Fondaparinux is an agent that has a well-established safety and efficacy profile in the treatment of non-ST segment elevation acute coronary syndromes. When used alone, however, it is associated with a higher incidence of thrombotic complications during invasive coronary procedures, requiring the supplementation of an anti-IIa agent. This study aimed to evaluate the efficacy and safety of percutaneous coronary intervention (PCI) in patients with non-ST segment elevation acute coronary syndromes that were previously treated with fondaparinux.
MethodsThis was a prospective, controlled registry, enrolling 127 consecutive patients submitted to an early invasive stratification during treatment with fondaparinux with the supplementation of intravenous unfractionated heparin at a dose of 85 U/kg at the time of PCI.
ResultsThe composite primary endpoint rate, including death, acute myocardial infarction, stroke, stent thrombosis, or emergency myocardial revascularisation was 3.2%. The cumulative incidence of major bleeding and vascular complications was 3.2%. There were no cases of guide catheter thrombosis or abrupt vessel closure.
ConclusionsPCI in patients with acute coronary syndromes receiving fondaparinux was associated with a low rate of major adverse cardiovascular ischaemic events and severe haemorrhagic complications. Supplementation with unfractionated heparin during the invasive procedures eliminated the risk of catheter-related thrombosis.
Fondaparinux em IntervençãoCoronária Percutânea no Tratamento da Síndrome Coronária Aguda
IntroduçãoConsiderado um fármaco com adequado perfil de eficácia e segurança no tratamento da síndrome coronária aguda sem supradesnivelamento do segmento ST, o fonda parinux, utilizado de forma isolada, associase a maior ocorrência de complicações trombóticas durante a realização de procedimentos coronários invasivos, requerendo a adição de agente com atividade anti-IIa. Este estudo teve como objetivo avaliar a eficácia e a segurança da intervenção coronária percutânea (ICP) em pacientes admitidos com diagnóstico de síndrome coronária aguda sem supradesnivelamento do segmento ST, previamente tratados com fondaparinux.
MétodosRegistro prospectivo, controlado, envolvendo 127 pacientes consecutivos submetidos a estratificação invasiva precoce na vigência de fondaparinux, com suplementação de heparina não fracionada intravenosa na dose de 85 U/kg no momento da ICP.
ResultadosA taxa do desfecho primário, composto por morte, infarto agudo do miocárdio, acidente vascular encefálico, trombose de stent ou revascularização miocárdica de urgência, foi de 3,2%. A incidência acumulada de sangramento grave e complicações vasculares foi de 3,2%. Não houve casos de trombose do cateterguia ou oclusão abrupta do vaso.
ConclusõesA ICP em pacientes com síndrome coronária aguda em uso de fondaparinux associase a baixas taxas de eventos cardiovasculares adversos isquêmicos e de complicações hemorrágicas graves. A suplementação com heparina não fracionada durante o procedimento invasivo elimina o risco de trombose do cateter.
Antithrombotic drug therapy, which consists of antiplatelet agents and anticoagulants, together with early invasive risk stratification, constitutes an important mainstay in the care of high-risk patients with non-ST-segment elevation acute coronary syndrome (NSTEACS).1 Increased life expectancy and the prevalence of comorbidities, together with the large number of new drugs with different anti-ischemic action mechanisms, have made it essential for therapeutic decisions to strive for a delicate balance of the efficacy/safety binomial, which is accomplished through careful clinical judgment.
Fondaparinux (Arixtra®, GlaxoSmithKline, Brentford, UK) is a synthetic pentasaccharide that indirectly inhibits factor Xa through plasma antithrombin. The administration of a single daily dose of 2.5mg subcutaneously shows a peak plasma concentration within 2 hours and a renal clearance half-life of 17 hours. Compared with enoxaparin in the randomised trial Fifth Organization to Assess Strategies in Acute Ischemic Syndromes (OASIS-5) that involved 20,078 patients with NSTEACS, fondaparinux promoted a significant reduction of severe bleeding at day nine (2.2% vs. 4.1%; P < 0.001); the impact on decreased mortality at day 30 (2.9% vs. 3.5%; P=0.02) and after six months (5.8% vs. 6.5%; P=0.05) is attributed to this finding, favourable to fondaparinux.2
However, among the 6,238 patients in the OASIS-5 study submitted to percutaneous coronary intervention (PCI), a higher incidence of thrombus formation was observed in the guide catheter of the fondaparinux group (0.9% vs. 0.4%); this complication, although rare, is associated with higher rates of acute myocardial infarction (AMI) and stroke at 30 days. Intravenous supplementation with unfractionated heparin (UFH) at the time of PCI was an adequate strategy to prevent this phenomenon, a finding that was subsequently validated in a large randomised study designed for this purpose.3
The present study aimed to evaluate the efficacy and safety of PCI in patients with a diagnosis of NSTEACS previously treated with fondaparinux in a ‘real-world’ clinical setting.
METHODSThis was a prospective controlled study involving consecutive patients who were hospitalised for unstable angina or myocardial infarction without ST-segment elevation and underwent early invasive stratification with PCI indication. The antithrombotic treatment of all patients at hospital admission consisted of 2.5mg fondaparinux administered subcutaneously once per day, a single oral dose of aspirin of 200 to 300mg, followed by 100mg/daily, a single 300 to 600mg dose of clopidogrel, followed by 75mg/daily, or a single 180mg dose of ticagrelor, followed by 90mg twice daily.
After obtaining the arterial access pathway, up to 5,000 U of UFH were administered through the introducer sheath when the radial or ulnar techniques were used and 2,500 U of UFH when the femoral technique was used. When the coronary angiography was finished and PCI was indicated, the patients received supplemental intravenous UFH prior to the insertion of the intracoronary guidewire, with a total dose of 85 U/kg or 60 U/ kg when the use of glycoprotein IIb/IIIa inhibitors was planned, aiming to achieve an activated coagulation time between 250 and 350 seconds. The procedure was performed following the recommendations of current guidelines.4 The arterial sheath was removed immediately after the procedure when the ulnar or radial pathways were used; or after two hours, or when the activated coagulation time was < 180 seconds when the femoral pathway was used.
The primary efficacy endpoint included the occurrence of cardiovascular death, AMI, stroke, stent thrombosis or emergency coronary artery bypass graft (CABG) surgery during the hospitalisation period. The primary safety endpoint consisted of severe bleeding, vascular complications at the arterial puncture site, abrupt occlusion of the target vessel, or catheter thrombosis.
In accordance with the classification of the Bleeding Academic Research Consortium,5 severe bleeding was defined as type 3 (3a, bleeding with a decrease in haemoglobin≥3 and < 5g/dL or transfusion of packed red blood cells; 3b, bleeding with a decrease in haemoglobin≥5g/dL or cardiac tamponade or bleeding requiring surgical intervention or intravenous vasoactive drugs; and 3c, intracranial haemorrhage or subcategories confirmed by autopsy, imaging exams or lumbar puncture or intraocular bleeding with vision impairment) or type 5 (5a, probable fatal bleeding; and 5b, definite fatal bleeding). Vascular complications at the site of the arterial puncture included retroperitoneal bleeding, compartment syndrome, hematoma≥5cm, pseudoaneurysm, arteriovenous fistula, infection, limb ischemia, asymptomatic arterial occlusion, adjacent nerve injury or the need for reconstructive vascular surgery. Abrupt occlusion of the target vessel was defined as thrombolysis in myocardial infarction (TIMI) flow grade 0–1 during PCI in a coronary artery with previously normal anterograde flow (TIMI 3). Catheter thrombosis was defined as the presence of newly visible and/or angiographic thrombus in the guide-catheter or any instruments used during the procedure.
Continuous variables were expressed as the mean and standard deviation, and categorical variables were expressed as absolute numbers and their percentages.
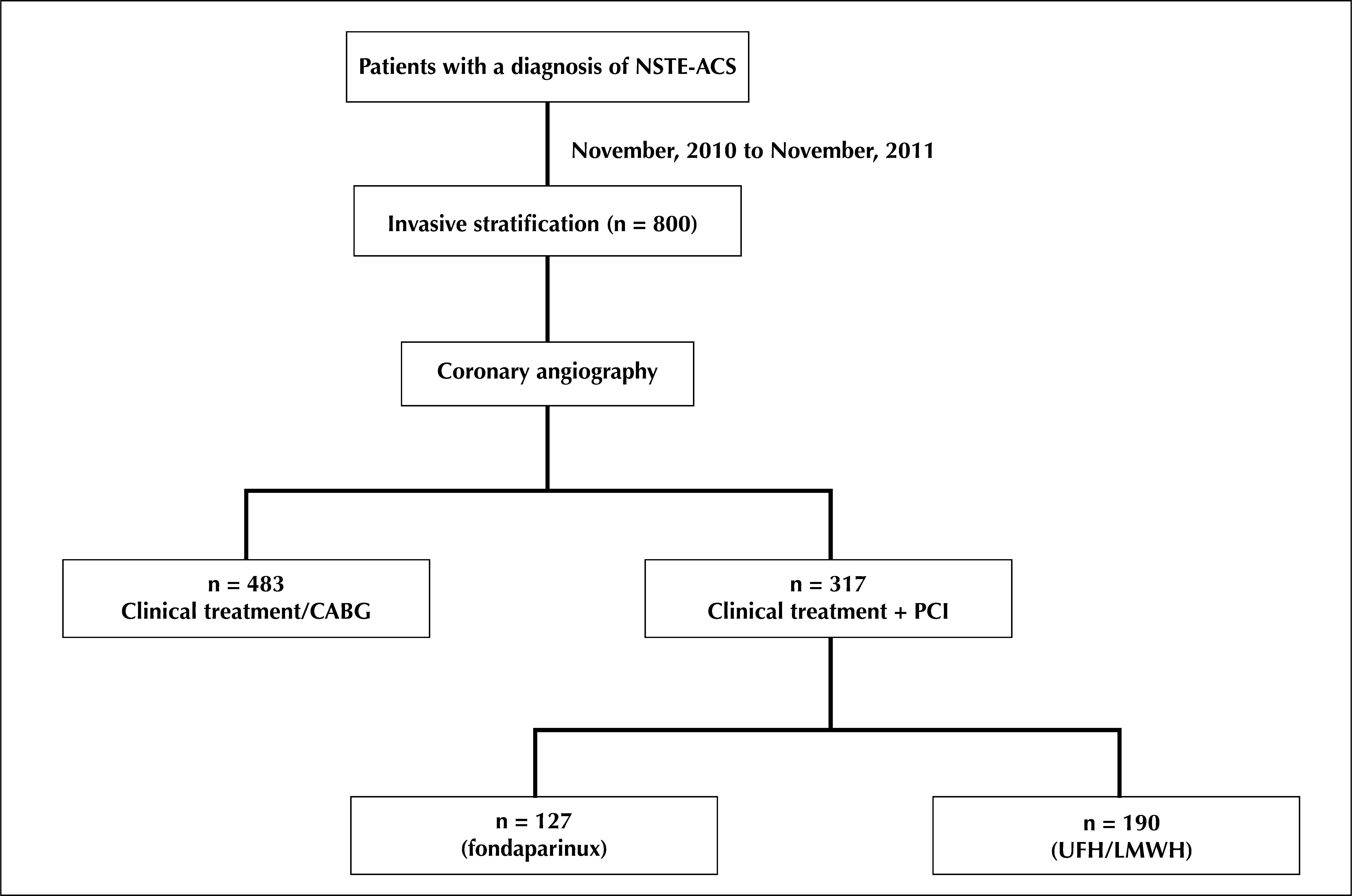
RESULTSFrom November, 2010 to November, 2011, 800 patients diagnosed with NSTEACS underwent early invasive stratification. Of these, 317 (39.6%) underwent PCI, of whom 127 (40.1%) used fondaparinux and dual antiplatelet therapy. These patients comprised the final sample (Figure 1).
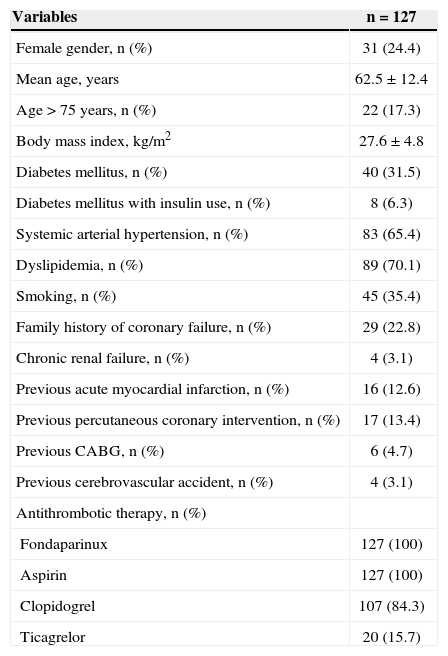
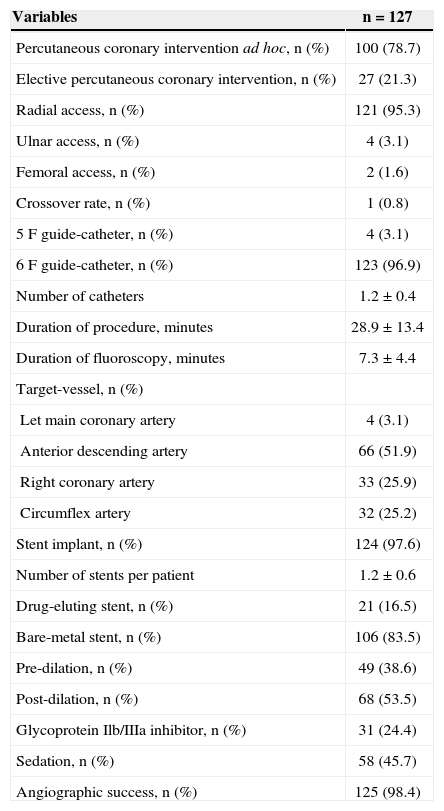
The mean age was 62.5±12.4years. Females comprised 24.4% of the study sample; 31.5% of the patients had diabetes mellitus (Table 1). Positive myocardial necrosis markers and ECG alterations suggestive of ischaemia (ST-segment depression or T-wave inversion > 3mm) were present in 68% and 89% of the sample, respectively, suggesting a high-risk population. The procedural characteristics are shown in Table 2. PCI immediately after coronary angiography (ad hoc) was performed in 78.7% of the cases. The radial access was the predominant pathway (95.3%). The left anterior descending artery was considered the target vessel in 51.9% of the procedures, and stents were implanted in 97.6% of the arteries. The mean hospital stay was 3.8±0.6days, and the duration of fondaparinux use was 2.3±0.3days.
Basal Demographic and Clinical Characteristics
| Variables | n=127 |
|---|---|
| Female gender, n (%) | 31 (24.4) |
| Mean age, years | 62.5±12.4 |
| Age>75 years, n (%) | 22 (17.3) |
| Body mass index, kg/m2 | 27.6±4.8 |
| Diabetes mellitus, n (%) | 40 (31.5) |
| Diabetes mellitus with insulin use, n (%) | 8 (6.3) |
| Systemic arterial hypertension, n (%) | 83 (65.4) |
| Dyslipidemia, n (%) | 89 (70.1) |
| Smoking, n (%) | 45 (35.4) |
| Family history of coronary failure, n (%) | 29 (22.8) |
| Chronic renal failure, n (%) | 4 (3.1) |
| Previous acute myocardial infarction, n (%) | 16 (12.6) |
| Previous percutaneous coronary intervention, n (%) | 17 (13.4) |
| Previous CABG, n (%) | 6 (4.7) |
| Previous cerebrovascular accident, n (%) | 4 (3.1) |
| Antithrombotic therapy, n (%) | |
| Fondaparinux | 127 (100) |
| Aspirin | 127 (100) |
| Clopidogrel | 107 (84.3) |
| Ticagrelor | 20 (15.7) |
n=number of patients.
Characteristics of the Procedures
| Variables | n=127 |
|---|---|
| Percutaneous coronary intervention ad hoc, n (%) | 100 (78.7) |
| Elective percutaneous coronary intervention, n (%) | 27 (21.3) |
| Radial access, n (%) | 121 (95.3) |
| Ulnar access, n (%) | 4 (3.1) |
| Femoral access, n (%) | 2 (1.6) |
| Crossover rate, n (%) | 1 (0.8) |
| 5F guide-catheter, n (%) | 4 (3.1) |
| 6F guide-catheter, n (%) | 123 (96.9) |
| Number of catheters | 1.2±0.4 |
| Duration of procedure, minutes | 28.9±13.4 |
| Duration of fluoroscopy, minutes | 7.3±4.4 |
| Target-vessel, n (%) | |
| Let main coronary artery | 4 (3.1) |
| Anterior descending artery | 66 (51.9) |
| Right coronary artery | 33 (25.9) |
| Circumflex artery | 32 (25.2) |
| Stent implant, n (%) | 124 (97.6) |
| Number of stents per patient | 1.2±0.6 |
| Drug-eluting stent, n (%) | 21 (16.5) |
| Bare-metal stent, n (%) | 106 (83.5) |
| Pre-dilation, n (%) | 49 (38.6) |
| Post-dilation, n (%) | 68 (53.5) |
| Glycoprotein Ilb/IIIa inhibitor, n (%) | 31 (24.4) |
| Sedation, n (%) | 58 (45.7) |
| Angiographic success, n (%) | 125 (98.4) |
n=number of patients.
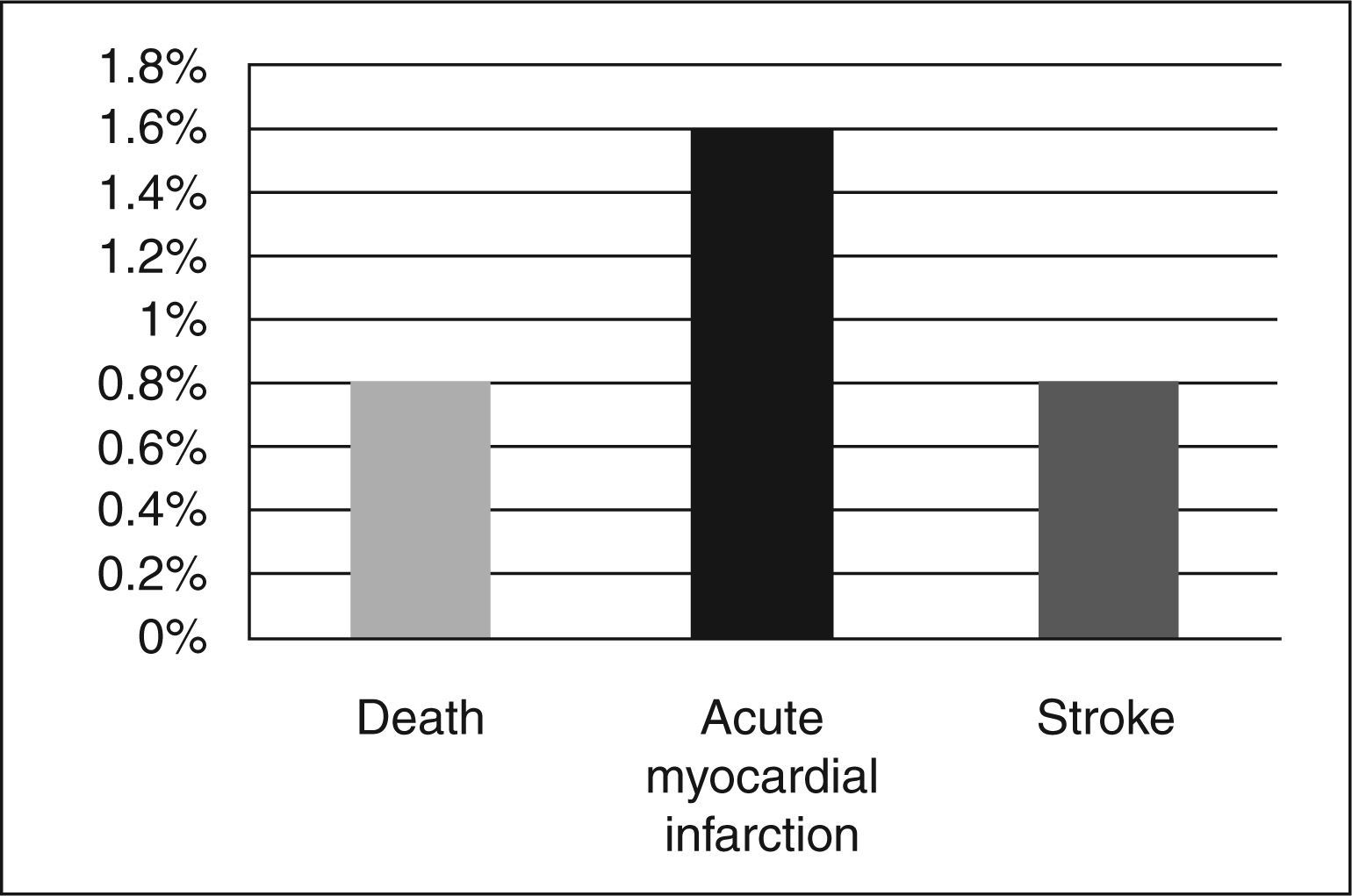
The overall incidence of adverse ischaemic cardiovascular events, which was the efficacy endpoint, was 3.2%. These events were due to one cardiovascular death (0.8%) secondary to refractory ventricular fibrillation, two periprocedural AMI (1.6%) confirmed by elevated creatine kinase MB fraction (CK-MB) > three times the maximum reference value and one (0.8%) ischemic stroke (Figure 2). There were no cases of stent thrombosis or the need for emergency CABG.
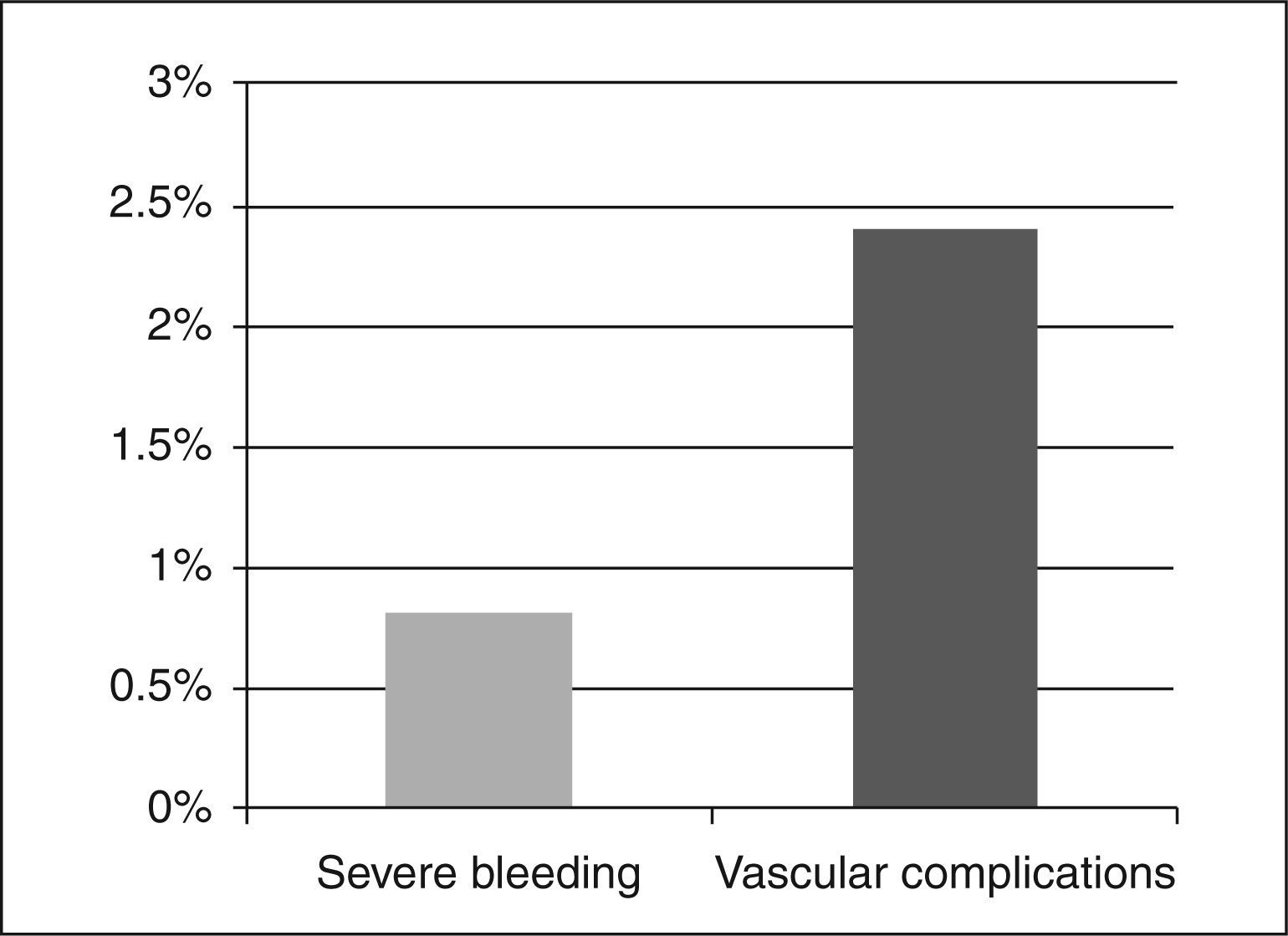
Regarding the safety endpoint, the cumulative incidence of events during hospitalisation was 3.2%, with one episode of severe bleeding type 3b (0.8%) and a decrease in haemoglobin of 5.3g/dL secondary to hematoma in the right upper limb and three haematomas (2.4%) > 5cm of subcutaneous location without clinical symptoms as vascular complications in the arterial puncture site (Figure 3). There were no cases of abrupt vessel occlusion or catheter thrombosis with the systematic supplementation of UFH prior to PCI.
DISCUSSIONIn patients diagnosed with NSTEACS who underwent PCI, a strong, consistent, and temporal association between the occurrence of severe bleeding and ischaemic events, including death, AMI, and stroke, can be observed.6 The prognostic impact of a severe bleeding episode is comparable to that of recurrent AMI on the subsequent mortality rate.7 Thus, strategies aimed at reducing or minimising the complication of maintaining the anti-ischaemic efficacy are important steps in therapeutic decision-making.8 Among these strategies, new commercially available antithrombotic agents, such as fondaparinux, have shown promise in clinical practice while also promoting this efficacy and safety profile.2,9,10
In the pre-specified subanalysis of the OASIS-5 study involving patients undergoing PCI, the use of fondaparinux reduced the risk of severe bleeding at nine days in 54% (2.4% vs. 5.1%; P < 0.00001) of cases, with a similar incidence of ischaemic events compared with enoxaparin, resulting in a higher net clinical benefit (death, AMI, stroke, severe bleeding: 8.2% vs. 10.4%; P=0.004).11 However, finding an increased risk of thrombus formation in the guide catheter, a condition unfavourable to fondaparinux, led to a conservative attitude regardinits acceptance in this group of patients.
The observation that UFH supplementation prior to PCI reduced the risk of this complication led to the randomised Fondaparinux Trial with Unfractioned Heparin During Revascularization in Acute Coronary Syndromes (FUTURA/OASIS-8), which aimed to determine the optimal dose of UFH to be administered during the procedure and to determine whether its addition would attenuate the benefits achieved with fondaparinux in reducing bleeding.3 That study, which involved 2,026 patients, concluded that a dose of intravenous UFH of 85 U/kg guided by the activated coagulation time did not increase bleeding and vascular complications compared to a fixed dose of 50 U/kg, with an almost null rate (0.1%) of guide catheter thrombosis and a tendency towards the reduction of ischaemic events.
Based on the results of these studies, treatment of NSTEACS with fondaparinux plus UFH when PCI is necessary has been considered the anticoagulant strategy with better efficacy and a better safety profile, garnering a class I recommendation and a level of evidence A, according to a recent European guideline.1 However, the use of stringent and often restrictive inclusion and exclusion criteria in randomised trials gives this selected population a status of non-representativeness in daily clinical practice. In this context, consecutive and controlled registries still provide an accurate picture of contemporary practice, and are sources of information that allow critical analysis when applying a therapeutic tool.
The present sample had a mean age > 60 years, of which 17% were aged > 75 years. Subjects had a high prevalence of positive myocardial necrosis markers and ischaemic electrocardiographic alterations. The antithrombotic drug therapy consisted of systematically administered fondaparinux and dual antiplatelet therapy. This therapy added to early invasive stratification and promoted a decrease of in-hospital incidence of ischaemic adverse cardiovascular events and episodes of severe bleeding. A low rate of bleeding complications was observed, which was dissimilar from the findings of major international records, which showed a rate of near 9% in a sample with similar risk.12,13 This finding is attributed to the fact that, in addition to the routine use of fondaparinux, the radial or ulnar accesses were the preferred pathways when performing invasive coronary procedures, which composed 98.4% of the cases. Consistent evidence indicates that the choice of radial access rather than femoral access, especially when the surgeon has experience with the technique, promotes the reduction of vascular complications, death, AMI, stroke, and major bleeding nonrelated to CABG surgery.14–16 Besides, by instituting supplemental UFH therapy immediately after the arterial sheath is placed, in addition to complementing the dose because of the need for PCI, no thrombotic complications related to the procedure or instruments were observed, and a higher risk of bleeding was thus avoided.
Study limitationsThe limitations of the present study included its observational nature, the absence of a control group, using alternative anticoagulant treatment, the limited number of patients (as it represents the authors’ initial report on their experience with a new drug), and the lack of follow-up with patients after hospital discharge once the high cumulative mortality at one year in this clinical setting was known.17
CONCLUSIONSIn patients with NSTEACS, i.e., those representative of the ‘real world’ who were previously treated with fondaparinux, the adjuvant infusion of UFH before PCI eliminated the risk of thrombus formation in the catheter due to its low rate of adverse ischaemic cardiovascular events and severe haemorrhagic complications.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.