Transcatheter aortic valve implantation is a well-established therapy in patients with severe aortic stenosis. There has been a progressive improvement in device technology associated with increased experience of the interventionists, resulting in safer procedures with better outcomes. The first second-generation device approved in Brazil, Lotus™ Valve System (Boston Scientific Corporation, Natick, USA), incorporates several of these new characteristics. This report describes the first two cases, both successfully performed in the country, carried out under local anesthesia and conscious sedation.
O implante percutâneo de bioprótese aórtica é a terapia estabelecida em pacientes com estenose aórtica grave. Houve um progressivo aperfeiçoamento na tecnologia dos dispositivos que, associada a maior experiência dos operadores, resultou em procedimentos mais seguros e com melhores resultados. O primeiro dispositivo de segunda geração aprovado no Brasil, o sistema de válvula Lotus™ (Boston Scientific Corporation, Natick, EUA), incorpora várias dessas novas características. Descrevemos aqui os dois primeiros casos realizados no país, conduzidos sob anestesia local e sedação consciente, ambos com sucesso.
Transcatheter aortic valve implantation (TAVI) is currently a viable treatment option for patients with severe symptomatic aortic stenosis (AoS) considered inoperable or at high risk for complications related to conventional surgical valve replacement. This technique has shown consistent results, with a significant reduction in mortality compared to drug therapy in inoperable patients,1 and with mortality rates similar to those of conventional surgery in high-risk patients.2 However, complications inherent to the procedures can impair the short and mid-term outcomes, limiting the use of this technique.
Correct positioning of the first-generation devices available in Brazil at the level of the aortic valve annulus can be quite challenging, being one of the main limitations of the technique; furthermore, valve displacement can lead to severe complications, including coronary occlusion.3 Incomplete prosthesis apposition may occur in the presence of significant amounts of calcium, in highly elliptical anatomies, or with suboptimal implantation, resulting in paravalvular regurgitation (PVR);4,5 it has been associated with increased mortality in several longitudinal studies.
With technological development, several improvements have been achieved in the devices, as well as in anesthetic management. The authors describe herein the first two procedures performed in Brazil using a second-generation device, the Lotus™ Valve System (Boston Scientific Corporation, Natick, USA) for percutaneous treatment of AoS performed under local anesthesia and conscious sedation.
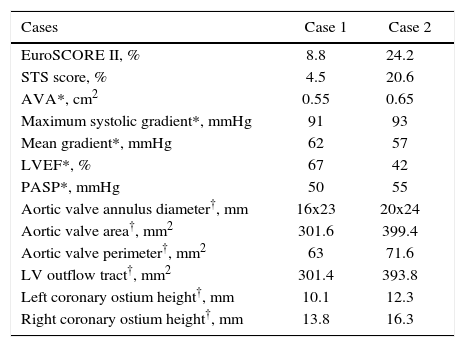
Case reportsCase 1An 88-year-old female patient was admitted at Hospital Brasil (Santo André, SP, Brazil) for congestive heart failure, with New York Heart Association functional class (NYHA) IV. The patient had a history of hypertension, diabetes mellitus, and coronary artery disease with angioplasty and drug-eluting stent implantation for lesion in the left anterior descending artery in 2014, and moderate AoS. The transthoracic echocardiography (TTE) at admission showed severe AoS; due to her advanced age and comorbidities, a percutaneous treatment was indicated. Contrast computed tomography was performed to evaluate the aortic root, valve annulus, and vascular access (Table 1).
Pre-procedure characteristics.
| Cases | Case 1 | Case 2 |
|---|---|---|
| EuroSCORE II, % | 8.8 | 24.2 |
| STS score, % | 4.5 | 20.6 |
| AVA*, cm2 | 0.55 | 0.65 |
| Maximum systolic gradient*, mmHg | 91 | 93 |
| Mean gradient*, mmHg | 62 | 57 |
| LVEF*, % | 67 | 42 |
| PASP*, mmHg | 50 | 55 |
| Aortic valve annulus diameter†, mm | 16x23 | 20x24 |
| Aortic valve area†, mm2 | 301.6 | 399.4 |
| Aortic valve perimeter†, mm2 | 63 | 71.6 |
| LV outflow tract†, mm2 | 301.4 | 393.8 |
| Left coronary ostium height†, mm | 10.1 | 12.3 |
| Right coronary ostium height†, mm | 13.8 | 16.3 |
STS: Society of Thoracic Surgeons; AVA: aortic valve area; LVEF: left ventricular ejection fraction; PASP: pulmonary artery systolic pressure; LV: left ventricle.
* Transthoracic echocardiogram.
† Cardiac CT.
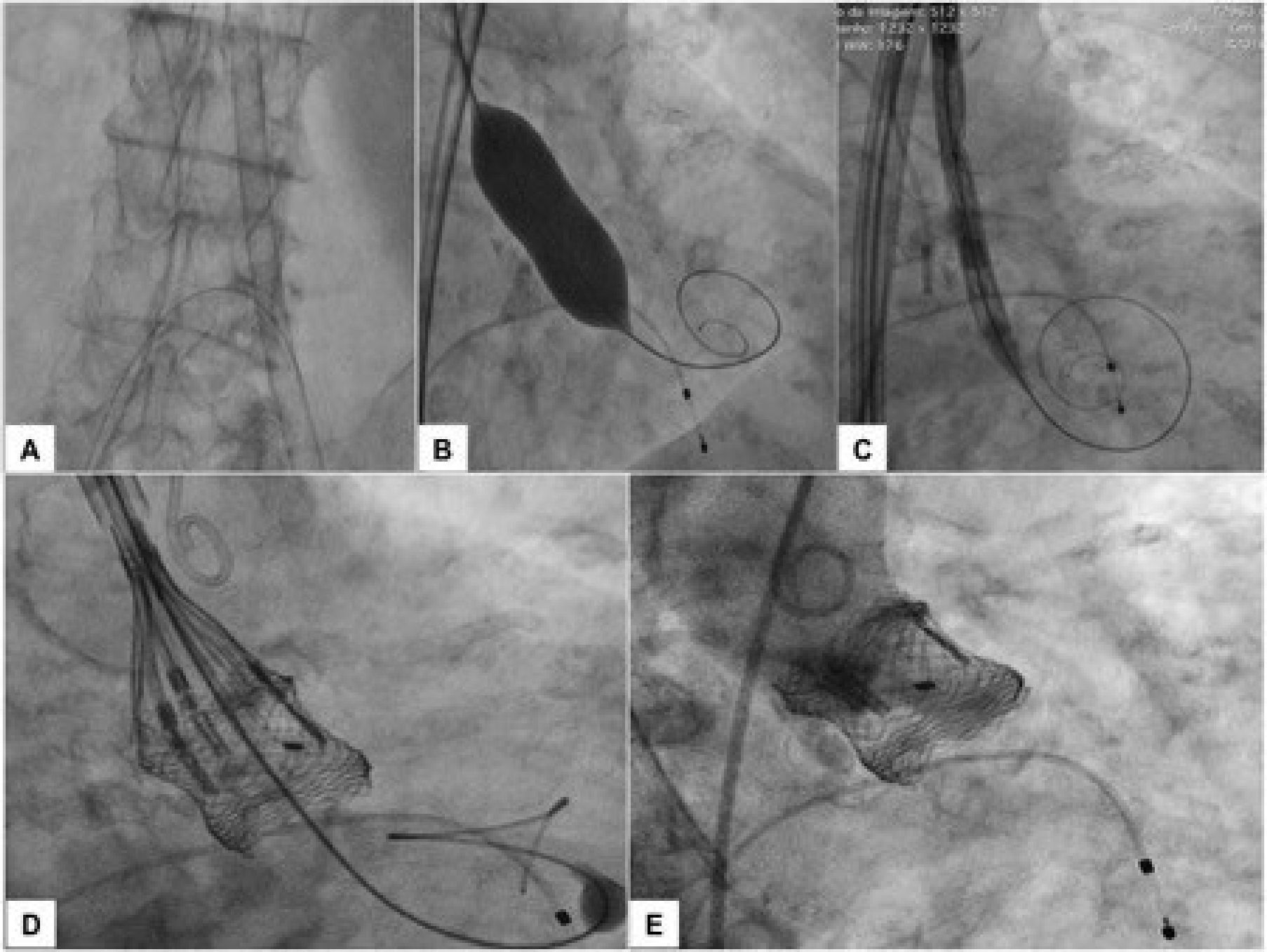
The procedure was performed under local anesthesia, conscious sedation, and without the need for transesophageal echocardiography. Heparin was administered at a dose of 150 IU/kg, in order to achieve an activated clotting time between 300 and 350seconds. Initially, the right femoral arterial access was achieved with a 7 F sheath for contralateral protection with a 0.018 × 300cm guidewire. The main arterial access was achieved percutaneously from the left femoral artery, using two Perclose Proglide® devices (Abbott Vascular, Redwood City, USA), followed by an 18 F sheath. Right femoral venous access was achieved percutaneously for implantation of a temporary pacemaker in the right ventricle. Subsequently, an aortography was performed to identify the view with the alignment of the three cusps on the same plane, which was used to release the prosthesis. The basal peak-to-peak transvalvular aortic gradient was 83mmHg. A rigid 0.035” Safari® Pre-Shaped TAVI Guidewire (Boston Scientific Corporation, Natick, USA) was maintained in the left ventricle and an 18 × 40mm valvuloplasty Cristal® balloon (Balt, Montmorency, France) was advanced over this guidewire, positioned in the aortic valve, and inflated to its nominal diameter under pacemaker-induced tachycardia. Next, a number 23 Lotus™ Aortic Valve System was advanced over the rigid guidewire and positioned at the level of the valve annulus. After angiograms were performed for position adjustment, the device was released. The angiographic control showed no regurgitation, whereas manometry showed no systolic transvalvular gradient (Fig. 1). The TTE performed at the end of the procedure confirmed the absence of aortic regurgitation, a peak gradient of 18mmHg and a mean gradient of 8mmHg. The patient was transferred to the room the next day and was discharged 4 days after the procedure.
(A) 18 F sheath and contralateral protection with 0.018 × 300cm guidewire. (B) 0.035” Safari® rigid guidewire in the left ventricle and 18 × 40mm valvuloplasty balloon in pacemaker-induced tachycardia. (C) Number 23 Lotus™ Valve System at the initial and expanded position. (D) Pre-release position. (E) Control aortography – optimal positioning and absence of aortic regurgitation.
An 87-year-old female patient was admitted at Hospital São Luiz, Unidade Morumbi (Sao Paulo, SP, Brazil) for congestive heart failure (CHF) in NYHA functional class IV. She had a long-term diagnosis of severe AoS, having repeatedly refused surgical treatment. Due to the high surgical risk and in accordance with the patient's preference, it was decided to perform a percutaneous treatment.
Contrast CT was performed to evaluate the vascular access, as well as the aortic valve and aortic root geometry (Table 1).
The procedure was performed under local anesthesia, conscious sedation, and without the need for transesophageal echocardiography. Initially, heparin was administered (150 IU/kg), and right femoral access was achieved with a 7 F sheath (contralateral protection). The main arterial access was achieved percutaneously from the left femoral artery, using two Perclose Proglide® devices (Abbott Vascular, Redwood City, USA), followed by an 18 F sheath. A temporary pacemaker was implanted through the right femoral vein. An aortography was performed to identify the view with the alignment of the three cusps on the same plane, which was used to release the prosthesis. The basal peak-to-peak transvalvular aortic gradient was 90mmHg. Valvuloplasty using an 18 × 40mm Cristal® balloon (Balt, Montmorency, France) was performed before the implantation of a number 23 Lotus™ Aortic Valve System, both advanced over the 0.035” Safari® rigid guidewire and positioned at the level of the valve annulus. After angiograms were performed for position adjustment, the device was released. Angiographic control and the final TTE showed no regurgitation. The post-procedural peak and mean gradients were 15mmHg and 9mmHg, respectively. The patient left the intensive care unit after 48hours and was discharged 5 days after the procedure.
DiscussionThe Lotus™ valve system has been recently introduced in Brazil for the treatment of severe symptomatic AoS. This is a bovine pericardial bioprosthesis with three leaflets attached to a nitinol mesh structure, with a radiopaque marker in a system mounted on a catheter for introduction and retrograde release through the femoral artery (Fig. 2). The valve is released by controlled mechanical expansion. It is pre-attached to a delivery system and features early leaflet operation, which contributes to a controlled and precise initial positioning, repositioning, or complete withdrawal at any time prior to release, even when 100% expanded, as in the case of malapposition or unfavorable complications (e.g., coronary occlusion). It has a single external adaptive seal made of polyurethane/polycarbonate, designed to adapt to irregular anatomical surfaces, occluding interstices between the prosthesis and the degenerated calcified leaflet material at the aorto-ventricular interface, thus minimizing PVR. There is no need for pacemaker-induced tachycardia during implantation. It is available in 23mm (18 F sheath), 25mm, and 27mm (both with 20 F sheath).
Its unique characteristic of early leaflet operation, even before being completely released, associated with the lack of need for rapid ventricular pacing by a pacemaker and its capacity to be fully recaptured after implantation and before its release, are characteristics that prioritize its choice for less invasive strategies, using local anesthesia and conscious sedation.
The REPRISE II6 (REpositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus Valve System: Evaluation of Safety and Performance) clinical trial, a prospective, multicenter, single-arm study in high-risk surgical patients with severe AoS, demonstrated the safety and efficacy of the procedure during successful valve implantation in all 120 patients, while achieving the primary outcome of device performance (mean gradient < 18mmHg at 30 days), with low mortality (4.2%) and incapacitating stroke (1.7%) rates, as well as absence of embolization, ectopic release of the valve, or implantation of an additional valve. Additionally, it showed marked functional class improvement (91% were in functional class I or II, at 30 days) with low rate of significant PVR (moderate PVR occurred in 1.0%, and severe PVR in none of the patients).
General anesthesia has some disadvantages, such as the cardiodepressant effect of general anesthetics, which can cause cardiovascular instability during anesthesia induction and during the procedure, particularly hypotension and bradycardia, and, consequently, the need for vasoconstrictors, which may be deleterious in patients with severe AoS.7 Local anesthesia, in turn, prevents uncontrolled hemodynamic changes, is better tolerated in patients with chronic pulmonary disease, and allows for a prompt monitoring of any neurological alteration. In addition to the possibility of a shorter-duration procedure, with a faster recovery and reduced hospital length of stay, which could reduce the risk of nosocomial infections and other complications associated with hospitalization,8 it has the additional advantage of cost reduction. However, the possibility of change into general anesthesia should be considered at any time during the procedure.
This strategy was assessed in a French registry9 (FRANCE 2 Registry Investigators) with 2,326 patients undergoing TAVI via femoral artery, using the CoreValve™ (Medtronic, Minneapolis, USA) and Edwards SAPIEN/SAPIEN XT (Edwards Lifesciences, Irvine, USA) prostheses and compared the clinical outcomes of those who underwent the procedure under general (1,377 patients) or local anesthesia (949 patients). There were no differences regarding device implantation success and survival rate at 30 days (97.6% vs. 97.0%; p = 0.41, and 91.6% vs. 91.3%; p = 0.69, respectively), while the incidence of PVR ≥ mild was significantly lower with general anesthesia (15.0% vs. 19.1%; p = 0.015), a finding that was not confirmed after group pairing (12.7% vs. 16.2%; p = 0.19). There are no studies that assessed this topic with the second-generation Lotus™ device.
In both cases, there was no need for vasoactive drugs during the procedure, and patients remained collaborative despite sedation, leading to a rapid recovery and early hospital discharge (at the fourth and fifth days). There was no need for permanent pacemaker implantation in any patient.
In conclusion, the cases described in this study demonstrate the performance of a prosthesis that allows greater stability during the procedure, accurate placement (with correction at any stage prior to its release), and adaptive seal with the potential to minimize PVR (and also decrease the need for TTE). Furthermore, this prosthesis enables the use of less invasive techniques, such as local anesthesia and conscious sedation.
Funding sourceNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.