The radial access is widely used for cardiac catheterization and angioplasty, with advantages such as decreased risk of bleeding and vascular complications. This case reports on a rare complication during cardiac catheterization by radial access: radial artery endothelial tissue avulsion through the catheter and its embolization to the left anterior descending artery, which was resolved by manual aspiration of the embolized fragment. This complication may be related to the specific anatomical characteristics of the patient's radial artery.
A via radial é amplamente utilizada para a realização de cateterismo cardíaco e angioplastia, com vantagens como a diminuição do risco de sangramento e de complicações vasculares. O presente caso relata uma complicação rara durante o cateterismo cardíaco por via radial: a avulsão de tecido endotelial da artéria radial pelo cateter e sua embolização para a artéria descendente anterior, a qual foi resolvida pela aspiração manual do fragmento embolizado. Tal complicação pode ter relação com as características anatômicas específicas da artéria radial do paciente.
The radial access approach has been increasingly used in diagnostic and therapeutic procedures, as it is associated with a decreased risk of bleeding, greater comfort to the patient, and early ambulation after the procedure, when compared with the femoral access.1–3 Particularly, the reduction in bleeding translates into a decrease in clinical events and even mortality reduction, especially in patients with ST elevation myocardial infarction (STEMI).4,5
Therefore, the knowledge and management of possible complications related to the radial access are important; among them, the authors report radial artery avulsion, a very rare complication, whose occurrence can lead to radial artery occlusion.6,7 Radial artery avulsion may be associated to the embolization of endothelium fragments with acute coronary occlusion, requiring immediate percutaneous intervention, as demonstrated in this case.
Case reportA 63-year-old female patient, with a history of diabetes mellitus, hypertension, and multivessel coronary artery disease with inferior wall STEMI in March 2012. At the time of admission, a coronary angiography was performed, showing right coronary artery (RCA) occlusion and obstructive lesions in the left anterior descending artery (LAD) and the marginal branch, with grade 3 collateral circulation from LAD to RCA. An unsuccessful attempt was made to perform angioplasty in the RCA, due to the impossibility of crossing the lesion with the guidewire. The patient was evaluated by the cardiology team, which indicated surgical revascularization.
The patient was discharged on the recommendation of returning after 60 days to be reassessed and to schedule the surgery, but she did not return to continue the treatment. In November 2014, due to chest pain on moderate physical effort, she returned to the outpatient clinic and a new coronary angiography was requested.
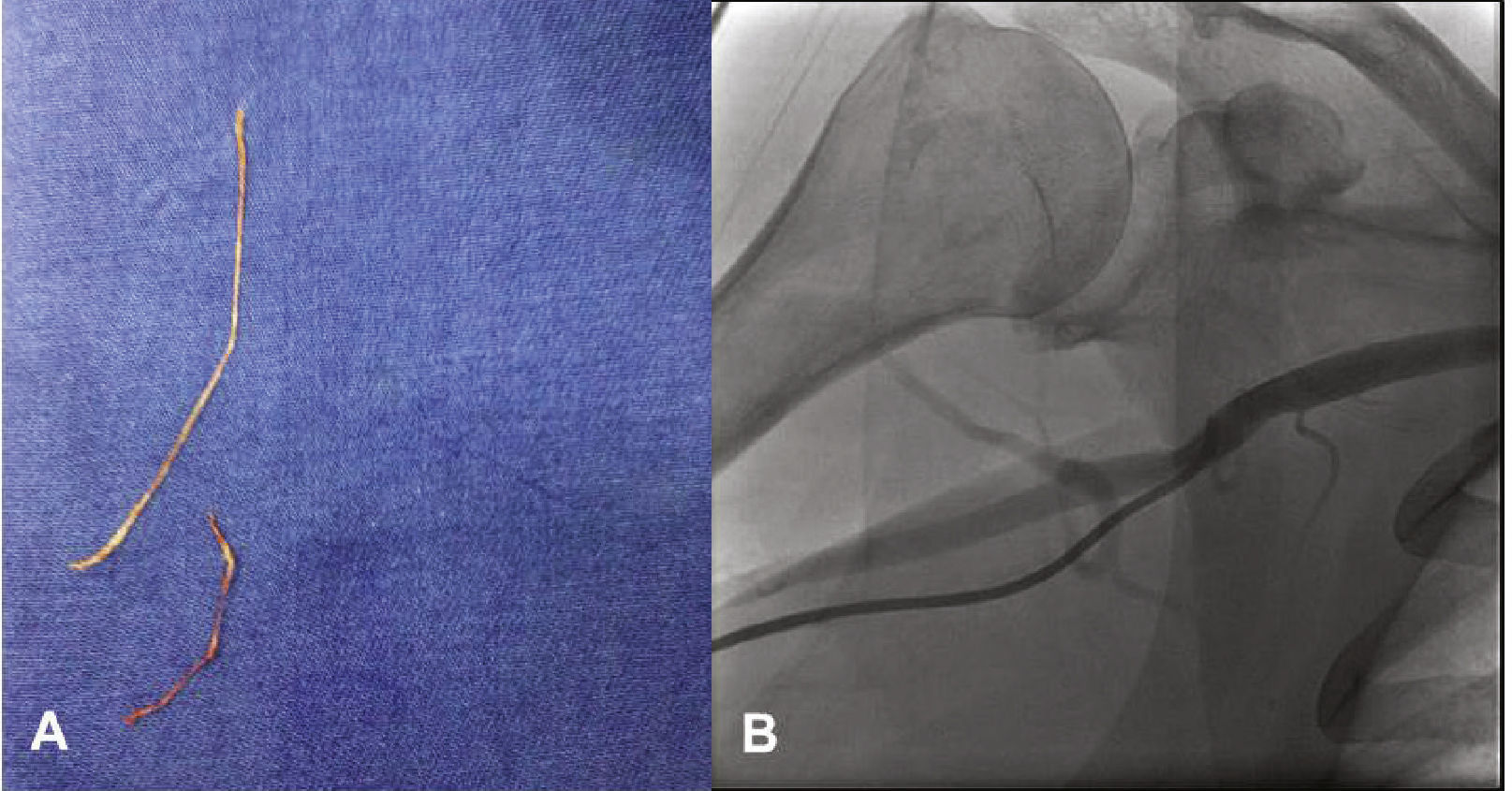
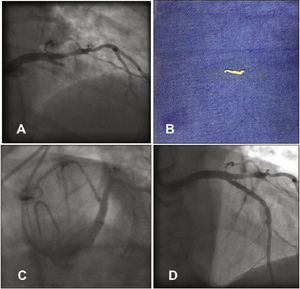
The procedure was performed using radial access, after a normal Allen test result, using a 6 F Glidesheath and 0.021”/45cm straight-tip guidewire that accompanies the sheath (Terumo Medical®, Tokyo, Japan). Heparin was administered at a dose of 5,000 IU through the sheath. When passing the Judkins JL 3.5 5 F catheter and 0.038” Stiff hydrophilic guidewire (Terumo Medical®, Tokyo, Japan) set through the right radial artery, the patient reported pain in the arm and difficulty was perceived when advancing the catheter through the right brachial artery; nonetheless, the catheter reached the aortic root. After guidewire removal, there was no blood reflux into the catheter and, therefore, the catheter was withdrawn and washed with 0.9% saline solution, followed by the elimination of tubular-like and whitish tissue (Fig. 1A). The same catheter was reintroduced and contrast injection was performed in the radial artery, which was shown to be a small-caliber vessel, originating from the right axillary artery (Fig. 1B). At this time, 40mg of papaverine, diluted in 10mL of 0.9% saline solution, were slowly administered intra-arterially for 2minutes. With the aid of the hydrophilic guide, the catheter once again reached the aortic root and left coronary angiography was performed in the anteroposterior view, disclosing LAD occlusion (Fig. 2A). At this point, the patient started reporting severe chest pain.
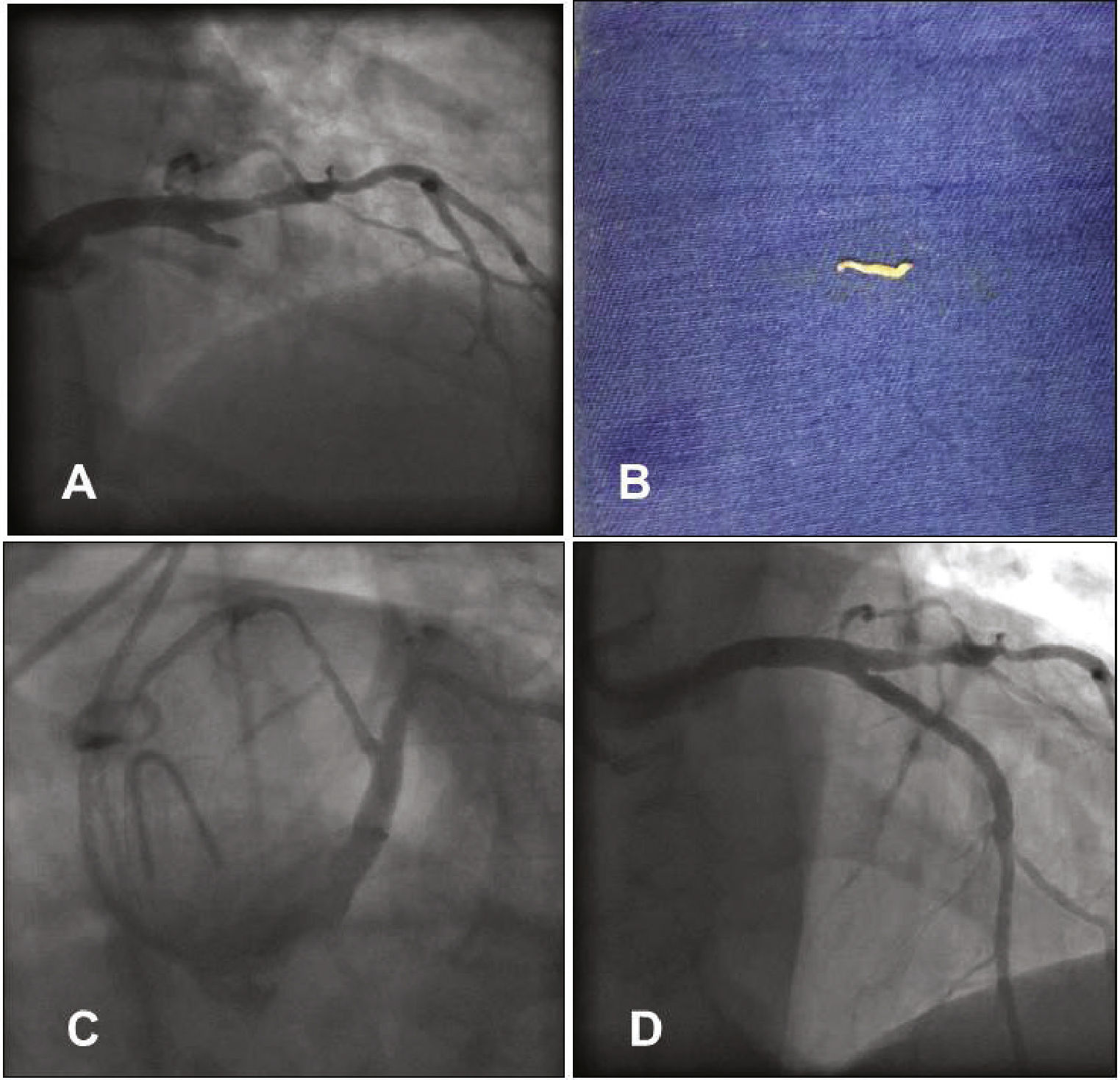
(A) Left coronary angiography showing left anterior descending artery proximal occlusion. (B) Tissue fragment removed from the left anterior descending artery by manual aspiration. (C) Left coronary angiography showing reperfusion of the left anterior descending artery after aspiration. (D) Control left coronary angiography after stent implantation.
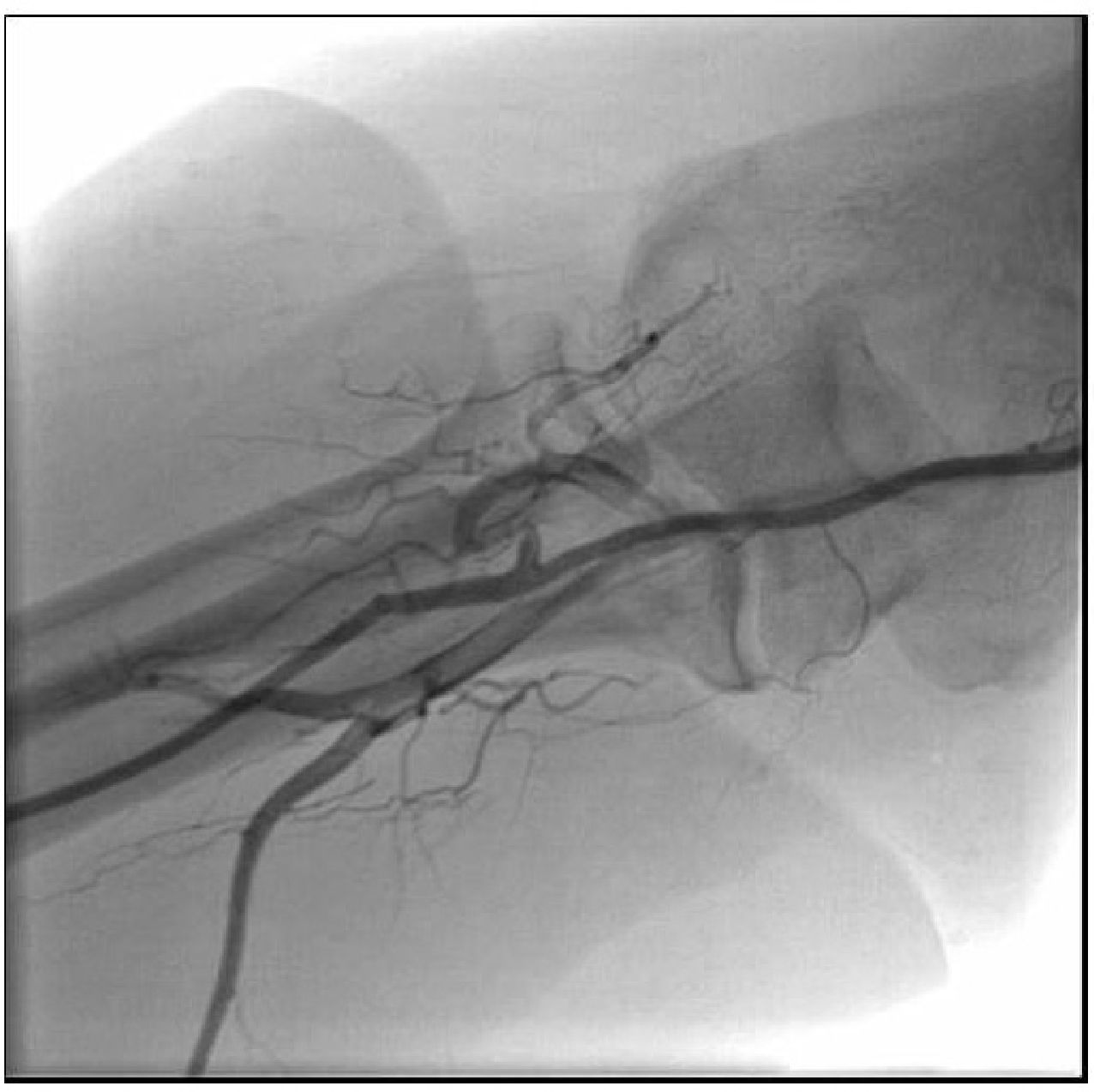
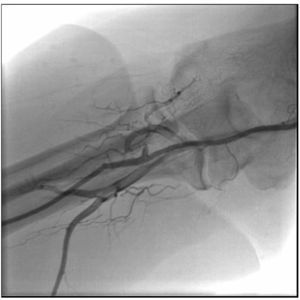
She was treated with intravenous (IV) heparin, complemented to reach 100 IU/kg, 600mg of oral clopidogrel and the diagnostic catheter was replaced by an EBU 3.5 6 F guide catheter (Medtronic, Minneapolis, USA) through the right radial artery. An Extra Support 0.014” Whisper guidewire (Abbott Vascular, Santa Clara, USA) was advanced to the distal LAD and then manual aspiration was performed using a Thrombuster II catheter (Kaneca Medix Corporation, Tokyo, Japan), which removed a small fragment similar to the one removed from the diagnostic catheter (Fig. 2B). The control coronary angiography showed LAD with Thrombolysis In Myocardial Infarction (TIMI) III flow and an 80% proximal segmental lesion (Fig. 2C), similar to the catheterization that had been performed in 2012. Pre-dilation was performed with a 2.5 × 20mm Pantera balloon (Biotronik Corporation, Berlin, Germany) at 8atm, followed by the 3.0 × 32mm Promus Element stent (Boston Scientific Inc., Natick, USA) implantation at 18atm. Post-implantation control coronary angiography showed a well-expanded stent, without dissections, and TIMI III flow (Fig. 2D). The right radial angiography performed after the procedure disclosed a patent radial artery (Fig. 3).
The patient remained asymptomatic during hospitalization, with no electrocardiographic alterations, with a peak troponin of 0.87 ng/mL and MB isoenzyme of creatine kinase (CKMB) mass of 3 ng/mL, and was discharged on dual antiplatelet therapy. At the clinical reassessment 2 months after hospital discharge, the patient remained asymptomatic, and the right radial pulse was present, only slightly decreased in comparison to the left radial pulse.
DiscussionThe present case differs from reports of radial artery avulsion reported in the medical literature by the simultaneous occurrence of endothelial fragment embolization to the coronary artery resulting in occlusion, which was resolved by using the aspiration catheter.
The most common complications of using the radial access when performing coronary angiography and coronary angioplasty are arterial spasm and thrombotic occlusion of the radial artery. Other more infrequent complications are bleeding at the access site, forearm hematomas, perforation of the radial or brachial artery, pseudoaneurysm, compartment syndrome, and abscess at the access site.1,8
Radial artery avulsion is a very rare complication; most case reports are related to the moment of radial sheath withdrawal6,7,9 and, less commonly, to spasm with guide catheter entrapment. In the present case, the radial artery, which was a small-caliber vessel, had an anatomical variation, originating at the axillary artery, a fact that led to the occurrence of spasm and resulted in the tissue fragment avulsion through the catheter tip.10
Among the radial artery anatomic variations, three of them – high bifurcation (axillary), curvature, and tortuosity of the radial artery – are associated with failure of the radial technique. The high bifurcation is frequently observed and can be divided into four types, according to variations in the radial artery diameter and anastomoses.1 In the third type, similar to that observed in the present report, the remaining or hypoplastic radial artery is a small-caliber vessel, whose diameter was too small even for 4 F catheter use. The alternative access approach is preferred in this extreme case, due to the pain at catheter progression associated with spasm and increased risk of perforation. Contralateral radial access is always a possibility to be considered in this context, as the forearm vasculature tends to be asymmetrical. Alternatively, the femoral access can be used.1
Coronary embolism may be caused by conditions such as infective endocarditis, atrial fibrillation, mural thrombus fragmentation in the left atrium or left ventricle, and atrial myxoma or thrombus originating in heart valve prostheses.11 In this case report, the embolism probably occurred due to a new tissue avulsion through the catheter tip, after washing and reintroduction, or alternatively during the first attempt at the left coronary catheterization due to the migration of this tissue fragment within the catheter. Manual thromboaspiration, usually used for thrombus aspiration in STEMI,12 was effective in adequately aspirating the embolus.
The radial artery avulsion was probably due to trauma caused by the catheter tip in a small-caliber vessel, with an additional subjacent spasm. The predictors of radial artery spasm are older age, short stature, small-caliber radial artery, female gender, diabetes, failure at the first attempt of arterial access, and pain.1 Measures to prevent and/or treat vasospasm are useful to reduce the chances of this complication, such as adequate local anesthesia, use of hydrophilic sheaths and catheters,13 gentle manipulation of catheters, use of sedation to reduce anxiety, and systematic prophylactic use of spasmolytic drugs. The use of nitroglycerin and verapamil can reduce the incidence of radial artery spasm to < 5% with 6 F catheters and to < 1%, with 5 F catheters.14 The use of a sheath with an extremely fine-tipped dilator also contributes to reduce trauma to the vessel wall.
One limitation of this report was not performing the histopathological analysis of the extracted fragments.
Funding sourceNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.