Inadequate anastomosis of aorto-coronary grafts to the coronary venous system represents an infrequent but potentially serious complication of coronary artery bypass surgery. Different surgical techniques and percutaneous devices have been described for the permanent correction of these fistulas. We report two cases of iatrogenic aorto-coronary fistulas successfully treated with percutaneous occlusion using the self-expandable nitinol device (vascular plug).
Oclusão Percutânea de Fístula Aorto-Coronária Venosa Adquirida Após Revascularização Miocárdica: ExperiênciaInicial com Plug Vascular Autoexpansível de Nitinol
Enxertos aorto-coronários anastomosados inapropriadamente no sistema venoso representam complicação infrequente, porém potencialmente grave, das cirurgias de revascularização miocárdica. Técnicas cirúrgicas e dispositivos percutâneos diversos têm sido descritos para a correção definitiva dessas fístulas. Relatamos aqui dois casos de fístulas aorto-coronárias iatrogênicas submetidas à oclusão percutânea com sucesso, utilizando-se dispositivo autoexpansível de nitinol (plug vascular).
Coronary fistulas are rare congenital or acquired conditions that may determine high output heart failure, pulmonary arterial hypertension or myocardial ischemia, secondary to left-right shunt or the “coronary theft” phenomenon.1
While an initial period of observation in asymptomatic patients has been the rule, the presence of symptoms or progressive increase in size of these anomalous connections determine the need for intervention – percutaneous or surgical.2-4
We report our initial experience of percutaneous embolization of arteriovenous fistulas (AVFs) acquired after coronary-artery bypass graft (CABG), using a self-expandable nitinol vascular occluder in two patients that presented with persistent angina symptoms after complete myocardial revascularization and optimal drug therapy.
CASE REPORTSCase 1Female patient, 65 years-old, with a history of type 2 diabetes mellitus, hypertension and chronic coronary artery disease (CABG performed in 2011), admitted in April 2013 due to acute coronary syndrome of intermediate risk (GRACE score: 96). Serial electrocardiograms showed regular sinus rhythm and no alterations consistent with acute ischemia; markers of myocardial necrosis were negative. Two-dimensional transthoracic echocardiography showed slight dilation of the right atrium and moderate in the left. The left ventricle showed small dilatation and slight reduction in the overall systolic function with due to anterior septal hypokinesia (mid-basal segment). The ejection fraction (Simpson) was 0.53 and systolic pulmonary artery pressure was 24mmHg.
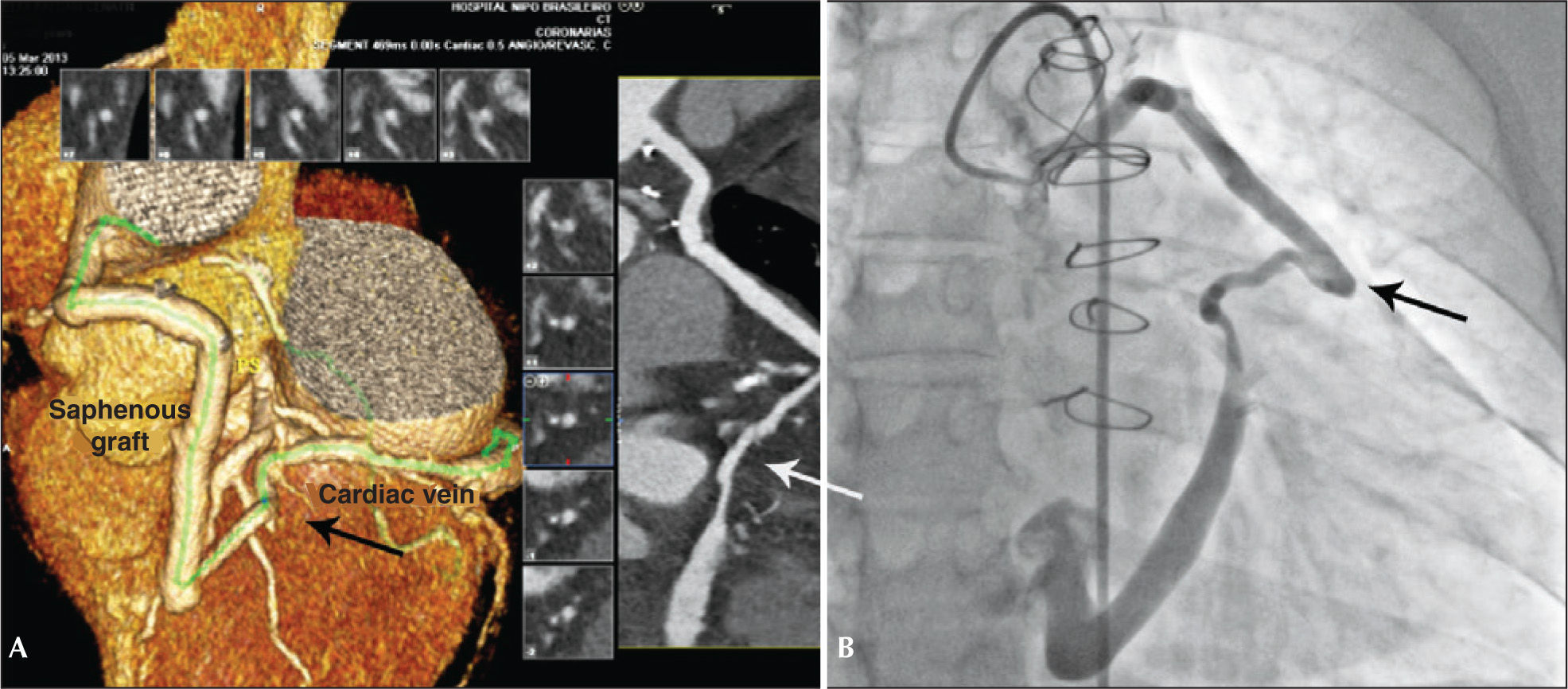
The coronary angiography showed a dilated saphenous vein graft connected to the great cardiac vein, with significant dilation of the coronary sinus (Figure 1A).
Coronary angiography confirmed the presence of the saphenous vein connection with the great cardiac vein, with additional evidence of segmental and severe obstructive lesion in the mid-third of the nonrevascularized left circumflex artery (Figure 1B). We chose to perform a percutaneous coronary intervention (PCI) of the left circumflex artery and outpatient programming for the AVF occlusion. Everolimus-eluting stenting was performed successfully using a 2.75×33mm Xience Prime® stent (Abbott Vascular, Santa Clara, United States).
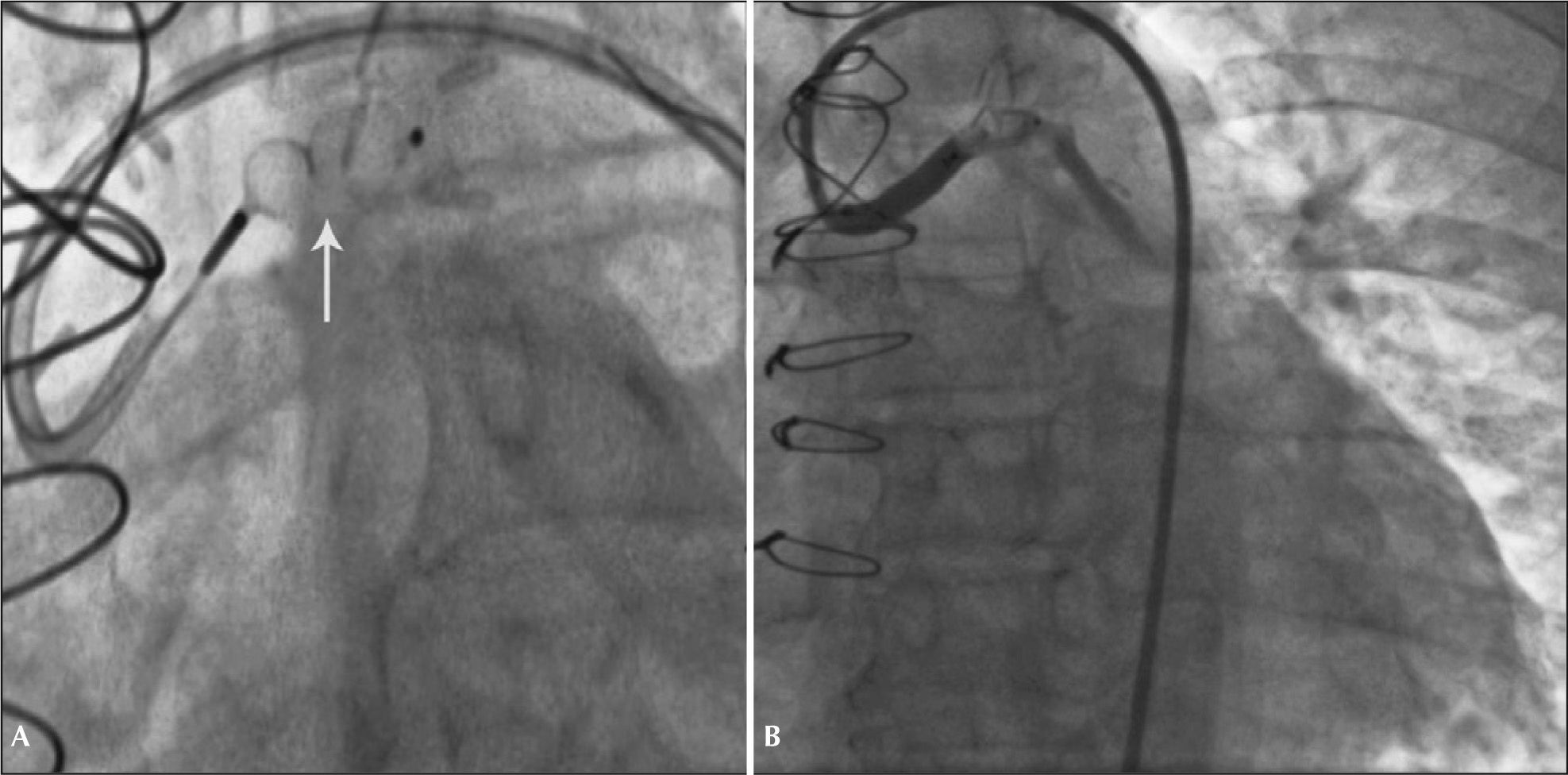
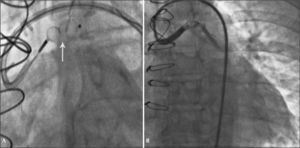
Fifteen days after the discharge, the patient had a new episode of prolonged angina at rest, with similar characteristics to those of the previous event, which resulted in a new admission to the coronary care unit. A new coronary angiography showed a patent stent and percutaneous occlusion of the AVF was then performed. Anticoagulation with unfractionated heparin at a dose of 100 U/kg and selective catheterization of the graft with a 7F Amplatz Right-2 guide catheter were carried out. Due to the proximal tortuosity, it was decided to place two 0.014” ChoICE® PT Extra Support Guide Wires (Boston Scientific Corporation, Natick, USA) distally, providing deep intubation of the guide catheter (Figure 2A).
Under fluoroscopic control, the 8.0×7.0mm Amplatzer® Vascular Plug II was advanced (St. Jude Medical, Minnetonka, USA) up to the middle segment of the graft, when the guidewires were retracted and then, the device was released. Control angiography showed total occlusion of the graft 5 minutes after the release of the prosthesis (Figure 2B).
Case 2Female patient, 45 years-old, with a history of hypertension, dyslipidemia and chronic coronary disease. She was submitted to PCI with drug-eluting stent implantation in left main coronary artery and left anterior descending and left circumflex arteries, in addition to two CABG procedures (the last in October 2012), with a current picture of atypical chest pain and dyspnea on exertion.
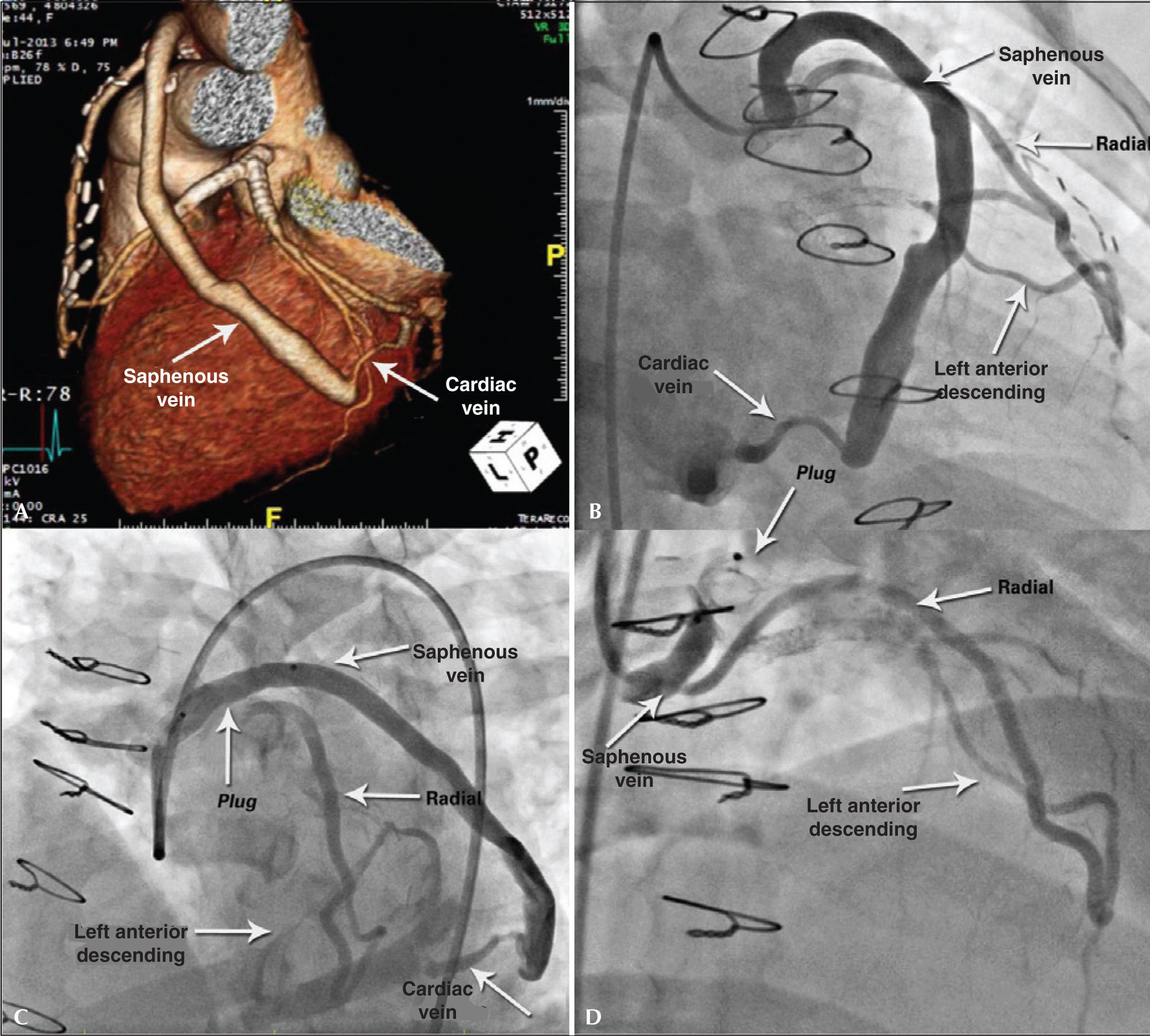
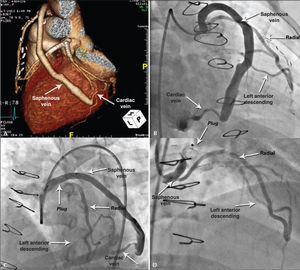
During the diagnostic investigation she was submitted to coronary angiography, which showed a Y graft from the radial artery to left anterior descending artery and saphenous vein grafts with distal anastomosis in the cardiac vein (Figure 3A).
– Case 2: Angiography demonstrating saphenous-cardiac vein anastomosis (A). Angiography showing the two branches of the Y graft, radialleft anterior descending and saphenous-cardiac vein (B). Immediate outcome with ample flow after plug release, which can be seen by the proximal and distal marks (C). Outcome after 30 days of procedure, showing the better positioned plug and occlusion of the branch to the cardiac vein, in addition to permeability of Y to left anterior descending artery (D).
Coronary angiography showed stents positioned in the left main coronary artery and in the proximal portions of the anterior descending and circumflex arteries, with severe focal in-stent restenosis at the left circumflex artery ostium, confirming the presence of AVF described on angiography (Figure 3B). Percutaneous occlusion of the AVF was performed during the same procedure. Selective catheterization of the graft was achieved with a 6F Amplatz Left-3 guide catheter under full heparinization, using a 0.014” extra-support guidewire for deep intubation. The selected device was the 10×7.0mm Amplatzer® Vascular Plug II (Figure 3C) positioned in bypass upstream of the emergence of the radial artery Y and released after removal of the guidewire. A slight slowing of the anterograde flow was observed in the saphenous graft, while maintaining its patency.
After a 30-day evolution, the patient was admitted to the interventional laboratory for treatment of in-stent restenosis in the left circumflex artery and reassessment of the treated graft. She was successfully submitted to PCI with a 3.0×15mm Pantera® Lux paclitaxel-eluting balloon (Biotronik, Berlin, Germany). Control angiography showed complete occlusion in anastomosis of coronary bypass with the cardiac vein, maintaining the flow at the anastomosis in Y to the left anterior descending artery (Figure 3D).
DISCUSSIONAcquired coronary fistulas resulting from the inadvertent insertion of aorto-coronary grafts (or of internal thoracic artery in situ) in cardiac veins represent a rare and potentially severe complication of CABG surgeries.3
In a recent review, Gardner et al.4 reported only 36 published cases, in which most AVFs involved the territory of the left anterior descending artery (66%) and diagonal branches (11%).
Distortions in ventricular anatomy (fibrosis and pericardial adhesions secondary to infarction, pericarditis or previous CABG), presence of intramyocardial trajectory, overlying epicardial fat and vascular collapse secondary to cardioplegic solution and clamping of the aorta may hinder the identification of the target vessel and predispose to distal anastomosis in the venous system.4
High-frequency continuous systolic murmur, heart failure of undefined cause and persistent angina after CABG should raise the possibility of iatrogenic AVF. Symptoms can manifest from the immediate postoperative period up to several years after the intervention.2,5
The natural history of these AVFs depends on the magnitude of the left-right shunt developed. Similarly to congenital fistulas, high-output cardiac failure, thrombotic events, progressive dilation with rupture and hemopericardium, pulmonary arterial hypertension, atrial and ventricular arrhythmias and infective endarteritis are possible complications.3,4 Some patients, however, remain asymptomatic and eventually undergo spontaneous AVF occlusion.6
Manifest myocardial ischemia by positive functional tests or as persistent angina has as its main mechanisms the limitation of flow in nonrevascularized territory and the “coronary theft” phenomenon (more evident in sequential grafts).1,5 The absence of retrograde myocardial perfusion, from the arterialized coronary sinus (in the absence of proximal vein ligation) may potentiate myocardial ischemia in these cases.7
Left heart catheterization (coronary angiography and manometry) remains the gold standard for the assessment of anatomy, hemodynamic repercussions and AVF therapeutic planning. CT angiography and cardiac MRI have shown to be invaluable, providing additional information about the trajectory, the drainage site and the association of the AVF with other structures.
There is no consensus regarding the initial modality of intervention in the group of symptomatic patients or those refractory to the initial medical therapy. Among the options described, the most often used are the AVF ligation associated with a new CABG or percutaneous embolization of the AVF (anterograde or retrograde through the coronary sinus). The latter modality uses coils, detachable balloons or covered stents, associated or not with PCI in the nonrevascularized coronary lesion.2,3,8
In the two cases described here, the percutaneous embolization of the acquired AVFs using the selfexpandable nitinol vascular plug was technically simple and successful, even in the presence of dual antiplatelet therapy. It is a cylindrical prosthesis consisting of an uncoated, low profile, nitinol mesh that does not require the use of a sheath and allows the repositioning and eventual removal of the device. The plug is compatible with 5 to 7 C guide catheters, depending on the selected prostheses, and it is recommended that it should be 30 to 50% larger than the target vessel diameter, in order to minimize the risk of distal embolization (Figure 4).
Initially devised for use in peripheral embolization, different types of plugs have been used with success and low incidence of complications in the treatment of vascular abnormalities associated with congenital heart disease. In 2007, Fischer et al.9 reported three cases of percutaneous occlusion of congenital AVFs using this vascular device. Mylonas et al.10 described the percutaneous occlusion of a 9cm diameter aneurysm in a degenerated saphenous vein graft to the obtuse marginal branch in an 85-year-old patient. We used an 8mm vascular plug, with immediate success of the procedure, confirmed by the control CT in 30 days.
Percutaneous embolization for the treatment of iatrogenic AVF after CABG has been shown to be a safe and effective method, thus becoming the method of choice in opposition to the surgical correction.4
We describe an alternative herein, not yet reported in the literature, to the classic use of detachable balloons or coils in the treatment of this particular condition. Percutaneous embolization with vascular occluder showed to be technically simple, albeit still a high-cost one. Late follow-up is needed to assess the durability of the occluding effect as well as the risk of complications, especially of distal embolization and vascular perforation.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
FUNDING SOURCENone.