias agudas, mas persistem pontos obscuros quanto à sua real eficácia, no contexto da não reperfusão, em pacientes tratados com intervenção coronária percutânea primária.
MétodosInvestigação multicêntrica que avaliou o impacto da administração precoce do inibidor da glicoproteína IIb/IIIa de baixo peso molecular tirofiban, em forma duplo-mascarada, controlada por placebo, sobre a ocorrência de não reperfusão, empregando métodos angiográficos e eletrocardiográfico para documentar (1) os fluxos coronário epicárdico, pelo escore TIMI, e microcirculatório, pelo escore MBG de opacificação e escoamento miocárdicos; (2) a resolução do supradesnivelamento do segmento ST, como índice final do sucesso da reperfusão.
ConclusõesSe comprovada redução da incidência de não reperfusão tanto 90 minutos como 24 horas após a intervenção coronária percutânea primária, este estudo-piloto, deve nortear a implementação de estudo mais abrangente, para investigar o possível impacto do bloqueio.
Primary percutaneous coronary intervention (PPCI), usually with stent implant, is considered the preferred reperfusion strategy, due to its association with high rates of recanalization of the occluded artery, reduction in ischemic myocardial damage progression and, more importantly, mortality reduction in ST-segment elevation myocardial infarction (STEMI).1 However, patency restoration of a previously occluded epicardial artery is not always accompanied by adequate reperfusion of the myocardium that depends on it.2 This no-reflow phenomenon (NRP) occurs in a variable proportion of patients (5% to 50%) and has negative impact on the known benefits of PPCI, namely: (a) early complications after myocardial infarction (arrhythmias, pericardial effusion, cardiac tamponade, and heart failure); (b) adverse left ventricular remodeling; (c) late readmissions for heart failure; (d) increased mortality rate.2,3 No-reflow is a complex and dynamic process that involves several physiopathological components such as distal atherothrombotic embolization, ischemic lesion, reperfusion lesion, and intrinsic susceptibility to coronary microcirculation combined with the effects of ischemia and reperfusion lesion.3,4 This phenomenon may or may not be reversible, depending on the net result of the functional and/or anatomical abnormalities of coronary microcirculation, as well as the therapeutic approach used for its prevention and treatment.
It can be diagnosed at different stages by various methods, among which are coronary angiography, electrocardiography, and noninvasive imaging methods, such as myocardial echocardiography with contrast and magnetic resonance.5,6
Coronary angiography with contrast is usually used to evaluate non-reperfusion during PPCI by gradation of the epicardial flow using the Thrombolysis In Myocardial Infarction (TIMI) score: TIMI 0 indicates no perfusion (without flow after coronary lesion); TIMI1 represents minimum perfusion (contrast surpasses the lesion, but shows no opacification of the vessel downstream); TIMI 2 indicates partial perfusion (contrast surpasses the lesion and there is opacification in the distal vessel, but with slower speed than in the adjacent vessels); TIMI 3 occurs in cases of complete perfusion (contrast surpasses the lesion at the same speed as in adjacent vessels). Gradation of the opacification aspect and myocardial outflow is indicated by the Myocardial Blush Grade (MBG) score: MBG 0 indicates no myocardial opacification and no myocardial outflow; MBG 1 indicates mild opacification and slow myocardial outflow; MBG 2 corresponds to moderate opacification and somewhat increased myocardial outflow, but still lower than that observed in other arteries without lesions; MBG 3 indicates normal opacification and myocardial outflow comparable to that observed in other arteries without lesions.7,8
Through the electrocardiogram (ECG), non-reperfusion is inferred by analyzing the ratio of the ST-segment elevation before and after treatment and its complete resolution (> 70%), which rules out the occurrence of NRP. Lower degrees of ST-segment resolution characterize the occurrence of NRP, and moderate (30% to 70%) to severe (< 30%) degrees of reperfusion are commonly observed.9,10
These integrated data stratify these patients in varying degrees of no-reflow. Angiographically, nonreperfusion is characterized if epicardial TIMI≤2, or when, even in cases of epicardial TIMI=3, the degree of opacification and myocardial outflow by MBG is not≥2. These aspects as well as diagnostic factors have prognostic consequences, as several studies have shown that patients with TIMI 3, MBG 2-3, and resolution of ST>70% have a better outcome compared to those with TIMI<3, MBG 0-1, and ST resolution<70%.10-12
Numerous therapeutic strategies have been used in the NRP approach in primary PCI, among which are the use of drugs such as inhibitors of the final common pathway of platelet aggregation (abciximab, tirofiban, and eptifibatide), vasodilators (dinitrates and trinitrates, adenosine, verapamil, and sodium nitroprusside), antiinflammatory drugs (statins), antithrombotic agents (heparin and bivalirudin), metabolic inhibitors (nicorandil), and mechanical devices, such as distal filter protection catheters and thrombus aspirators. These investigations have reported inconsistent or merely promising results, probably due to the fact that the physiopathology of this phenomenon is multivariate, with several key elements in every clinical situation.3,13-19 In fact, as observed in the context of patients with myocardial infarction without ST-segment elevation, the use of glycoprotein IIb/IIIa inhibitors (GPI IIb/IIIa) in the presence of dual platelet therapy with acetylsalicylic acid and clopidogrel did not appear to have a beneficial effect in terms of reducing severe events, in addition to increasing the risk of bleeding.20
Similarly, in STEMI patients, the use of such agents that block the final common pathway of platelet aggregation still lacks a solid scientific basis. It should also be noted; however, that use of low molecular weight GPI IIb/IIIa (eptifibatide and tirofiban) in STEMI has increased in recent years, after studies that showed encouraging results equivalent to those previously obtained with the high molecular weight inhibitor abciximab. Additionally, eptifibatide and tirofiban have shown, as opportune advantages, both faster reversibility of platelet aggregation inhibition after cessation of use and lower cost.12-14
The general use of this antiplatelet agent group is class IIa or IIb recommendation in the most recent consensuses (American and European) STEMI and PPCI. According to the latest American consensus on STEMI, GPI IIb/IIIa have a class IIa recommendation, as it is considered acceptable to start treatment with abciximab, tirofiban, or eptifibatide as an adjunct therapy to PPCI with or without clopidogrel, or for stent implant in patients receiving unfractionated heparin. The same consensus recommends, as class IIb, their use in situations preceding the interventional cardiology lab, (ambulance and emergency rooms) in patients with STEMI who will be submitted to PPCI.21-23
In contrast, the latest European consensus on STEMI recommends as class IIa the use of GPI IIb/IIIa only as an optional therapy in patients with high thrombotic load, slow flow, or no flow. In this consensus, the indication of GPI IIb/IIIa remained restrictive (only as class IIb) for routine use in patients without contraindications and using unfractionated heparin. Similarly, the upstream use of these drugs, i.e., before the interventional cardiology environment, also had class IIb indication in high-risk patients referred for PPCI.22
In a recent study, with over 300,000 STEMI patients undergoing PPCI, the use of GPI IIb/IIIa was>70% and, coincidentally, the incidence of NRP was very low (2.3%), although its diagnosis was performed exclusively by the angiographic criterion of TIMI epicardial flow.24
It should be remembered that in early observational studies, as well as in recent randomized trials, tirofiban showed low rates of complications such as bleeding and thrombocytopenia.13
In summary, the administration of GPI IIb/IIIa is usually recommended to treat NRP when detected angiographically. However, there remain significant questions about the true value and significance of its early use (before or just at the beginning of the PPCI procedure) to prevent the onset of non-reperfusion, especially in patients already treated with dual platelet inhibition (combined use of acetylsalicylic acid and clopidogrel, according to current practice).
METHODSThis will be a multicenter, prospective, randomized, double-blinded (during the phases of procedures and analysis of results) study, which will include patients that will receive tirofiban or placebo infusion intravenously, with a STEMI diagnosis, and who will have been referred for PPCI.
The project was approved by the Research Ethics Committee of Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo, process Nº 2,495/2010. Subsequently, the approval of the Research Ethics Committee was extended to hospitals São Joaquim and Santa Casa, both in Franca (SP), and to Instituto do Coração do Triângulo Mineiro, in Uberlândia (MG). Patients who meet the inclusion criteria will be offered an informed consent to be signed by themselves or their legal guardians.
Inclusion criteria are: age>18 years; chest pain>20 minutes with ST elevation≥1mm in two contiguous leads, or with presumably new left bundle branch block on the electrocardiogram; and period of up to 12 hours of symptom onset for the implementation of PPCI.
Exclusion criteria are: cardiogenic shock; myocardial infarction in the same territory; hemorrhagic diathesis; use of fibrinolytics; coma after brain anoxia; known thrombocytopenia or leukopenia; severe liver dysfunction; severe renal failure (creatinine>3.0mg/dL); contraindication to the use of aspirin, thienopyridines, or heparin; life expectancy<1 year; major surgery<3 months; stroke<30 days; history of intracerebral disease (aneurysm and arteriovenous malformation); severe trauma<6 weeks; oral anticoagulant use; and participation in another research protocol.
During the randomization of these patients and before drug administration protocol, routine blood tests (complete blood count, full coagulation profile, blood glucose, glycated hemoglobin [Hb], creatinine, urea, full lipid profile, potassium, sodium, CK–MB, and troponin) will be performed.
The study endpoint will be the diagnosis of NRP, sequentially supported by angiographic methods (analysis of coronary angiography and PPCI by TIMI and MBG scores) and the electrocardiographic method (quantitative analysis of ST-segment elevation before PPCI, at 90 minutes after PPCI, and 24 hours after the procedure).
The following will be recorded in the study database: demographic, clinical, and angiographic characteristics; and data on the percutaneous therapeutic intervention and its results: age, gender, ethnicity, weight, height, body mass index, history of diabetes mellitus, arterial hypertension, dyslipidemia, smoking status (current; previous – if>1 month, or never), obesity, family history of coronary artery disease, previous myocardial infarction, previous percutaneous coronary intervention in another artery, previous coronary artery bypass graft surgery, current anterior wall infarction, previous heart failure, and current heart failure; time of symptom to first medical care, symptom to health care in this institution, symptom to drug administration, symptom to diagnostic catheterization, symptom to balloon, institutional medical care to balloon, diagnostic procedures, therapeutic procedure; prior use of aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, statins, nitrate, and illicit drug use; history of known coronary heart disease; systolic blood pressure<100mm Hg, heart rate>100bpm, Killip-Kimball classification, vascular access (radial, brachial, and femoral), artery associated with the current STEMI (right coronary artery, left circumflex artery, left anterior descending artery, and left main coronary trunk), probable artery associated with lesions<50%; pre- and post-procedure TIMI flow scores 0, 1, 2, and 3; MBG 0, 1, 2, and 3; type of lesion A, B, or C; presence of single-vessel, two-vessel, or three-vessel disease; angiographic occurrence of no-reflow; use of intracoronary drugs to treat NRP in the catheterization laboratory (trinitrates, adenosine, verapamil, and sodium nitroprusside); acceptable resolution of no-reperfusion; use of manual thrombus aspiration; persistent pain after the procedure (1 to 10); morphine; stent implantation (one, two, or three), stent type and dimensions, direct stent implantation, balloon pre-dilatation and balloon post-dilatation, stent deployment pressure, post-stent balloon pressure, intervention performed only with balloon (without stent); peak CK-MB, troponin, left ventricular ejection fraction; occurrence of procedural failure; pre- and post-procedure ST-segment deviation (mm); and the occurrence of severe, moderate, and minimal bleeding. The following TIMI criteria will be used for bleeding events: (1) severe bleeding, if intracerebral hemorrhage, or if the clinical picture is due to a decrease in Hb>5g/dL or hematocrit (Ht)>15%; (2) moderate bleeding if clinical picture of hemorrhage with a decrease of Hb of 3-5g/dL and Ht of 9-15%; (3) minimal bleeding, if clinical picture of hemorrhage with Hb decrease<3g/dL or Ht<9%; moderate and severe thrombocytopenia.
Also during the initial phase of the diagnostic cardiac catheterization, peripheral intravenous infusion of saline without (placebo) or with tirofiban, according to the result of the random allocation of patients to one of the two experimental groups will be implemented. Intravenous administration of tirofiban will be conducted as follows: loading dose of 25μg/kg and a maintenance dose for 12 hours of 0.15μg/kg/min. Intravenous unfractionated heparin will be administered initially at a dose of 100 U/kg body weight when the patient is allocated to the infusion of placebo, and a dose of 70 U/kg body weight when the infusion is tirofiban. This procedure of heparin dose adjustment will also be performed in double-blind fashion regarding doctors and patients, and it will be the responsibility of the nursing staff to maintain the requirements of a double-blind study. Acetylsalicylic acid at a loading dose of 300mg, and clopidogrel at an initial dose of 600mg will be routinely employed prior to the PPCI procedure. The other anti-ischemic drugs (nitrates and beta-blockers) will be used as usual. The use of other drugs to treat NRP, when angiographically verified (nitroglycerin, adenosine, verapamil, and sodium nitroprusside), will be left to the PPCI surgeons’ discretion in the interventional cardiology laboratory. The use of manual thrombus aspiration devices will also be left to the surgeons’ discretion.
Sample size calculationFor sample size calculation, this study will consider the estimate based on the literature that NRP in STEMI patients undergoing PPCI occurs at an incidence of approximately 70%. The study hypothesis to be tested is that the incidence reduction will be approximately 50% in the tirofiban group, that is, a decrease in the occurrence of NRP to 35%. In summary:
- •
Hypothesis: 50% reduction in NRP incidence in the active group (with tirofiban);
- •
Placebo group: estimated incidence of NRP=70% represented by P1;
- •
Tirofiban group: estimated incidence of NRP=35% represented by P2;
- •
Alpha=5%; corresponds to the probability of false positive result (0.05);
- •
Beta=20%; corresponds to the probability of false negative result (0.20);
- •
f (α, β) is the constant resulting from arbitrary values conferred to a and b and its value is 7.9, according to the statistical table;
With P1=70%; P2=35%; f (α, β)=7.9, the sample shall be calculated according to the formula:
The sample size was calculated, then, as 28 patients in each branch of the study, for a total number of 56 patients.25 Thus:
CONCLUSIONSConsidering the above-mentioned data, it has potential clinical relevance the systematic investigation of the effects of early administration of the low molecular weight glycoprotein IIb/IIIa inhibitor tirofiban (the only one available in Brazil for clinical use) in the context of management of patients with myocardial infarction with ST-segment elevation and its association with the emergence and consequences of the no-reflow phenomenon.
Thus, the purpose of this study is to evaluate whether early systematic inhibition of the final common pathway of platelet aggregation using tirofiban reduces the occurrence of no-reflow phenomenon during and after primary percutaneous coronary intervention in patients with myocardial infarction with ST-segment elevation. To achieve this goal, an initial group of approximately 60 patients will be divided into experimental group (tirofiban) and placebo, in randomized order. In an unprecedented manner, the occurrence of no-reflow phenomenon will be evaluated both 90 minutes after the primary percutaneous coronary intervention, as well as at 24h after the procedure, through the successive application of angiographic and electrocardiographic criteria.
This study may guide future implementation of other, more comprehensive, multicenter investigations to determine whether the eventual prevention of the occurrence of no-reflow phenomenon after treatment by primary percutaneous coronary intervention in patients with STEMI by administering tirofiban will result in survival benefit in the medium and long term.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
FUNDING SOURCESNone.