Endovascular repair of aortic diseases is a well-established therapeutic alternative for patients with the appropriate anatomy and/or high surgical risk, as it provides lower morbidity and mortality rates. This study aimed to analyse the outcomes of asymptomatic patients undergoing endovascular treatment of thoracic aortic dissections with an aortic diameter > 5.5cm or endoleaks. Technical success, therapeutic success, morbidity, mortality, and perioperative complication and reintervention rates were assessed.
MethodsThe present retrospective study, which was performed at a reference centre from January, 2010 to July, 2011, analysed consecutive patients undergoing endovascular repair of chronic complicated type B aortic dissections based on the Stanford classification.
ResultsTwenty-six patients were treated. The mean age was 56.4±7 years, and 61.5% were males. Technical and therapeutic success rates were 100% and 74%, respectively. The perioperative mortality was 7.6%, and the mortality rate in the first year of follow-up was 19.3%. The reintervention rate was 15.3%.
ConclusionsIn the present study, endovascular treatment of chronic type B aortic dissections proved to be a feasible method associated with acceptable perioperative complication rates. The therapeutic success and reintervention rates indicated the necessity for stringent and careful clinical follow-up of these patients.
Tratamento Endovascular da DissecçãoCrônica de Aorta Tipo B Complicada
IntroduçãoA correção endovascular das doenças aórticas está bem estabelecida como alternativa terapêutica para pacientes com anatomia adequada e/ou alto risco cirúrgico, proporcionando menores taxas de morbidade e mortalidade. Nosso objetivo foi analisar os resultados do tratamento de pacientes assintomáticos submetidos a tratamento endo-vascular de dissecções de aorta torácica complicadas, seja por diâmetro aórtico > 5,5cm ou vazamentos. Avaliamos o sucesso técnico, o sucesso terapêutico, a morbidade e a mortalidade, e as taxas de complicações perioperatórias e de reintervenções.
MétodosEstudo retrospectivo, realizado em um centro de referência, no período de janeiro de 2010 a julho de 2011, em que foram analisados pacientes consecuti-vos submetidos a correção endovascular de dissecção crônica de aorta tipo B complicada pela classificação de Stanford.
ResultadosForam tratados 26 pacientes. A média de idade foi de 56,4±7 anos e 61,5% eram do sexo masculino. Os sucessos técnico e terapêutico foram de 100% e 74%, res-pectivamente. A mortalidade perioperatória foi de 7,6% e a taxa de mortalidade no primeiro ano de seguimento foi de 19,3%. A taxa de reintervenção foi de 15,3%.
ConclusõesEm nosso estudo, o tratamento endovascular da dissecção crônica de aorta tipo B demonstrou ser um método viável e associado a aceitáveis taxas de complicações perioperatórias. As taxas de sucesso terapêutico e de reintervenções obtidas demonstram a necessidade de seguimento clínico rigoroso e atento desses pacientes.
Stanford type B aortic dissection, which does not involve the ascending aorta, causes high rates of morbidity and mortality in its complicated form, occurring in younger patients and resulting in death from direct complications of the disease.1 Spontaneous resolution is rare.2 The risk factors that are often associated with this disease include hypertension, cardiovascular disease, lung diseases, and kidney dysfunction. Stanford type B aortic dissection predominates in the male gender (3:1), and approximately 30% of patients with type B dissections develop some type of complication.
Endovascular or surgical treatment is indicated when there is rapid increase in the aortic diameter, signs of rupture (mediastinal bruises, pleural effusion), ischaemic syndromes or intractable pain. In such situations, this intervention has superior results compared with clinical treatment.3 The overall mortality for surgical repair of type B aortic dissections is approximately 30%, reaching 50% when surgery occurs in emergency situations.1,4,5
Endovascular devices allow for a treatment that is less invasive than surgery, preventing aortic clamping.6 The stents occlude the dissection orifices, reorganise the vessel layers, and prevent blood entry between the vessel layers, leading to decompression, thrombosis, and fibrosis of the false lumen, thus contributing to favourable aortic remodelling and fewer adverse clinical events.3 Endovascular treatment yields lower rates of blood transfusion, shorter hospital stays, reduced length of intensive care unit stays, and lower costs. Moreover, this therapy allows for reperfusion of ischaemic vascular beds in complicated dissections, with lower risks than found in open surgery.7 The main disadvantage of this technique is that it predisposes the patient to an increased number of reinterventions in the medium- and long-term.8,9
Endovascular treatment of chronic type B aortic dissections is still controversial. The primary debate refers to the possibility of remodelling not occurring after occlusion of the inlet orifice, either because of the incapacity of the prosthesis to expand completely or due to the incapacity of the previously formed haematoma to be reabsorbed by the blood vessel wall.2
The aim of this study was to evaluate the clinical outcomes of asymptomatic patients undergoing endovascular repair of complicated type B aortic dissections by analysing the technical success, therapeutic success, morbidity and mortality, complications, and rate of reinterventions.
METHODSStudy typeA retrospective, observational, longitudinal study was conducted at a reference centre for cardiovascular diseases from January of 2010 to July of 2011. In total, 26 patients undergoing endovascular repair of type B aortic dissections were evaluated.
Inclusion and exclusion criteriaPatients with type B aortic dissections with aortic diameters≥55mm, as well as patients who were previously treated with stents that developed type I or III endoleaks, were included.
The study excluded patients with a proximal aortic neck containing thrombi or calcifications > 50% of the neck diameter, an external iliac artery diameter < 7mm or a creatinine clearance < 30mL/min.
A cardiac and/or anaesthetic risk assessment was not considered in the inclusion or exclusion of the patients.
Surgical techniqueAll procedures were performed in the Haemodynamics Laboratory of the Endovascular Intervention Centre (Centro de Intervenções Endovasculares – CIEV) of the Instituto Dante Pazzanese de Cardiologia (São Paulo, SP, Brazil).
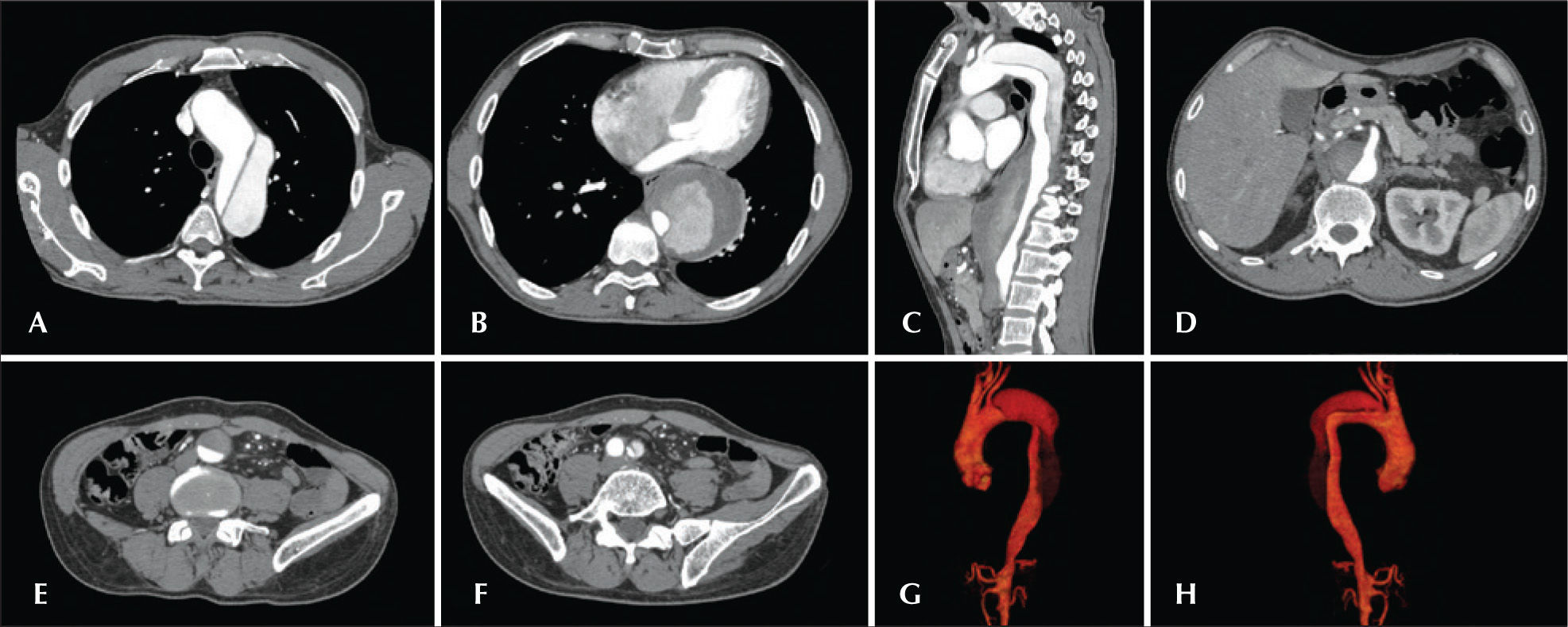
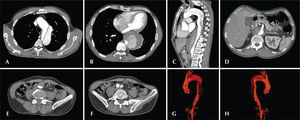
For all cases, the diagnosis and treatment schedule were based on the angiotomography results; pre-operative arteriography was considered to be an optional diagnostic method. All of the tomography scans were reconstructed using OsiriX software version 3.2 for Macintosh (Department of Medical Imaging and Information Science of the University Hospital of Geneva – Geneva, Switzerland) in three-dimensional mode and multiplanar reconstruction mode; subsequently, the diameters, as well as the dissection angles and extensions of the proximal and distal aortic neck of the aorta were obtained (Figure 1).
– Angiotomography with multiplanar and three-dimensional reconstruction. A, axial view demonstrating entry of the dissection at the origin of the left subclavian artery. B, the largest aortic diameter. C, sagittal view. D, the superior mesenteric artery originating from the true lumen. E, abdominal aortic involvement. F, left iliac artery dissection. G, three-dimensional reconstruction in the left anterior oblique view. H, three-dimensional reconstruction in the right anterior oblique view.
All patients received general inhalational anaesthesia with cerebrospinal fluid monitoring in cases of second treatment of aortic aneurysms and in cases in which preoperative carotid-subclavian or carotid-carotid bypasses were performed.
After anaesthesia induction and appropriate antibiotic prophylaxis (1.5g cefuroxime), treatment was initiated by open surgical dissection (unilateral) of the common femoral artery and by contralateral femoral or brachial puncture, based on the type of intervention being performed.
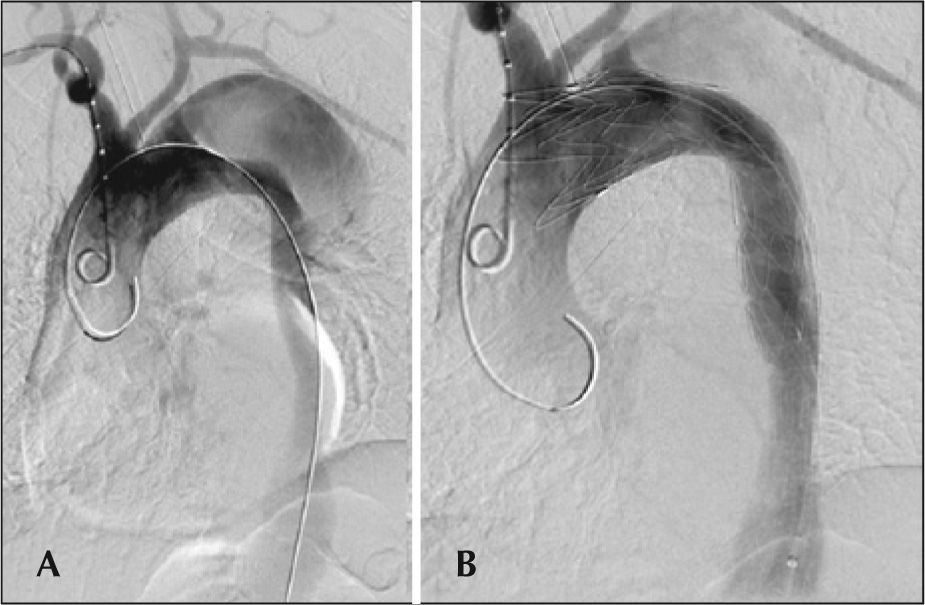
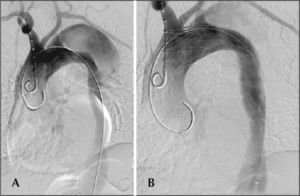
The radiographic control was performed using Artis flat panel equipment (Siemens – Erlangen, Germany). The following devices were used: Valiant® (Medtronic – Minneapolis, MN, USA), Zenith TX2® (Cook Medical – Bloomington, IN, USA), TAG® (Gore Medical – Flagstaff, AZ, USA), Hercules® (Microport – Shanghai, China), and Relay® (Bolton Medical – Sunrise, FL, USA). Intraoperative control arteriography was performed in all patients (Figure 2). The immediate postoperative period occurred in the intensive care unit in all cases.
Postoperative follow-upAll of the patients were followed for 15 days, 30 days, 180 days, and 360 days after correction. In this study, angiotomographies performed 30 days after the intervention and at the end of the first year were analysed. Outpatient follow-up and annual tomographic images were maintained in all patients.
Outcomes and definitionsThe following were considered to be primary outcomes:
- -
Technical success: when stent release occurred in the affected area, with or without the presence of endoleaks or other events that could adversely affect the development of aortic disease.
- -
Therapeutic success: when stent release occurred without leaks or other events that could promote the development of aortic disease.
- -
Perioperative mortality: considered to be all deaths recorded within the first 30 days after the procedure.
- -
Procedural complications: classified as intraoperative (occurring in the catheterisation laboratory during the intervention) and in-hospital (occurring during hospitalisation, outside the catheterisation laboratory, and within 30 days after the intervention). The following outcomes were considered to be complications: local bleeding (retroperitoneal or inguinal haematoma); inadvertent occlusion of the subclavian artery; peripheral embolisation to the lower limbs with acute arterial occlusion; occurrence of paraplegia or paraparesis; infections in the surgical site, lower respiratory tract, or stent; acute renal failure, defined as an increase > two times the baseline creatinine level prior to the procedure; and death.
- -
Reintervention: interventions performed to maintain appropriate functioning of the stent or resolution of complications associated with the intervention.
The following were considered to be secondary outcomes: initial or primary endoleaks (originating during the initial procedure or diagnosed within the first 30 days) and secondary endoleaks (diagnosed 30 days after the initial procedure).
The anchorage sites for the thoracic aortic endoprosthetic fixation were analysed based on the classification of Ishimaru.10
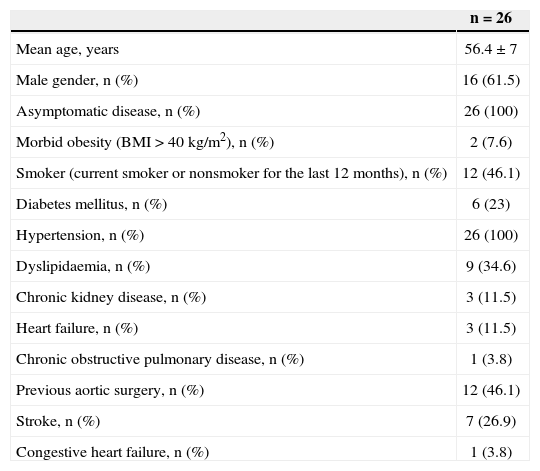
RESULTSIn total, 26 patients treated for type B aortic dissection were included, of whom 15 (57.6%) had treatment indications due to diameters > 55mm, and 11 patients (42.3%) presented late endoleaks. The clinical and demographic characteristics of the study population are listed in Table 1.
Demographic Characteristics and Clinical Data of the Study Population n=26
| n=26 | |
|---|---|
| Mean age, years | 56.4±7 |
| Male gender, n (%) | 16 (61.5) |
| Asymptomatic disease, n (%) | 26 (100) |
| Morbid obesity (BMI>40 kg/m2), n (%) | 2 (7.6) |
| Smoker (current smoker or nonsmoker for the last 12 months), n (%) | 12 (46.1) |
| Diabetes mellitus, n (%) | 6 (23) |
| Hypertension, n (%) | 26 (100) |
| Dyslipidaemia, n (%) | 9 (34.6) |
| Chronic kidney disease, n (%) | 3 (11.5) |
| Heart failure, n (%) | 3 (11.5) |
| Chronic obstructive pulmonary disease, n (%) | 1 (3.8) |
| Previous aortic surgery, n (%) | 12 (46.1) |
| Stroke, n (%) | 7 (26.9) |
| Congestive heart failure, n (%) | 1 (3.8) |
BMI=body mass index; n=number of patients.
All patients were asymptomatic and treated electively. The mean duration of the procedure was 67 minutes (49 to 104 minutes), and the mean hospital stay was 9.9 days, with an eight-day range. Inhalational anaesthesia was used in all cases, with cerebrospinal fluid monitoring in 14 cases (53.1%).
The anchorage sites for the thoracic aortic endoprosthetic fixation were distributed, based on the classification of Ishimaru,10 in six cases (23%) in zone 2, 14 cases (53.8%) in zone 3, and six cases (23%), in zone 4. All of the patients with anchorage in zone 2 underwent subclavian artery bypass surgery before the procedure.
The devices used were Valiant® in 10 cases (38.5%), Zenith TX2® in seven cases (27%), TAG® in six cases (23%), Relay® stent in two cases (7.6%), and Hercules® in one case (3.8%).
Technical success was achieved in all cases. Therapeutic success occurred in 20 patients (74%). The only cause of treatment failure was the occurrence or persistence of an endoleak, based on the intervention indication. Of the six patients who did not achieve initial therapeutic success, four (15.4%) were from the second treatment group, and two (7.6%) were from the primary treatment group.
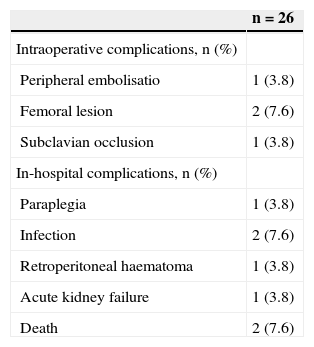
The complication rate was 30.7%, and the most frequent intraoperative complications were femoral artery lesions in two patients (7.6%) and inadvertent occlusion of the subclavian artery in one patient (3.8%). The in-hospital postoperative complications consisted of surgical site infection in two patients (7.6%), retroperitoneal haematoma in one patient (3.8%), acute kidney failure in one patient (3.8%), and one case (3.8%) of paraplegia in a patient who had undergone a preoperative carotid-subclavian bypass and selective cerebrospinal fluid drainage (Table 2).
Analysis of Intra- and Perioperative Complications
| n=26 | |
|---|---|
| Intraoperative complications, n (%) | |
| Peripheral embolisatio | 1 (3.8) |
| Femoral lesion | 2 (7.6) |
| Subclavian occlusion | 1 (3.8) |
| In-hospital complications, n (%) | |
| Paraplegia | 1 (3.8) |
| Infection | 2 (7.6) |
| Retroperitoneal haematoma | 1 (3.8) |
| Acute kidney failure | 1 (3.8) |
| Death | 2 (7.6) |
n=number of patients.
The total rate of primary endoleaks was 23%, and all of the cases were type Ia. There were no cases of type II or III endoleaks or of stent migration during the patient follow-up. Two high-risk surgical patients presented spontaneous resolution of the leaks within three and six months of computed tomography follow-up, respectively. The reintervention rate in one year was 15.3%, resulting from treatment of type I leaks.
Perioperative mortality was 7.6% (n=2), with one death secondary to sepsis from a lower respiratory tract infection and the other death due to cardiopulmonary arrest secondary to Chagas cardiomyopathy. At the one-year follow-up, three additional deaths were observed: two cases of aortic redissection (at three months and four months after surgery), and one patient with ischaemic cardiomyopathy who progressed to acute pulmonary oedema (11 months after the procedure). The annual survival rate during follow-up was 80.7%.
DISCUSSIONEndovascular treatment of thoracic aortic disease is currently the treatment of choice for selected cases.1,3,11 Although acknowledged, the procedure yields variable results that depend on the analysed population. The anatomical and physiopathological differences between aneurysms and aortic dissections affect surgical techniques, translating into different results. Due to the instability and frailty of the aortic wall in thoracic aortic dissections, proper occlusion of the inlet might not be effectively achieved. In patients with aneurysms, anatomic distortion (characterised by increased tortuosity of the aortic arch), and a higher incidence of peripheral atherosclerotic stenoses often limit the efficacy of the release system.
The technical success rate in the present study was 100%, that is, the stent was positioned and released at the desired site in all cases. Brazilian authors have demonstrated technical success rates of 98% in 130 patients treated for type B aortic dissection and true aneurysms.12 The EUROpean collaborators on Stent-graft Techniques for abdominal aortic Aneurysm Repair (EUROSTAR)13 study observed a primary technical success rate of 89% in patients with aortic dissections.
The therapeutic success rate in the present study was 73%, due to the presence of only type I endoleaks. This type of leak is the most frequent complication associated with endovascular repair of aortic dissections, and can eventually result in clinical failure. The percentages reported in the literature range from 0% to 44%.14–16 In this study population, the rate of primary leaks was 23%. An inlet less than 2cm from the left subclavian artery and located in the lesser curvature of the aortic arch is one of the factors predisposing to stent kinking and leaks during control angiography, as well as previous aortic surgical or endovascular manipulation. In the present study, 11 patients (42.3%) exhibited leaks as indications for the endovascular procedure, of which four patients had undergone previous aortic surgery and seven patients had undergone prior endovascular treatment.
Of the patients with type I endoleaks, two individuals presented a small leak volume during arteriography, which resolved spontaneously within three to six months of follow-up. These data suggest better rates of therapeutic success without hasty interventions, as well as fewer future reinterventions.
There were no cases of secondary leaks. Considering the cases with spontaneous resolution, the reintervention rate was 15.3% at the one-year follow-up. The ‘spontaneous resolution’ of type I leaks is actually caused by thrombosis of small leaks, which can evolve with the continuous transmission of pressure to the false lumen. The Evaluation of the Medtronic Vascular Talent Thoracic Stent Graft System for the Treatment of Thoracic Aortic Aneurysms (VALOR) study17 demonstrated annual leak rates of 17%, with type I leaks in 6.3%, type II leaks in 9.5%, and type III leaks in 1.9% of the cases.
In the present study, it was observed that the therapeutic success rate for the type Ia leak was lower than that for type Ib. Thus, only 43% of Ia leaks could be repaired. To explain these data, it was observed that 23% of the endoprostheses were anchored in zone 2. All of these patients underwent a carotid-subclavian bypass. The coverage of the subclavian artery was generally well tolerated, and few patients developed dizziness or claudication of the left upper limb. In the VALOR study, 5% of the patients underwent a carotid-subclavian bypass. Many groups electively perform this bypass, belatedly, in cases of symptom onset.17,18
A complication rate of 30.7% was observed in the present study; the femoral artery lesion was the most prevalent complication, in 7.6% of the cases. The large profile devices associated with the hight number of patients who underwent retreatment contributed to these rates in the present study. The series published by the Arizona Heart Institute demonstrated complications in only 38% of patients with mild dissections.19 In the present study, one case of permanent paraplegia was (3.8%). There were no cases of stroke. Prophylactic cerebrospinal fluid drainage may be useful in high-risk patients, i.e., patients with diseases of the thoracic aorta and associated abdominal aorta, histories of open or endovascular repair of abdominal aortic aneurysms, requirements for iliac conduits to advance the stent due to a history of transient paralysis of the lower limbs, and the incapacity to undergo cerebrospinal fluid drainage in less than an hour after stent implantation if paraparesis or paraplegia are expected. The main disadvantage of preoperative drainage is potential spinal haematoma formation administering heparin during the intervention. In this case, the cerebrospinal fluid drainage should be suspended.20 In the present study, all patients submitted to a second treatment for repair of previous leaks and those patients who had been submitted to a previous surgical bypass underwent cerebrospinal fluid monitoring and drainage whenever increases in cerebrospinal fluid pressure levels were detected. In most series and comparisons between endovascular and surgical treatments for Stanford type B aortic dissections, endovascular treatment decreases the incidence of definitive paraplegia.21 However, in centres of excellence in the treatment of dissection with rates of postoperative paraplegia < 5%, endovascular treatment might not be superior in this regard.11 Overall, permanent paraplegia occurs in approximately 2% to 3% of patients after endovascular treatment. The EUROSTAR study revealed a 0.8% incidence of paraplegia in patients undergoing endovascular treatment for aortic dissections.13
The perioperative mortality rate in the present study was 7.6%, with an annual survival rate of 80.7%. Thirty days after the intervention, three deaths had occurred: two cases due to redissection and one case due to acute pulmonary oedema. In the EUROSTAR study, the in-hospital mortality was 8.4% in patients treated for dissection, and the annual survival rate was 90%, whereas in the Arizona Heart Institute series, the survival rate was 85%.13,19 The present study demonstrated a survival rate similar to that reported in other publications.
Prophylactic endovascular treatment of patients with stable type B aortic dissections should not be prescribed. The INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) study compared the clinical or endovascular treatment of 140 patients with chronic, stable, and asymptomatic dissections, and revealed no differences in mortality from any cause. The survival rate was 95.6% in the clinical treatment group vs. 88.9% in the endovascular treatment group at the two-year follow-up. The progression and death caused by rupture was similar in both groups, while the aortic remodelling was 91.3% in patients from the endovascular treatment group vs. 19.4% in the clinical treatment group. However, the clinical follow-up showed a significant number of patients who had to migrate to endovascular treatment.22 In patients such as of the present study, i.e., symptomatic patients with complications (malperfusion syndrome, progression of dissection, increased aneurysmal dilation, and impaired blood pressure control), the indication for endovascular treatment is already established in the literature.21,23
Concerning the group with chronic type B dissections, the literature also raises questions about the long-term survival of these patients, as well as the need for endovascular treatment of aortic remodelling. Moreover, questions have been raised about the endovascular treatment of type B dissections in patients with Marfan syndrome. Regarding this type of correction, there are concerns about the capacity of the diseased vessel to withstand the radial force of the device, which might cause additional dilation or dissection of juxtaimplant segments. No evidence has yet confirmed this hypothesis, but the recommended precautions include avoiding the use of oversized prostheses, post-dilatation balloons, or uncoated stents in the extremities. In the present study, no patients had Marfan syndrome. The surprising scarcity of reports regarding patients with this syndrome (especially in cases with acute dissection) in the published series on endovascular treatment of thoracic diseases might demonstrate a group selection bias that is related to poor initial results.24
Study limitationsThe limitations of this study included the small number of patients analysed, its retrospective nature and the lack of a comparative control group of patients with chronic type B dissections who were clinically treated. These factors limit the conclusions drawn and the comparison with larger studies.
The inclusion of patients in the second treatment due to endoleaks worsened the acute results of the total series, as the success rate was lower in this group.
CONCLUSIONSIn the present study, endovascular treatment of complicated, chronic, type B aortic dissections proved to be a feasible method associated with acceptable rates of perioperative complications. The rates of therapeutic success and reinterventions that were attained demonstrate the requirement for stringent and careful clinical follow-up of these patients.
The long-term benefit of endovascular therapy for complicated, chronic, type B aortic dissection is the major challenge to be achieved. Further studies are necessary to better assess this benefit.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.