For several years the percutaneous closure of patent ductus arteriosus has been a reliable and effective technique for most of the morphologic variants described by Krichenko. Type B, or window-type, patent ductus arteriosus remains a challenge due to the higher risk of device embolizations and incomplete occlusions. We report the successful use of AMPLATZER™ septal occluder in three patients with window-type patent ductus arteriosus, two cases treated with a 5-mm device and one case with a 7-mm device. The AMPLATZER™ device designed for the occlusion of atrial septal defects is effective for the percutaneous treatment of this morphological variant of patent ductus arteriosus.
Fechamento do Canal Arterial Persistente Tipo Janela Com o Oclusor Septal AMPLATZER™
Há vários anos, a oclusão percutânea do canal arterial persistente é uma técnica factível e eficaz na maioria das variantes morfológicas descritas por Krishenko. O tipo B, em janela, caracterizado por ser curto, permanece um desafio, devido ao maior risco de embolizações das próteses e das oclusões incompletas. Descrevemos aqui o uso bem-sucedido de oclusores septais AMPLATZER™ em três pacientes com canal arterial em janela, dois casos tratados com dispositivos de 5mm e um com o de 7mm. O dispositivo AMPLATZER™ desenhado para a oclusão da comunicação interatrial mostrou-se eficaz para o tratamento percutâneo dessa variante morfológica de canal arterial persistente.
Percutaneous exclusion of patent ductus arteriosus (PDA) is a feasible and effective technique in most morphological variants described by Krichenko et al.1 However, type B or window-type PDA, characterized as short, remains a challenge due to the higher risk of prosthesis embolization and incomplete occlusions. To solve this limitation in adults, double disk devices originally designed for the occlusion of septal defects have been used, such as CardioSEAL®, Starflex® and, more recently, AMPLATZER™.2−6
This is a report of three cases with window-type PDA treated with AMPLATZER™ Septal Occluder (AGA Medical Corp., Goleen Valley, United States) designed to treat atrial septal defects.
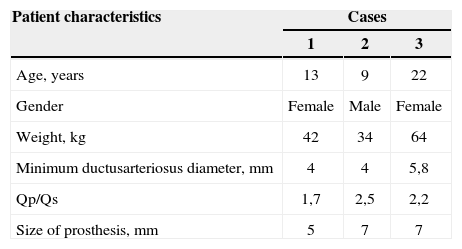
CLINICAL CASESThree patients, two females, with continuous murmur in the second left intercostal space, broad pulse, electrocardiographic and radiological data of left ventricular volume overload and pulmonary hyperflow were evaluated by echocardiography. The presence of patent ductus arteriosus with continuous left-to-right flow and left ventricular dilation was observed (Table 1).
All guardians or tutors signed the consent form and the patients were taken to the laboratory of hemodynamics for percutaneous occlusion of the ductus arteriosus. Patients were submitted to general anesthesia, and arterial and femoral venipuncture; after receiving heparin at a dose of 100 U/kg, full hemodynamic assessment was performed, which detected high pulmonary blood pressure in the third case (49/32mmHg, with a mean of 41mmHg) and normal in the other two. The descending aortogram with modification of the lateral projection given by the caudal angulation, confirmed the presence of very short ductus arteriosus (Table 1) and pulmonary diameters>3mm, as well as planar aortic ampullas (Figure 1A and 2A). Due to the characteristics of the PDAs, it was decided that the AMPLATZER™ septal occluder would better adapt to the observed morphology, which is why we chose to use these devices, estimating a size 1 to 2mm above the minimum ductus diameter.
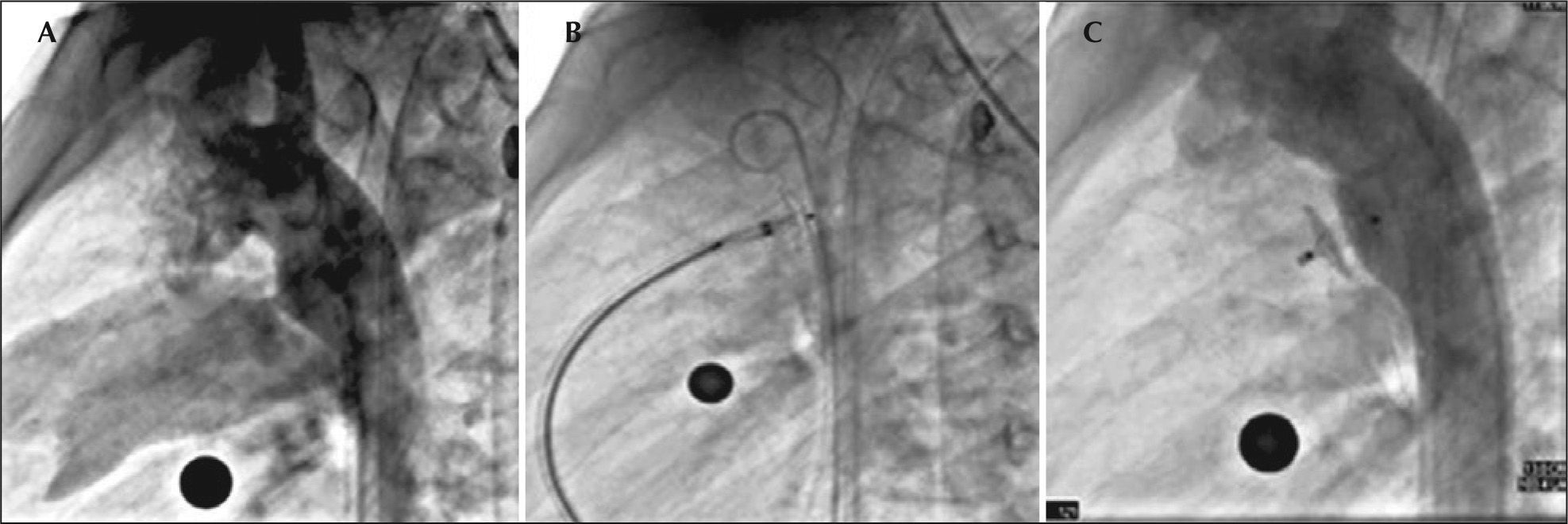
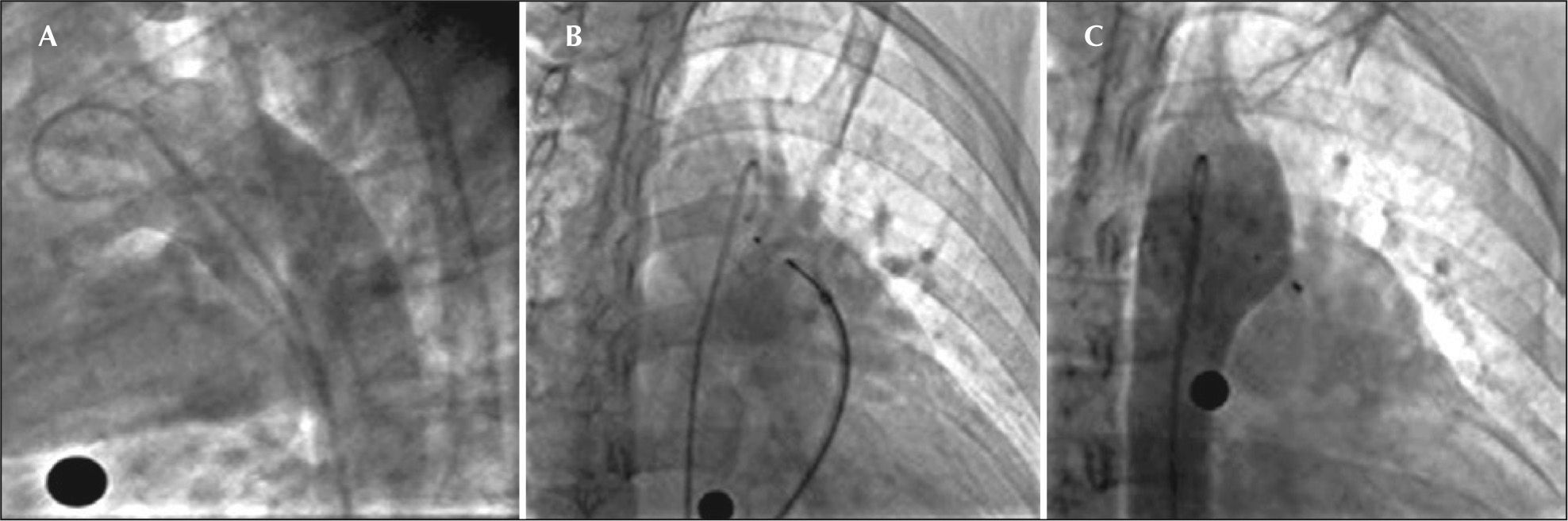
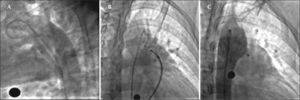
– Angiography in the right oblique view showing passage of the contrast to the pulmonary artery through the ductus arteriosus (A). Opening of the left disk in the aortic ampulla (B). Control angiography in right oblique view, posterior to the implant, showing correct device position without residual shunt (C).
In the first two cases, the cannulation of the ductus arteriosus was performed from the pulmonary artery to the descending aorta using a wedge pressure catheter (Arrow, Reading, United States), whereas in the third case, it was necessary to perform the cannulation from the aorta and create an arteriovenous loop through the ductus arteriosus, and, later, position a 0.035×260cm Amplatz Super Stiff™ Guidewire that allows placing the 6F-releasing system in the descending aorta. The selected AMPLATZER™ septal occluder was loaded and taken to the descending aorta under fluoroscopy, releasing the left disc of the device. Subsequently, the system was gently retracted until the disc was positioned in the ductus ampulla and the correct location was verified through the angiographic control, maintaining gentle cable traction when removing the sheath, exposing the central waist within the ductus arteriosus and the right disc in pulmonary artery. As shown in Figures 1B and 2B, with the cable still attached to the device, we verified the absence of pressure gradients at the aortic level and in the left pulmonary artery, releasing the device. Lastly, the angiographic evaluation showed proper positioning of the devices (Figures 1C and 2C). Patients were discharged on the following day, without complications and advised to undergo endocarditis prophylaxis for 6 months thereafter. The echocardiograms performed at 1, 3, 6 and 12 months after the implant confirmed adequate device positioning with no associated obstructions.
DISCUSSIONPercutaneous occlusion of most angiographic variants of patent ductus arteriosus can be performed with a variety of devices, including coils with and without controlled release, as well as AMPLATZER™ I and II occluders.7−9 These may be used also to treat the most unusual anatomical variants.10 Unquestionably, the window-type PDA continues to show limitations to occlusion, basically when the minimum ductus diameter is>3mm. The short extension of these defects, associated with a low length/diameter ratio complicates the stabilization of the previously mentioned occluders, which generates a high risk of embolization. Furthermore, device protrusion into the aorta or into the pulmonary artery can occur, causing obstruction of these structures. These factors are associated with high failure rate for the percutaneous closure of type B ductus arteriosus.11,12
In PDA closure, the use of double-disc devices such as AMPLATZER™ for muscular ventricular septal defect was initially described in adults with large ductus arteriosus and pulmonary artery hypertension.2−4 Subsequently, Stone et al.6 described, also in adults, the implant of an AMPLATZER™ Septal Occluder device for the treatment of a large and short ductus. On the other hand, and almost simultaneously, Bialkowski et al.2 described the use of StarFlex® and CardioSEAL® devices as an option for closing the window-type PDA, reporting the successful treatment of two cases (a 62-year-old woman and a 17-year-old boy weighing 72kg). Unlike the cases described above, our patients had no significant pulmonary hypertension; however, the short length of the PDA forced us to consider using a device that could ensure adequate stabilization with total defect occlusion. Additionally, our patients were younger and weighed less. The advantages of using the AMPLATZER™ device are due to its double disc system with a short central waist, which ensures an adequate configuration to address the morphology of this PDA variant, resulting in an optimal occlusion without the risk of embolization. Additionally, the delivery and release system profile allows its implant in pediatric patients, as demonstrated by our cases. On the other hand, its disadvantage is its higher cost, in comparison with other generally used occluders.
Therefore, we conclude that in pediatric patients with window-type patent ductus arteriosus, the use of the AMPLATZER™ septal occluder can be safe and effective in the percutaneous treatment of these defects.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
FUNDING SOURCENone.