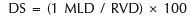The embolization of atheroma/thrombus fragments during percutaneous coronary intervention (PCI) causes microcirculatory perfusion disturbances. The new MGuard™, a mesh-based bare-metal stent, demonstrated efficacy in the prevention of embolic complications during primary PCI. However, the late clinical outcome of patients treated with the MGuard™ stent remains unknown.
MethodsA series of 65 patients with de novo coronary lesions treated with MGuard™ stent was analyzed. Baseline clinical data, procedure and late clinical follow-up (mean duration, 2.6±1.4years) data were collected retrospectively by a review of medical records and/ or direct telephone contact.
ResultsMean age was 66.1±13.7years, 32.3% of patients were diabetic, 49.2% had a previous acute myocardial infarction (AMI), and 44.6% presented with acute coronary syndrome. Two thirds of the lesions were located in a saphenous vein graft, almost half of the lesions had thrombus and most were classified as type B2/C. The MGuard™ stent was successfully implanted in all cases. At the end of the procedure, TIMI 3 flow was achieved in 93.4% and angiographic success was 91.8%. In the late clinical follow- up, adverse event rates included cardiac death in 6.2%, nonfatal AMI in 9.2%, target lesion revascularization in 9.2% and definite/probable stent thrombosis in 1.5%.
ConclusionsThe late follow-up of patients with complex coronary lesions treated with the MGuard™ stent demonstrated low rates of target lesion revascularization and stent thrombosis.
Evolução Tardia de Pacientes com Lesões Coronárias ComplexasTratados com o Novo Stent Metálico MGuard™
IntroduçãoA embolização de fragmentos de ateroma/trombo durante a intervenção coronária percutânea (ICP) ocasiona distúrbios de perfusão da microcirculação. O novo stent MGuard™, que é revestido com uma rede de polietileno, demonstrou eficácia na prevenção de complicações embólicas durante a ICP primária. No entanto, a evolução clínica tardia de pacientes tratados com o stent MGuard™ permanece desconhecida.
MétodosUma série de 65 pacientes portadores de lesões coronárias de novo tratados com o stent MGuard™ foi analisada. Os dados clínicos basais, do procedimento e do seguimento clínico tardio (média de tempo, 2,6±1,4 anos) foram coletados retrospectivamente por meio da revisão de prontuários médicos e/ou contato telefônico direto.
ResultadosA média de idade foi de 66,1±13,7 anos, 32,3% eram diabéticos, 49,2% tinham infarto agudo do miocárdio (IAM) prévio, e 44,6% apresentaramse com síndrome coronária aguda. Dois terços das lesões estavam localizados em pontes de safena, quase metade tinha presença de trombo e a maioria foi classificada como tipo B2/C. O stent MGuard™ foi implantado com sucesso em todos os casos. Ao final do procedimento, fluxo TIMI 3 foi alcançado em 93,4% e o sucesso angiográfico foi de 91,8%. No seguimento tardio, as taxas de eventos adversos incluíram óbito cardiovascular em 6,2%, IAM não-fatal em 9,2%, revascularização da lesãoalvo em 9,2% e trombose de stent definitiva/provável em 1,5%.
ConclusõesO seguimento tardio de pacientes com lesões coronárias complexas tratados com o stent MGuard™ demonstrou baixas taxas de revascularização da lesãoalvo e de trombose do stent.
Distal embolisation of the atherothrombotic material during percutaneous coronary intervention (PCI) is an undesirable occurrence that leads to microvascular obstruction and reduction of myocardial perfusion,1 which may lead to periprocedural infarction, and consequently decrease in ventricular function and increase in cardiac mortality.2−4 Several therapeutic approaches, including the use of dedicated devices, have been tested in different clinical settings in order to prevent embolic complications. The results, however, were not consistent. Additionally, some percutaneous devices were associated with increased costs and procedure time.
The MGuard™ stent (Inspire MD – Tel Aviv, Israel) is a new low-profile metallic device, specifically designed for the prevention of distal embolisation during PCI, since its metal struts are covered by a double polymer network. Previous studies involving complex lesions with high thrombogenic potential (acute coronary syndrome, saphenous graft degeneration, and thrombus, among others) have demonstrated the efficacy of the MGuard™ stent in preventing embolic complications.5,6 Furthermore, the MGuard™ stent was superior to conventional stents (non-covered) in relation to target-vessel reperfusion in patients with acute myocardial infarction with ST-segment elevation (STEMI) undergoing primary PCI.7
Despite the promising clinical results obtained with the MGuard™ stent in several clinical series, the late evolution of patients with coronary artery disease treated with this device remains unknown. Therefore, this study aimed to evaluate the late follow-up (> six months) of a series of patients treated with the MGuard™ stent in the daily practice of a public tertiary institution.
METHODSStudy design and target populationThe current analysis constitutes a retrospective observational study conducted in a single center (Instituto Dante Pazzanese de Cardiologia, São Paulo, SP, Brazil). The study population included 65 patients (66 coronary lesions) submitted to PCI, whether elective or emergency, with MGuard™ stent implantation during the routine of the Invasive Cardiology Department between 2007 and 2012. In general, the MGuard™ stent was considered for the treatment of patients with de novo coronary lesions in native vessel or bypass graft who had angiographic characteristics associated with instability, such as thrombus, ulcers and filling defects, and/or in the presence of acute coronary syndrome. The study patients were selected from the local database, and data collection was performed through review of medical records, medical reports, and angiograms, as well as by telephone contact.
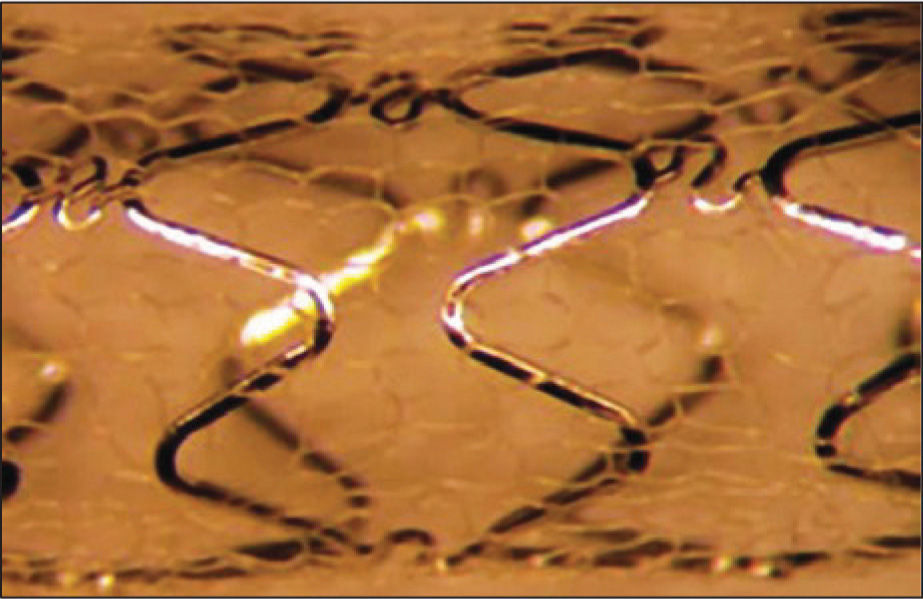
Study deviceThe characteristics and specifications of the MGuard™ device have been previously reported.6 In summary, the MGuard™ stent incorporates a 316L, laser-cut, stainless steel metallic platform, coated or covered with a double network of microscopic polyethylene terephthalate fibres, which are attached to metal struts (Figure 1). This network acts by trapping the material contained between the stent and the vessel wall, thus preventing the distal embolisation of atherothrombotic materials during implantation. The release system is low-profile balloon-expandable, compatible with 5F guide catheters.
ProcedureIn general, PCIs were performed according to current guidelines. Pre- and/or post-dilation was performed at the surgeon’s discretion, as was the use of glycoprotein IIb/IIIa inhibitors. MGuard™ stents were available in diameters between 2.5mm and 4mm (with 0.5-mm intervals) and extensions between 12mm and 40mm. The treatment of non-target lesions in the target vessel or non-target vessel with bare-metal stent during the index procedure could be performed if necessary.
The pre-procedure dual antiplatelet therapy consisted of acetylsalicylic acid 100 to 200mg/day and loading dose of clopidogrel 300mg, at least 24 hours before the procedure, or 600mg if < 24 hours. After the intervention, acetylsalicylic acid 100 to 200mg/day was prescribed indefinitely, and clopidogrel 75mg/ day was maintained for at least six months. During the PCI, antithrombin therapy consisted of unfractionated heparin at a dose of 100IU/kg (or 70IU/kg in case of glycoprotein IIb/IIIa inhibitor use), aiming to achieve an activated clotting time > 250sec (or between 200 and 250sec, in case of glycoprotein IIb/IIIa inhibitor use).
Angiographic analysisThe qualitative and quantitative angiographic analyses were performed offline by an experienced surgeon, following a pre-defined protocol. In general, the preand post-procedure cineangiographies were performed after intracoronary administration of nitroglycerin (50–200μg) in at least two corresponding orthogonal views, and were stored in DICOM digital format. The morphological characteristics and the complexity of the lesions were defined according to the criteria of the modified classification system of the American College of Cardiology/American Heart Association (ACC/AHA). The coronary flow was determined according to the criteria of the Thrombolysis in Myocardial Infarction (TIMI) study. Angiographic complications during the procedure (dissection, slow flow, distal embolisation, no reflow phenomenon) were determined according to pre-established criteria and definitions. The analysis of quantitative coronary angiography (QCA) was performed using a dedicated computer program and semi-automatic detection of lumen borders (QAngio XA; Medis Medical Imaging Systems – Leiden, The Netherlands). The tip of the guide catheter filled with contrast was used for calibration. The minimum lumen diameter and the reference vessel diameter, obtained by the interpolated reference, were used to calculate the diameter of the stenosis using the following formula:
where DS=diameter of the stenosis, MLD=minimum lumen diameter, and RVD=reference vessel diameter. Immediate gain was defined as the difference in minimal lumen diameter pre- and post-procedure (post-procedural MLD – pre-procedure MLD). QCA analyses were reported for the intra-stent segment and intra-segment, which included the intra-stent segment plus 5mm of the proximal and distal borders of the stent.
Qualitative and quantitative angiographic analyses were performed in 61 of the 66 lesions, as the angiographic films were not available in five cases. All analyses were performed in the QCA laboratory of Instituto Dante Pazzanese de Cardiologia.
CLINICAL FOLLOW-UP AND DEFINITIONSThe late follow-up was performed by medical chart review and/or direct telephone contact. The mean clinical follow-up was 945 days (2.6±1.4years). The primary objective was to evaluate the rate of major adverse cardiac events (MACE), defined as the occurrence of cardiovascular death, non-fatal acute myocardial infarction (AMI), and target vessel revascularisation. All deaths were considered cardiovascular unless a noncardiac cause could be clearly established. Non-fatal AMI was defined as an increase greater than three times the laboratory reference values of creatine kinase MB fraction (CK-MB), which did not involve death of the patient. Target lesion revascularisation was defined as new revascularisation by PCI or coronary artery bypass graft (CABG) of the segment previously treated with MGuard™ stent. Target vessel revascularisation was defined as new revascularisation by PCI or CABG of the vessel previously treated with MGuard™ stent. Stent thrombosis was defined according to the criteria of the Academic Research Consortium (ARC) as: definitive (presence of acute coronary syndrome with angiographic or anatomopathological confirmation of stent occlusion); probable (sudden death occurrence < 30 days after the index procedure or AMI in the treated myocardial territory without angiographic confirmation of stent occlusion); and possible (sudden death occurrence > 30 days after the index procedure). Stent thrombosis was also classified according to the temporal occurrence, including the acute (< 24 hours post-procedure), subacute (between 24 hours and 30 days post-procedure), late (between one month and 12 months post-procedure), and very late (> 12 months post-procedure) phases. Angiographic success was defined as the achievement of TIMI 3 flow without dissection and residual stenosis < 50% in the treated segment after the procedure.
Statistical AnalysisQualitative variables were shown as frequencies and percentages, and quantitative variables as means and standard deviations. The occurrence of MACE as a function of time is described by Kaplan-Meier curves.
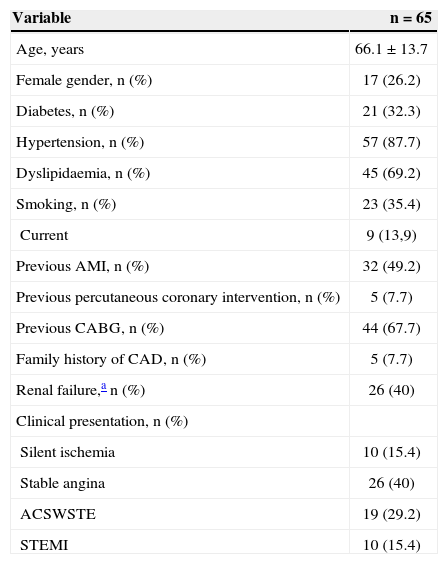
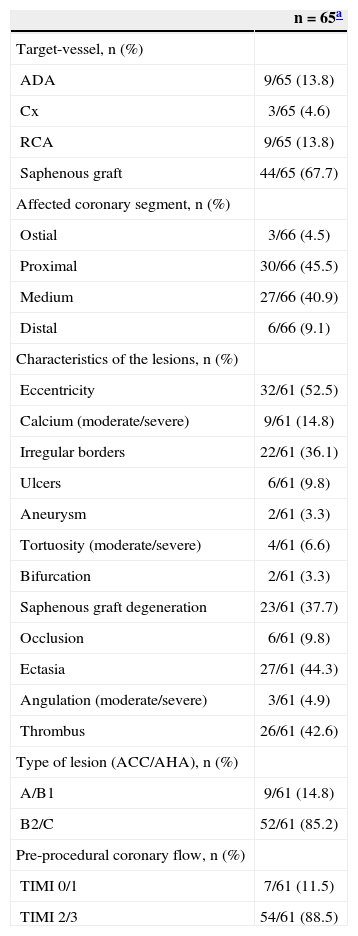
RESULTSMean age was 66.1±13.7years, 26.2% of patients were females, 32.3% had diabetes, and 49.2% had previous AMI. Regarding clinical presentation, 44.6% of the patients had acute coronary syndrome, including 15.4% with STEMI (Table 1). Of the 66 initially considered lesions (one patient had two lesions treated in two distinct target vessels), 44 lesions (67.7% of cases) were located in bypass grafts. Regarding the characteristics of the lesions (n=61), there was a high prevalence of thrombi (42.6%) and saphenous grafts degeneration (37.7%). Consequently, high-complexity lesions (type B2/C) were observed in most cases (85.2%). Furthermore, the prevalence of TIMI flow 0 or 1 before the procedure was 11.5% (Table 2).
Basal clinical characteristics
| Variable | n=65 |
|---|---|
| Age, years | 66.1±13.7 |
| Female gender, n (%) | 17 (26.2) |
| Diabetes, n (%) | 21 (32.3) |
| Hypertension, n (%) | 57 (87.7) |
| Dyslipidaemia, n (%) | 45 (69.2) |
| Smoking, n (%) | 23 (35.4) |
| Current | 9 (13,9) |
| Previous AMI, n (%) | 32 (49.2) |
| Previous percutaneous coronary intervention, n (%) | 5 (7.7) |
| Previous CABG, n (%) | 44 (67.7) |
| Family history of CAD, n (%) | 5 (7.7) |
| Renal failure,a n (%) | 26 (40) |
| Clinical presentation, n (%) | |
| Silent ischemia | 10 (15.4) |
| Stable angina | 26 (40) |
| ACSWSTE | 19 (29.2) |
| STEMI | 10 (15.4) |
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; CAD, coronary artery disease; ACSWSTE, acute coronary syndrome without ST-segment elevation; STEMI, myocardial infarction with ST-segment elevation.
Angiographic data
| n=65a | |
|---|---|
| Target-vessel, n (%) | |
| ADA | 9/65 (13.8) |
| Cx | 3/65 (4.6) |
| RCA | 9/65 (13.8) |
| Saphenous graft | 44/65 (67.7) |
| Affected coronary segment, n (%) | |
| Ostial | 3/66 (4.5) |
| Proximal | 30/66 (45.5) |
| Medium | 27/66 (40.9) |
| Distal | 6/66 (9.1) |
| Characteristics of the lesions, n (%) | |
| Eccentricity | 32/61 (52.5) |
| Calcium (moderate/severe) | 9/61 (14.8) |
| Irregular borders | 22/61 (36.1) |
| Ulcers | 6/61 (9.8) |
| Aneurysm | 2/61 (3.3) |
| Tortuosity (moderate/severe) | 4/61 (6.6) |
| Bifurcation | 2/61 (3.3) |
| Saphenous graft degeneration | 23/61 (37.7) |
| Occlusion | 6/61 (9.8) |
| Ectasia | 27/61 (44.3) |
| Angulation (moderate/severe) | 3/61 (4.9) |
| Thrombus | 26/61 (42.6) |
| Type of lesion (ACC/AHA), n (%) | |
| A/B1 | 9/61 (14.8) |
| B2/C | 52/61 (85.2) |
| Pre-procedural coronary flow, n (%) | |
| TIMI 0/1 | 7/61 (11.5) |
| TIMI 2/3 | 54/61 (88.5) |
65 patients for a total of 66 lesions/coronary segments, of which 61 were available for morphological and quantitative analyses. ADA, anterior descending artery; Cx, circumflex artery; RCA, right coronary artery; ACC/AHA, American College of Cardiology/American Heart Association; TIMI, Thrombolysis in Myocardial Infarction.
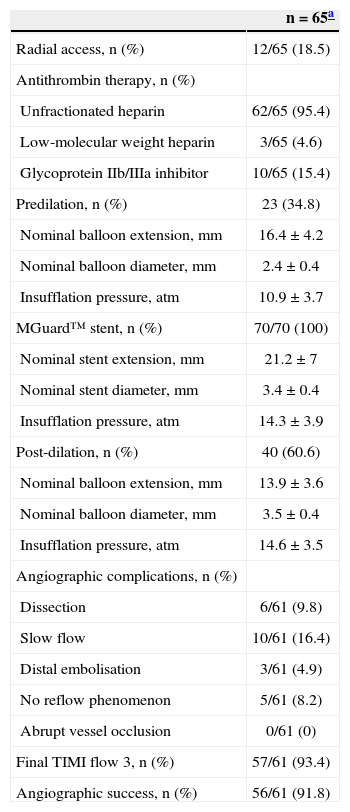
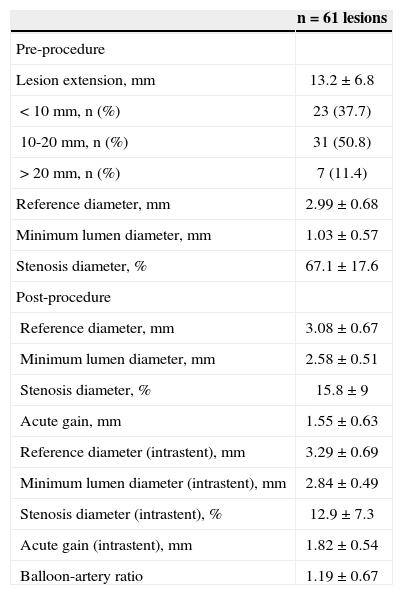
During the procedure, no filters were used for distal protection in any case. Lesion pre-dilation was performed in 34.5% of cases; a total of 70 MGuard™ stents were implanted in 66 lesions (1.06±0.2 lesions per stent), and post-dilation was performed in 60.6% of lesions. The values of length, diameter, and nominal inflation pressure values of balloons and stents used are shown in Table 3. Angiographic complications were observed in a relatively large number of patients: slowing of antegrade coronary flow (16.4%) and no reflow (8.2%) were the main complications observed. At the end of the procedure, TIMI 3 flow was achieved in 93.4% of cases, and angiographic success was 91.8%. Table 4 shows the data from QCA (n=61). Mean lesion length and reference vessel diameter before the procedure were 13.2±6.8mm and 2.99±0.68mm, respectively. At the end of the procedure, mean stenosis diameter was < 20%.
Data on the procedure
| n=65a | |
|---|---|
| Radial access, n (%) | 12/65 (18.5) |
| Antithrombin therapy, n (%) | |
| Unfractionated heparin | 62/65 (95.4) |
| Low-molecular weight heparin | 3/65 (4.6) |
| Glycoprotein IIb/IIIa inhibitor | 10/65 (15.4) |
| Predilation, n (%) | 23 (34.8) |
| Nominal balloon extension, mm | 16.4±4.2 |
| Nominal balloon diameter, mm | 2.4±0.4 |
| Insufflation pressure, atm | 10.9±3.7 |
| MGuard™ stent, n (%) | 70/70 (100) |
| Nominal stent extension, mm | 21.2±7 |
| Nominal stent diameter, mm | 3.4±0.4 |
| Insufflation pressure, atm | 14.3±3.9 |
| Post-dilation, n (%) | 40 (60.6) |
| Nominal balloon extension, mm | 13.9±3.6 |
| Nominal balloon diameter, mm | 3.5±0.4 |
| Insufflation pressure, atm | 14.6±3.5 |
| Angiographic complications, n (%) | |
| Dissection | 6/61 (9.8) |
| Slow flow | 10/61 (16.4) |
| Distal embolisation | 3/61 (4.9) |
| No reflow phenomenon | 5/61 (8.2) |
| Abrupt vessel occlusion | 0/61 (0) |
| Final TIMI flow 3, n (%) | 57/61 (93.4) |
| Angiographic success, n (%) | 56/61 (91.8) |
Quantitative coronary angiography
| n=61 lesions | |
|---|---|
| Pre-procedure | |
| Lesion extension, mm | 13.2±6.8 |
| < 10mm, n (%) | 23 (37.7) |
| 10-20mm, n (%) | 31 (50.8) |
| > 20mm, n (%) | 7 (11.4) |
| Reference diameter, mm | 2.99±0.68 |
| Minimum lumen diameter, mm | 1.03±0.57 |
| Stenosis diameter, % | 67.1±17.6 |
| Post-procedure | |
| Reference diameter, mm | 3.08±0.67 |
| Minimum lumen diameter, mm | 2.58±0.51 |
| Stenosis diameter, % | 15.8±9 |
| Acute gain, mm | 1.55±0.63 |
| Reference diameter (intrastent), mm | 3.29±0.69 |
| Minimum lumen diameter (intrastent), mm | 2.84±0.49 |
| Stenosis diameter (intrastent), % | 12.9±7.3 |
| Acute gain (intrastent), mm | 1.82±0.54 |
| Balloon-artery ratio | 1.19±0.67 |
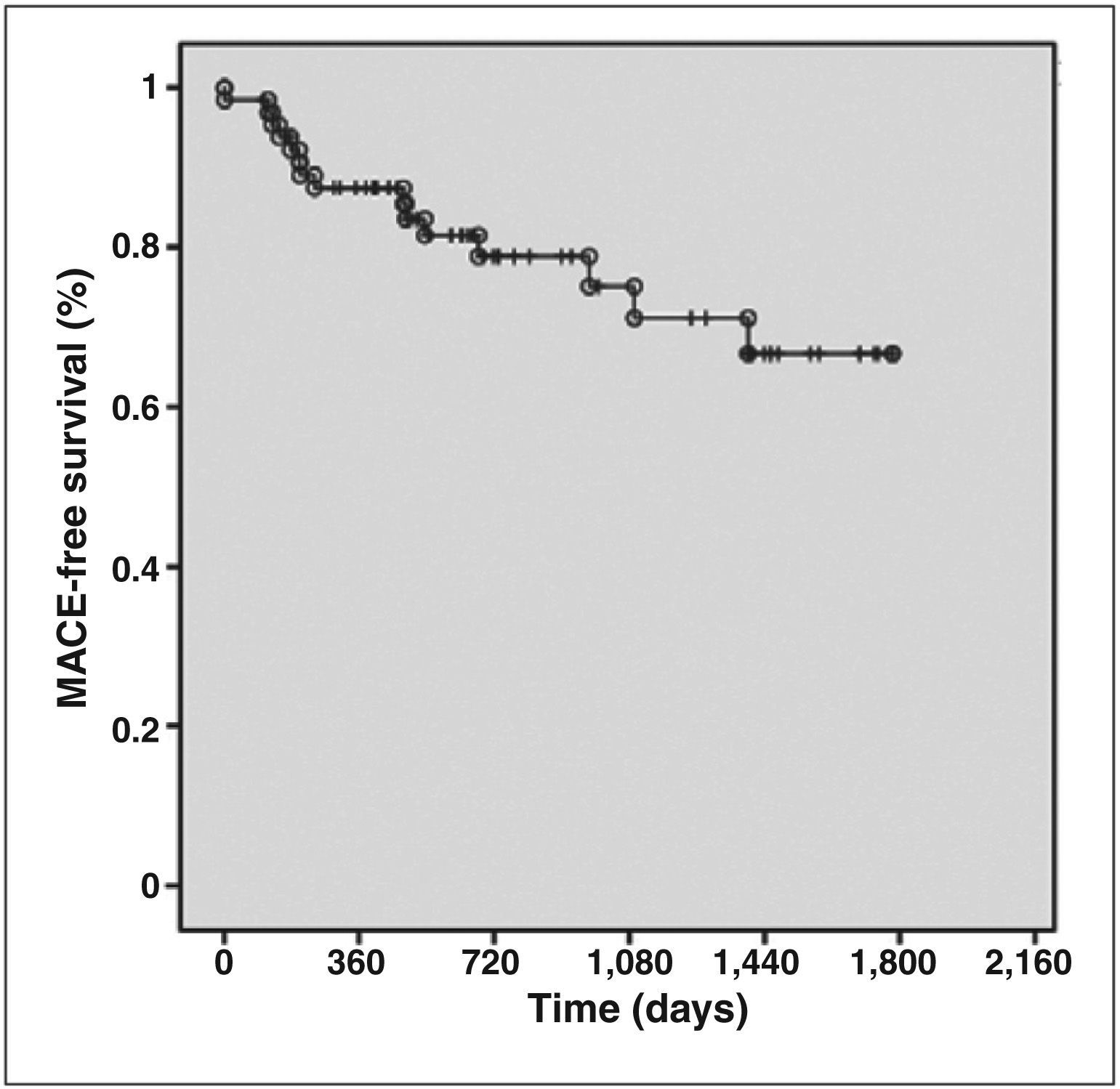
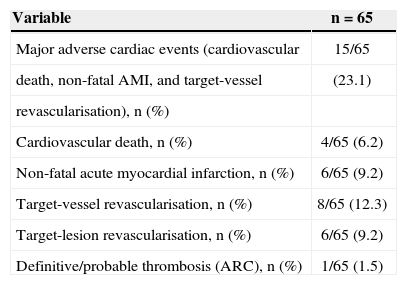
During the in-hospital phase, six patients had periprocedural myocardial infarction (five without Q-wave and one with Q-wave), but no other adverse events were observed until discharge. At the late clinical follow-up (mean duration 2.6±1.4years), cumulative rates of adverse events after discharge included cardiovascular death in 6.2% of patients, non-fatal AMI in 9.2%, target-vessel revascularisation in 12.3%, target-lesion revascularisation in 9.2%, and MACE in 23.1%. Regarding stent thrombosis, the events classified as definitive/ probable occurred in 1.5% of patients (Table 5). Figure 2 shows the MACE-free survival curve.
Clinical events (hierarchical classification)
| Variable | n=65 |
|---|---|
| Major adverse cardiac events (cardiovascular | 15/65 |
| death, non-fatal AMI, and target-vessel | (23.1) |
| revascularisation), n (%) | |
| Cardiovascular death, n (%) | 4/65 (6.2) |
| Non-fatal acute myocardial infarction, n (%) | 6/65 (9.2) |
| Target-vessel revascularisation, n (%) | 8/65 (12.3) |
| Target-lesion revascularisation, n (%) | 6/65 (9.2) |
| Definitive/probable thrombosis (ARC), n (%) | 1/65 (1.5) |
AMI, acute myocardial infarction; ARC, Academic Research Consortium.
Several authors have published studies demonstrating the MGuard™ performance in different scenarios. Grube et al.5 published a prospective study of 41 patients to evaluate the feasibility and safety of the MGuard™ stent during PCI. Clinical and laboratory monitoring were performed with cardiac biomarkers, electrocardiography (ECG), and coronary angiography at six months. In approximately half of the patients (56%), the target vessel was the bypass graft; however, no patients received glycoprotein IIb/IIIa inhibitors or distal embolic protection devices. Nevertheless, angiographic and procedure success rates were 100% and 95%, respectively. At the six-month follow-up, 19.5% of patients underwent revascularisation of the target vessel. At the very late follow-up of these patients (12 to 27 months), there was only one additional target-vessel revascularisation. In the present analysis, which included 65 patients with complex clinical and angiographic profiles, 67.7% of cases involved bypass graft (half with degeneration morphology), 15.4% received glycoprotein IIb/IIIa inhibitors, and none used a distal embolic protection device. At the end of the procedure, the angiographic success rate was 91.8%. At the very late follow-up (2.6±1.4years), 12.3% of patients were submitted to target-vessel revascularisation. These late outcomes suggest acceptable rates of target-vessel revascularisation, considering the location (bypass graft) and the complexity of the lesions, as well as the absence of a drug component in the MGuard™ stent. A study by Costa et al.,6 which included 30 patients with angiographic study protocol at six months, showed late lumen loss of 1.05±0.77mm in bypass grafts (n=16) and 0.95±0.66mm (n=14) in native vessels treated with the MGuard™ stent. These values are similar to those found in studies with conventional bare-metal stents used in clinical practice and justify, at least in part, the rates of target-lesion/ target-vessel revascularisation observed in the abovementioned studies. Another relevant aspect is the absence of negative impact in terms of neointimal hyperplasia formation, due to the presence of the polymer coating on the MGuard™ stent, designed to prevent migration of thrombus to the distal bed during PCI.
Several studies also evaluated the use of the MGuard™ stent in patients during acute coronary syndrome. Stone et al.7 published a prospective randomized study (Safety and Efficacy Study of MGuard Stent After a Heart Attack – MASTER), with 433 patients to evaluate the benefit of the MGuard™ stent in patients with STEMI submitted to PCI within 12 hours of symptom onset. The primary outcome was complete resolution of the ST-segment elevation, defined as a reduction > 70%. There were no differences in baseline patient characteristics between the groups; however, the group submitted to MGuard™ stenting had higher rates of TIMI 3 flow after the procedure (91.7% vs. 82.9%; P=0.006), and higher rate of complete resolution of ST-segment elevation (57.8% vs. 44.7%; P=0.008). Subgroup analysis showed no differences between them. Regarding clinical outcomes after 30 days, there was a tendency to decreased cardiovascular mortality in the MGuard™ group (0 vs. 1.9%; P=0.06), with no statistically significant difference for the other assessed outcomes. Still in the setting of acute coronary syndromes, Lindefjeld et al.8 published an analysis of 15 patients undergoing MGuard™ stenting in situations of high thrombotic burden (53% during STEMI). No distal embolic protection filters were used, although 40% of the PCIs were performed in saphenous vein bypass grafts, and TIMI 3 flow was obtained in all cases. In the present study, the rate of TIMI 3 flow after the procedure (93.4%) was similar to that in the group treated with the MGuard™ stent from the MASTER study (91.7%). These findings can be explained by the high prevalence of thrombus and embolic complications in the studies. Notably, outcomes with the MGuard™ stent were superior when compared to the control group in the MASTER study, suggesting efficacy of this stent in preventing distal embolisation when compared to conventional treatment.
The effectiveness of distal embolic protection devices in preventing thrombotic complications during PCI in saphenous vein bypass grafts has been extensively validated and incorporated into clinical practice. However, the use of such devices can be laborious and often impossible to perform, and adds time and cost to the procedure. It is noteworthy that such devices were not used during PCI in vein grafts in several studies with the MGuard™ stent; nevertheless, this measure does not appear to have compromised the results. The possibility of a therapeutic approach of lesions with high thrombotic burden, involving degenerated vein grafts, with a low-profile stent and without the need for embolic protection devices is undoubtedly very attractive and desirable. Esteves et al.9 published a comparative, non-randomized trial on PCI in saphenous vein bypass grafts with 38 patients (16 in the MGuard™ group and 22 in the bare-metal stent group, with use of distal embolic protection filter), which demonstrated that these devices were similar for the occurrence of MACE at 30 days. Conversely, some consider that both devices could act synergistically, especially in cases with high thrombotic content. Even though most reports with the MGuard™ stent demonstrate relatively high rates of procedural success despite the non-use of embolic protection devices in saphenous vein bypass grafts, there have been no formal recommendations for its isolated use to date, due to the lack of comparative analyses. Therefore, further, properly designed studies are needed in order to assess this possibility.
CONCLUSIONSThe late follow-up of patients with complex coronary lesions treated with the MGuard™ stent showed low rates of target-lesion revascularisation and stent thrombosis.
CONFLICT OF INTERESTSThe authors declare no conflicts of interest.