Atrial fibrillation (AF) increases the risk of thromboembolic events caused by emboli originating in the left atrial appendage (LAA). Mechanical methods for LAA occlusion have been developed as an alternative to oral anticoagulation. The aim of this study was to present an initial experience with the AMPLATZER® Cardiac Plug.
MethodsPatients with permanent or paroxysmal AF and with contraindications or complications of oral anticoagulation were included. Patients with LAA anatomy and measures compatible with the occluder, and without thrombi, were selected through transesophageal echocardiography.
ResultsA total of 14 procedures were performed in 13 patients (5M:8F), with mean age of 66.7 years. Significant bleeding and previous strokes were found in 69.2% and 53.8%, respectively. AF was permanent in 84.6% and paroxysmal in the remainder. The mean diameters of the ostium and the landing zone were 23.9mm and 20.8mm, respectively. Bilobulated LAA was observed in 76.9%. Procedures were possible in all cases. Sixteen devices were used in 13 patients, a ratio of 1.2:1, and only one patient required a second device for LAA occlusion. The mean follow-up was 12.2 months. All LAA remain closed, with no residual defect to date. There was only one late death, unrelated to the procedure.
ConclusionsLAA occlusion using the AMPLATZER® Cardiac Plug device was shown to be safe and effective in this small series of patients. The initial results are encouraging and indicate the transcatheter closure of the LAA as an alternative to oral anticoagulation therapy in selected patients.
A fibrilação atrial (FA) aumenta o risco de eventos tromboembólicos por êmbolos originados em apêndice atrial esquerdo (AAE). Métodos mecânicos para a oclusão do AAE foram desenvolvidos como alternativa à anticoagulação oral. O objetivo deste trabalho foi apresentar uma experiência inicial com o AMPLATZER® Cardiac Plug.
MétodosIncluímos pacientes com FA permanente ou paroxística, que apresentavam contraindicações ou complicações derivadas da anticoagulação oral. Pacientes com anatomia e medidas do AAE compatíveis com o oclusor, e sem trombos foram selecionados por meio de ecocardiograma transesofágico.
ResultadosForam realizados 14 procedimentos em 13 pacientes (5M:8F), com média de idade de 66,7 anos. Sangramento significativo e acidentes vasculares cerebrais prévios foram encontrados em 69,2% e em 53,8%, respectivamente. A FA era permanente em 84,6% e paroxística no restante da amostra. Os diâmetros médio do óstio e da zona alvo mediram 23,9mm e 20,8mm, respectivamente. AAE bilobulados foram observados em 76,9%. Os procedimentos foram possíveis em todos os casos. Dezesseis dispositivos foram usados em 13 pacientes, numa razão de 1,2:1, e apenas 1 paciente precisou de um segundo dispositivo para oclusão do AAE. O tempo médio de acompanhamento foi de 12,2 meses. Todos os AAE permanecem fechados e sem defeito residual até o momento. Houve apenas um óbito tardio não relacionado ao procedimento.
ConclusõesA oclusão do AAE com o dispositivo de AMPLATZER® Cardiac Plug mostrou ser segura e eficaz nesta pequena série de pacientes. Os resultados iniciais são encorajadores e apontam para o fechamento transcateter do AAE como alternativa para a anticoagulação oral em pacientes selecionados.
Atrial fibrillation (AF) of non-valvular origin is one of the most common type of arrhythmia, affecting 1 to 2% of the overall adult population.1 It is present in up to 14% of patients older than 65 years and its incidence doubles every decade.2 Being associated with a high risk of cardioembolic events, particularly stroke, AF accounts for approximately 15% of all ischemic strokes.3 The risk of stroke in patients with AF increases up to five times when compared to patients in sinus rhythm.4
The prevention of embolic phenomena of AF is traditionally achieved through the continued use of oral anticoagulants (OAC). The most often used OAC are vitamin K antagonists, which are effective, reducing the incidence of stroke in up to 60% and death in up to 25% of patients that remain within the therapeutic range.5 The continued use of these drugs brings several difficulties and inconveniences to patients, resulting in a large number of untreated individuals. The main problems reported are the risk of bleeding or previous episodes of significant bleeding in individuals with predisposing conditions; extreme frailty; the low rate of adherence to treatment; harmful drug interactions; the oscillation of therapeutic levels of the drug; and unwanted side effects.6–9
The knowledge that more than 90% of emboli in AF originate from thrombi in the left atrial appendage (LAA) led to the development of options for mechanical obliteration of this structure as an alternative therapy to OAC to prevent cerebral thromboembolic phenomena.10–12
In this article, the authors report their initial experience with LAA occlusion in a single center, using the first dedicated prosthesis developed for this purpose and approved for clinical use in Brazil.
MethodsRecords of all patients referred for percutaneous closure of the LAA in the Interventional Cardiology Department of Structural and Congenital Defects of Hospital Federal dos Servidores do Estado in the city of Rio de Janeiro (RJ) were retrospectively analyzed.
Patients with permanent or paroxysmal AF and who had contraindications or complications caused by the continued use of OAC were selected. Selection for the procedure was attained by transesophageal echocardiography (TEE), which included patients whose atrial appendages had anatomical characteristics and diameters compatible with the standard occluder used in this service (12.6mm to 28.5mm)13 and who did not have thrombi inside the LAA.
The prosthesis used was the AMPLATZER® Cardiac Plug (ACP, AGA Medical Corp., Minneapolis, USA), which is a self-expanding device made of nitinol, lined with polyester fabric. It consists of a cylindrical lobe available in diameters of 16 to 30mm, with 2-mm increments, which corresponds to the nominal size of the prosthesis. Attached to it by a flexible connector pin is a disc; the disc is 4mm larger than the lobe in 16 to 22-mm prostheses, and 6mm larger in 24 to 30-mm prostheses.
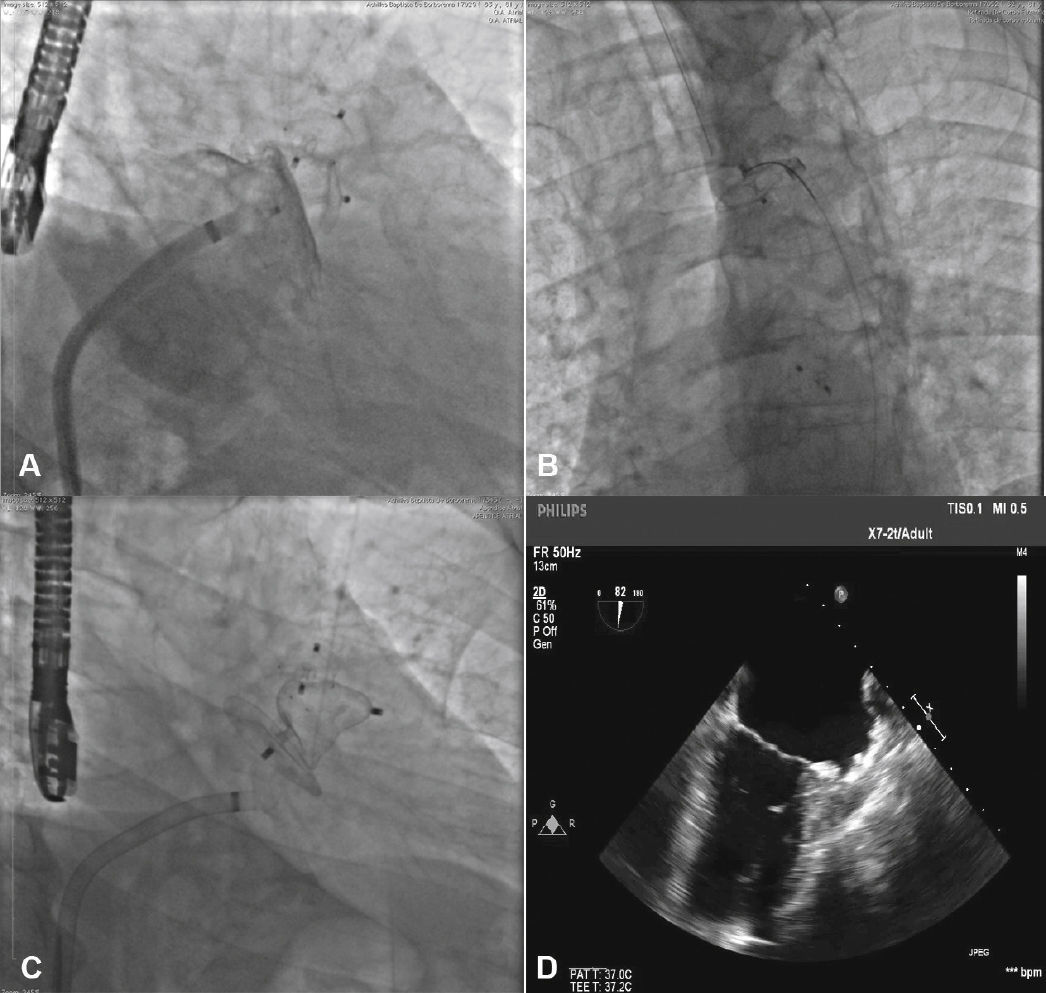
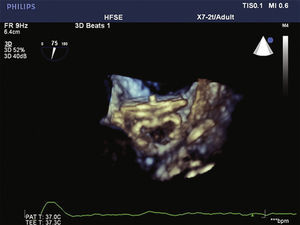
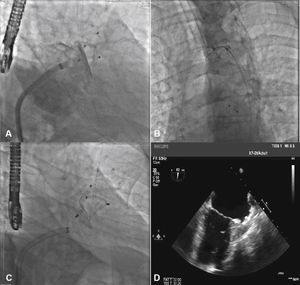
To increase the safety of the procedure and reduce the risk of device displacement, six pairs of thin stabilizing hooks are fastened to the lobe, identified by radiopaque markers, which help to secure the lobe to the LAA body in the landing zone (Fig. 1).
All procedures were performed under general anesthesia and tracheal intubation, after a minimum fasting period of 8hours, under fluoroscopic and transesophageal echocardiography guidance in the interventional cardiology laboratory.
Unfractionated heparin was administered at doses of 5,000 to 10,000 IU after obtaining transseptal access. Supplemental doses of 2,500 to 5,000 IU were administered every 30minutes when the procedures lasted longer than 1 hour. Antimicrobial prophylaxis with 2g of intravenous cefazolin was routinely administered. The patients were submitted to right and left heart catheterization through femoral vein puncture. Left atrial access was obtained by transseptal puncture with the standard technique using a Brockenbrough needle.
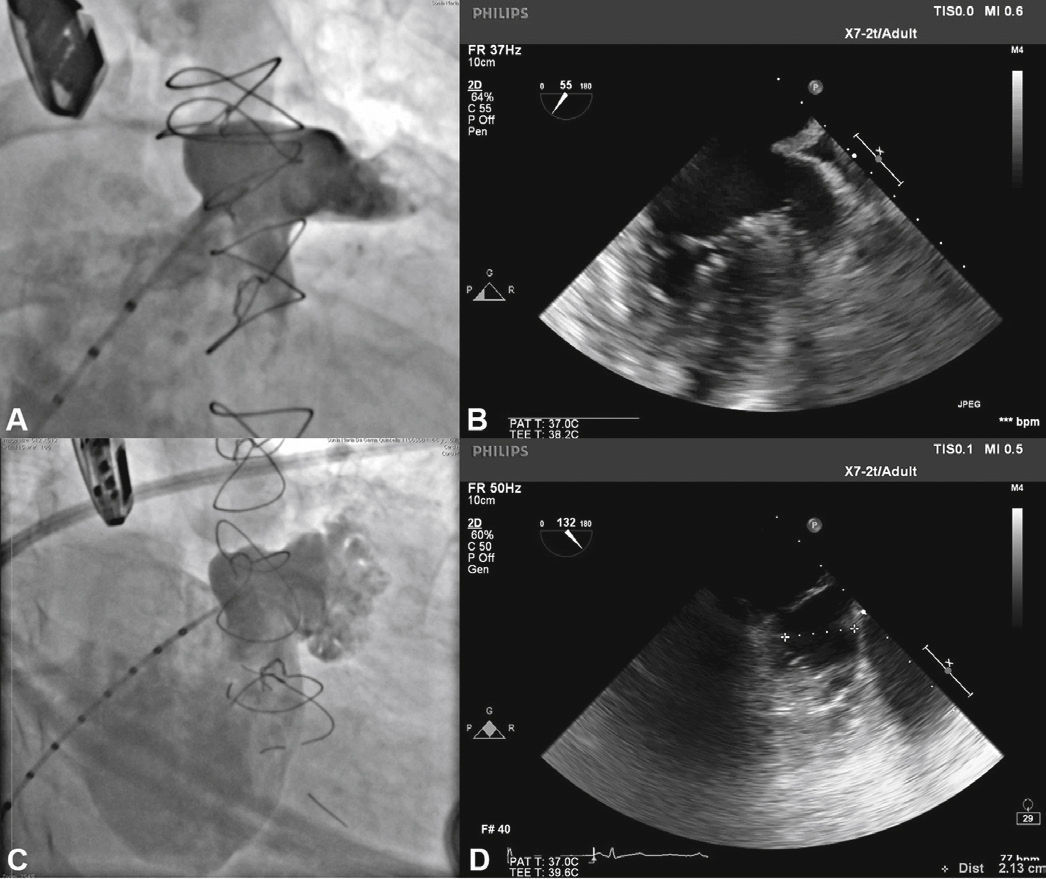
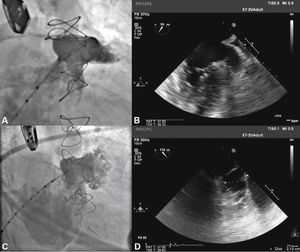
With the aid of a calibrated 5 F pigtail catheter, the LAA was catheterized and injections were performed in the right anterior oblique (RAO) view, with cranial and caudal angulation, to determine the anatomic type and to obtain measures of the ostium and the landing zone of the occluder device's lobe. The measures and anatomy were compared with those obtained with the TEE, with the largest measurements utilized to select the prosthesis to be implanted (Fig. 2).
Correspondence between angiographic and echocardiographic images. In A, the left atrial appendage seen in the cranial-right anterior oblique view shows similarity with the echocardiographic image obtained with the transducer at 55° in B. In C, appendage angiography in the right anterior oblique view with caudal angulation shows a better depiction of the more terminal trabecular portion of the left atrial appendage, which is also seen in D in the echocardiographic image at 132°.
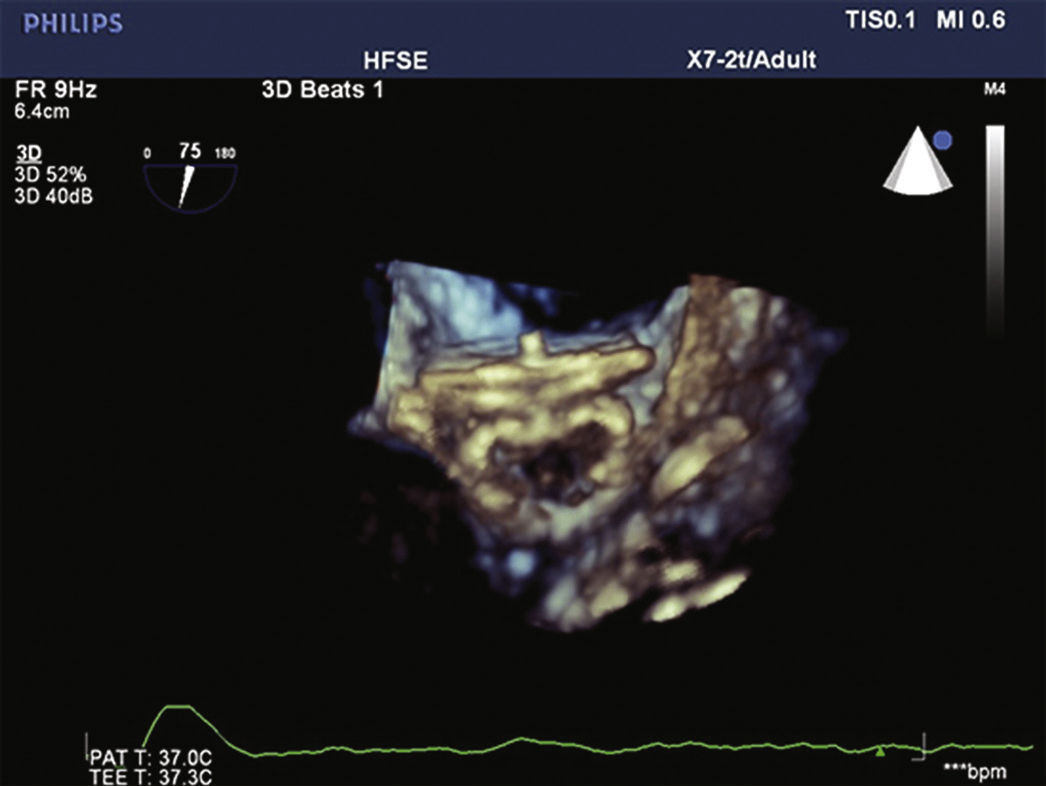
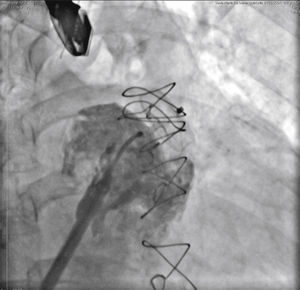
The pigtail catheter was removed and replaced by a long guide wire (260cm) with a curved tip (J), rigid or super rigid, inside the LAA. Over it, a long, double-curve AGA sheath was introduced, with a diameter compatible with the chosen occluder. The sheath was positioned as coaxially as possible to the LAA axis and the position was assessed using manual contrast injections, through the hemostatic valve. The chosen device was introduced through the sheath, previously loaded in the delivery system, whose lobe diameter was 2 to 4mm larger than the largest measure obtained from the implantation landing zone. The device lobe was ideally implanted maintaining two-thirds of the lobe in a distal position to the left circumflex coronary artery location, visualized by TEE. Subsequently, while maintaining slight traction on the delivery cable, the sheath was retracted, externalizing the disk in order to occlude the LAA ostium. The tension on the cable was maintained for a few minutes (tug test) to ensure that the device was well adhered to the LAA body, minimizing the risk of embolization or displacement. The evaluated signs of good positioning were the degree of compression of the occluder lobe, separation between the disc and the lobe, the concave shape of the disc and its position at the ostium entrance, and lobe positioning with at least two-thirds within the left circumflex coronary artery (Fig. 3).
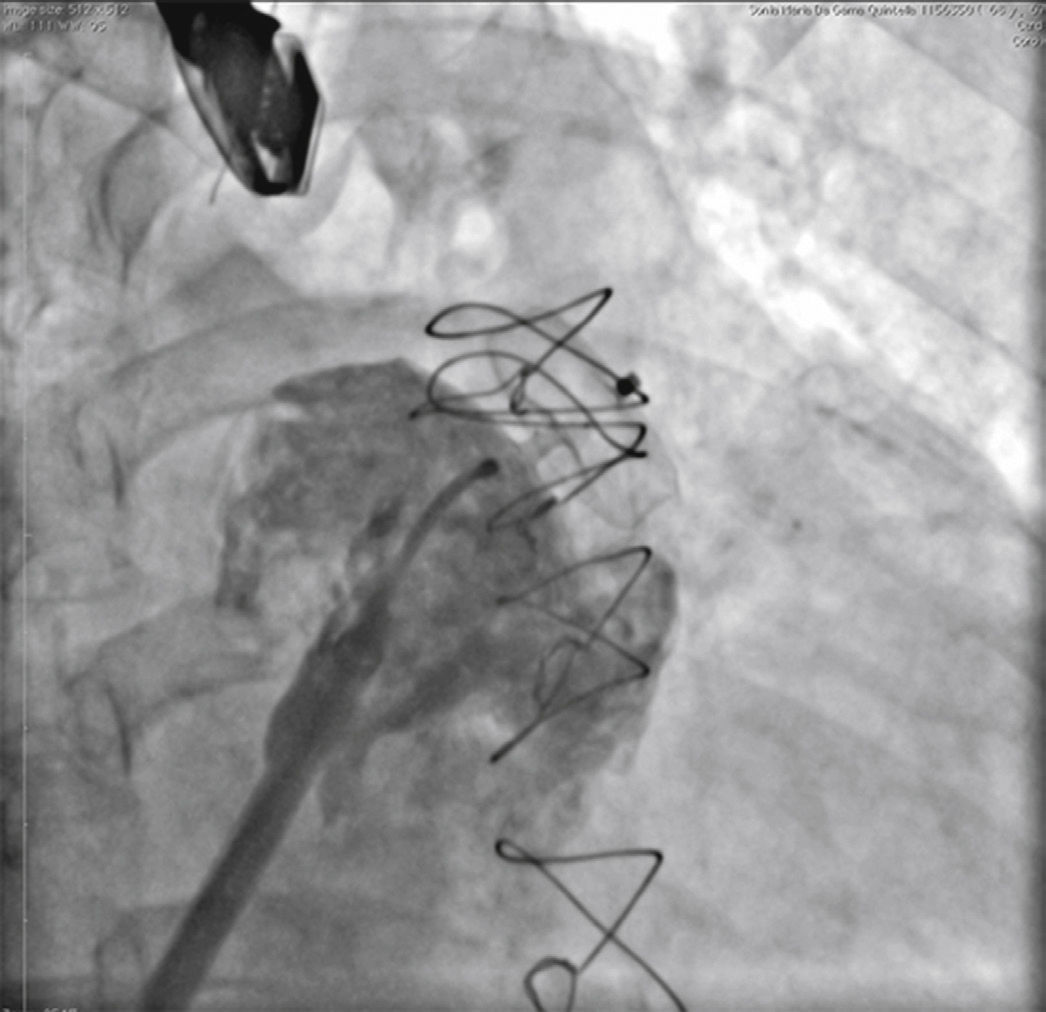
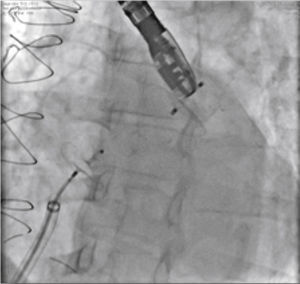
New injections were performed through the delivery system, to confirm the correct positioning of the device with complete occlusion of the LAA ostium (Fig. 4). After it was considered to be satisfactory, the prosthesis was released from the delivery cable by unscrewing it from the delivery system. Immediately after the implantation, the absence of periprosthetic residual flow and pericardial effusion was verified through the TEE.
Patients were awakened in the interventional laboratory and taken to the intensive care unit for postoperative care. Another dose of 1g of cefazolin was administered 6hours after the procedure. Patients were discharged on the day after the implantation, after being submitted to a control transthoracic echocardiogram (TTE).
At the follow-up, all patients were instructed to use dual antiplatelet therapy with 200mg of acetylsalicylic acid associated with 75mg of clopidogrel bisulfate. Medical evaluations followed by echocardiographic assessment were performed after 1 and 3 months, when clopidogrel was discontinued. After 6 months, control TEE was performed and patients maintained the continuous use of acetylsalicylic acid.
ResultsFrom March 2014 to August 2015, 14 percutaneous occlusions of the LAA were performed in 13 patients. Eight patients were females. Age ranged from 46 to 83 years (66.7 ± 11.8 years).
Significant bleeding during OAC use was found in nine (69.2%) patients. Gastrointestinal bleeding was identified in six patients, and a retinal, genitourinary and intracranial bleeding in the remainder.
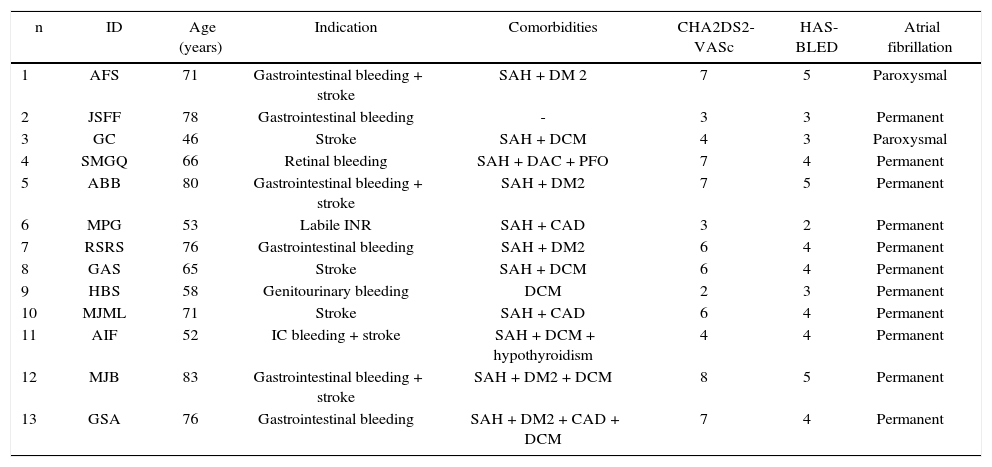
Seven (53.8%) patients had suffered a previous stroke. Most patients (84.6%) had permanent AF; in the others, the arrhythmia was paroxysmal. Clinical data and the main comorbidities found are described in Table 1.
Clinical data of patients submitted to left atrial appendage occlusion.
| n | ID | Age (years) | Indication | Comorbidities | CHA2DS2-VASc | HAS-BLED | Atrial fibrillation |
|---|---|---|---|---|---|---|---|
| 1 | AFS | 71 | Gastrointestinal bleeding + stroke | SAH + DM 2 | 7 | 5 | Paroxysmal |
| 2 | JSFF | 78 | Gastrointestinal bleeding | - | 3 | 3 | Permanent |
| 3 | GC | 46 | Stroke | SAH + DCM | 4 | 3 | Paroxysmal |
| 4 | SMGQ | 66 | Retinal bleeding | SAH + DAC + PFO | 7 | 4 | Permanent |
| 5 | ABB | 80 | Gastrointestinal bleeding + stroke | SAH + DM2 | 7 | 5 | Permanent |
| 6 | MPG | 53 | Labile INR | SAH + CAD | 3 | 2 | Permanent |
| 7 | RSRS | 76 | Gastrointestinal bleeding | SAH + DM2 | 6 | 4 | Permanent |
| 8 | GAS | 65 | Stroke | SAH + DCM | 6 | 4 | Permanent |
| 9 | HBS | 58 | Genitourinary bleeding | DCM | 2 | 3 | Permanent |
| 10 | MJML | 71 | Stroke | SAH + CAD | 6 | 4 | Permanent |
| 11 | AIF | 52 | IC bleeding + stroke | SAH + DCM + hypothyroidism | 4 | 4 | Permanent |
| 12 | MJB | 83 | Gastrointestinal bleeding + stroke | SAH + DM2 + DCM | 8 | 5 | Permanent |
| 13 | GSA | 76 | Gastrointestinal bleeding | SAH + DM2 + CAD + DCM | 7 | 4 | Permanent |
ID: patient identification; SAH: systemic arterial hypertension; DM2: type 2 diabetes mellitus; DCM: dilated cardiomyopathy; CAD: coronary artery disease; PFO: patent foramen ovale; INR: international normalized ratio.
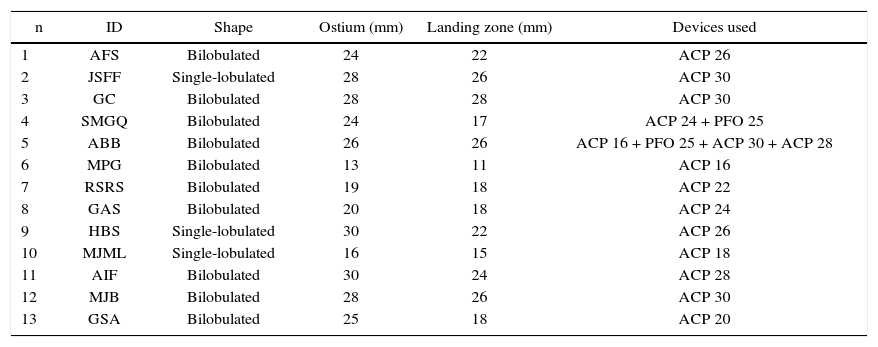
Regarding the shape, bilobulated appendages were present in 76.9% of cases, whereas the others were single-lobulated. Ostial diameter ranged from 13 to 30mm (23.9 ± 5.4mm) and the landing zone from 11 to 28mm (20.8 ± 5.1mm).
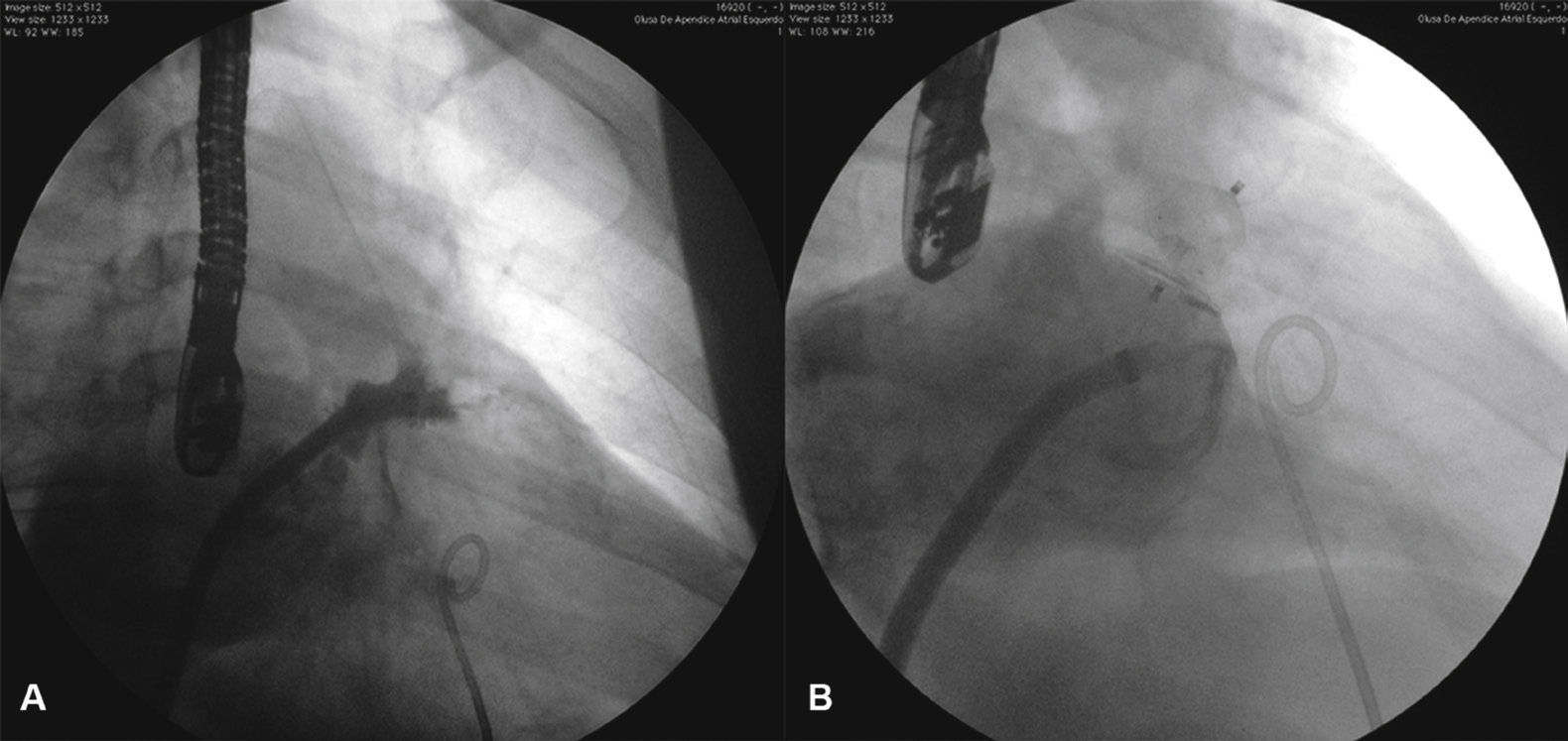
The procedure was successful in all cases. Sixteen devices were used for occlusion in the 13 patients, at a ratio of 1.2 device per patient. The other AMPLATZER® PFO Occluder (PFO) prosthesis of 25mm was used to occlude the foramen ovale of a patient (SMGQ, case 4) who did not require transseptal puncture and had her LAA occluded with ACP through the foramen ovale (Fig. 5).
Images from case 4. The AMPLATZER® 24-mm Cardiac Plug device is seen occluding the left atrial appendage and the AMPLATZER® 25-mm PFO Occluder device is positioned through the patient's foramen ovale. For the implant of the AMPLATZER® PFO Occluder device, the procedure utilized the same double curve sheath that was used for the implant of the AMPLATZER® Cardiac Plug device.
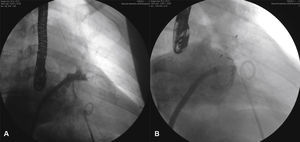
Only one patient required more than one prosthesis to occlude the LAA (ABB, case 5). He had his occlusion procedure performed as a live case during Rio.Interv 2014. At the time, a proximal accessory lobe was occluded in the anterosuperior position with a 16-mm ACP, and a 25-mm PFO prosthesis was used for the remainder of the appendage body, with immediate success. After 2 days, the PFO prosthesis migrated to the transverse aorta, from which it was percutaneously removed with a loop snare catheter. As the main body of the appendage was uncovered after embolization of the PFO prosthesis, a second procedure was offered and carried out 5 months after the index procedure. Initially, an attempt was made to occlude the LAA with a 30-mm ACP, which showed to be oversized and was finally replaced by a 28-mm ACP, which adequately occluded the appendage (Fig. 6). In the remaining patients, the LAA was occluded with a single device. Fifteen ACP and two PFO prostheses were used for LAA occlusion (Table 2).
Images from case 5. In A, the accessory lobe occluded with the AMPLATZER® 16-mm Cardiac Plug and the appendage body occluded with AMPLATZER® 25-mm PFO Occluder can be observed. In B, the AMPLATZER® PFO Occluder is embolized into the aortic arch, being captured by the loop catheter. In C, at the end of the final procedure, the accessory lobe is completely occluded by AMPLATZER® 16-mm Cardiac Plug and the body of the left atrial appendage by the second AMPLATZER® 28-mm Cardiac Plug device. In D, echocardiographic control image after the procedure, showing the ostium of the left atrial appendage occluded by AMPLATZER® 28-mm Cardiac Plug device. Observe the increase in echocardiographic density in the left atrial appendage corresponding to AMPLATZER® 16-mm Cardiac Plug device, which is barely visible in this image.
Characteristics of occluded appendages and devices used.
| n | ID | Shape | Ostium (mm) | Landing zone (mm) | Devices used |
|---|---|---|---|---|---|
| 1 | AFS | Bilobulated | 24 | 22 | ACP 26 |
| 2 | JSFF | Single-lobulated | 28 | 26 | ACP 30 |
| 3 | GC | Bilobulated | 28 | 28 | ACP 30 |
| 4 | SMGQ | Bilobulated | 24 | 17 | ACP 24 + PFO 25 |
| 5 | ABB | Bilobulated | 26 | 26 | ACP 16 + PFO 25 + ACP 30 + ACP 28 |
| 6 | MPG | Bilobulated | 13 | 11 | ACP 16 |
| 7 | RSRS | Bilobulated | 19 | 18 | ACP 22 |
| 8 | GAS | Bilobulated | 20 | 18 | ACP 24 |
| 9 | HBS | Single-lobulated | 30 | 22 | ACP 26 |
| 10 | MJML | Single-lobulated | 16 | 15 | ACP 18 |
| 11 | AIF | Bilobulated | 30 | 24 | ACP 28 |
| 12 | MJB | Bilobulated | 28 | 26 | ACP 30 |
| 13 | GSA | Bilobulated | 25 | 18 | ACP 20 |
ID: patient identification; ACP: AMPLATZER® Cardiac Plug; PFO: patent foramen ovale.
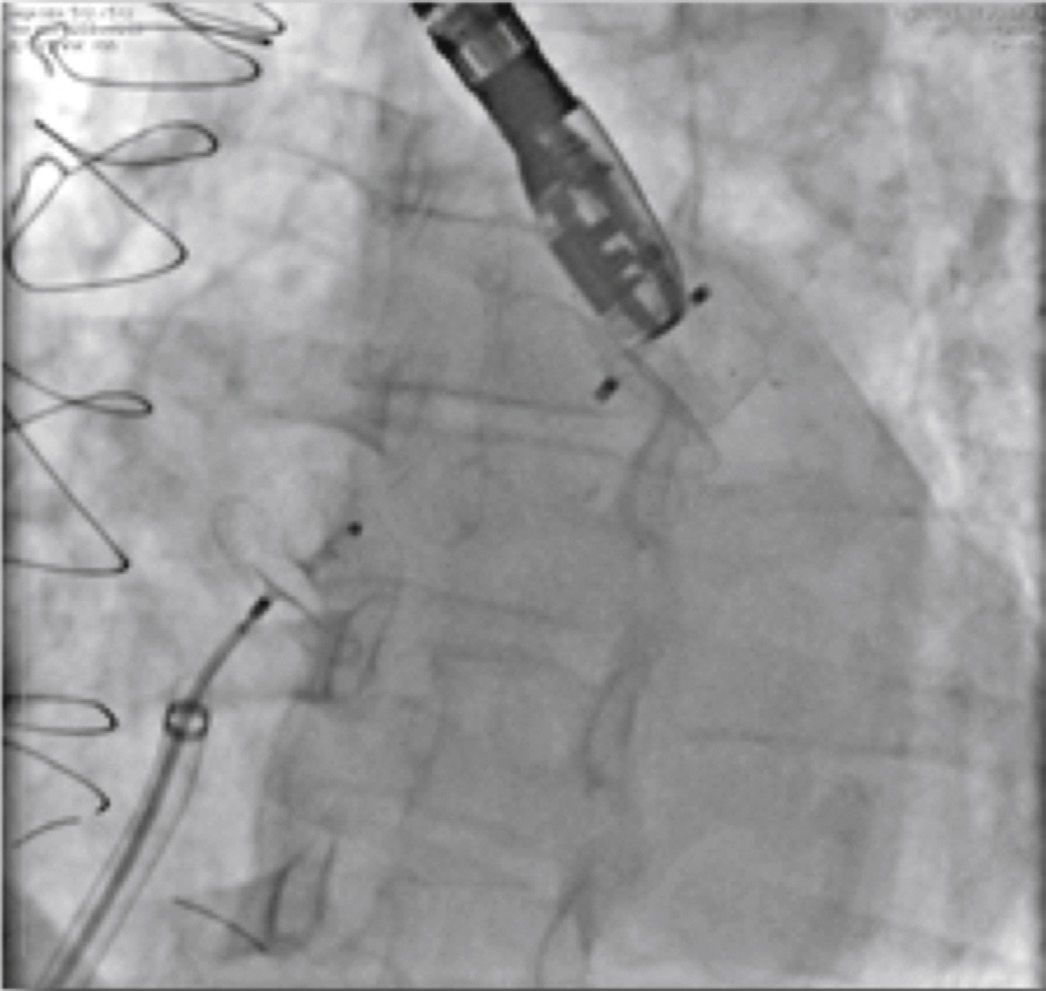
There was a single periprocedural complication (MPG, case 6). The patient had a narrow and shallow appendage, which was perforated by the hydrophilic guide at the beginning of the procedure. She had pericardial effusion, which was promptly drained at the interventional cardiology laboratory. After hemodynamic stabilization of the patient, the procedure continued and the LAA was occluded with a 16-mm ACP (Fig. 7). The effusion was contained after appendage occlusion and the patient was transferred to the intensive care unit in good condition for post-procedure care. At the follow-up, she had persistent pericarditis treated with colchicine and corticosteroids; she achieved remission after 30 days and has remained asymptomatic since then.
Images from case 6. In A, angiography performed through the double curve sheath, showing the small perforation at the tip of the left atrial appendage with contrast extravasation into the pericardial cavity. In B, after hemodynamic stabilization of the patient by draining the pericardial effusion with a pigtail catheter, the left atrial appendage totally occluded by the AMPLATZER® 16-mm Cardiac Plug device can be observed.
Follow-up was obtained in all cases. The mean time was 12.2 months (5 to 19 months) and only one patient had not yet reached the 6-month follow-up period. All atrial appendages remained closed, and no patient had periprosthetic leaks nor thromboembolic events to date.
There was one late death, which was unrelated to the procedure. The patient (GC, case 3) had severe dilated cardiomyopathy and died of refractory heart failure 11 months after the procedure. The prosthesis remained correctly positioned and there was no periprosthetic regurgitation.
DiscussionThe first, fundamental step in the occlusion procedure is obtaining access to the left atrium. Typically, this is accomplished through transseptal puncture with a Brockenbrough needle.14 To access and manipulate the long sheath coaxially inside the LAA, it is important that the transseptal needle cross the atrial septum in the posteroinferior position.13 Visualization by TEE allows the interventionist to perform the transseptal puncture precisely in the desired place, favoring procedural success.15 Additionally, gaining access to the left atrium via atrial septal defects has been described for LAA occlusion. The transseptal puncture is avoided and the technique does not prevent the completion of the procedures in most cases.16,17
The anatomy and the spatial location of the LAA are extremely variable, resulting in unique descriptions (cauliflower, cactus, chicken wing, windsock),13 which were not used in this study. The anatomical analysis of the LAA, in this study, was performed through transesophageal echocardiography and angiography. A correlation was observed between the echocardiographic images obtained with the transducer positioned between zero and 60° and the angiograms performed in RAO view with cranial angulation, whereas the images obtained with the transducer positioned between 90° and 130° were better correlated with angiograms obtained in RAO view with caudal angulation, which more precisely delineated the terminal part of the trabecular portion of the appendages. In the limited experience reported in this article, the RAO view was preferably used with caudal angulation for device implantation, as it has demonstrated the highest measured values, and the latter guided the choice of prostheses used.
The LAA, together with its great anatomical variability, is an extremely delicate and friable structure, which may explain the incidence of pericardial effusion observed in the occlusion procedures, ranging from 1.2 to 3.7%.18–21
Extreme care should be exercised when manipulating it and some basic rules need to be followed, such as using only round-tip guide wires (J) and striving to maintain the long delivery sheath as coaxial as possible to the long axis of the appendage, away from its walls. Care must be taken at all times during the procedure with the LAA borders, avoiding at all costs reaching its extremity with the dilator or long sheath. The learning curve of this procedure is significant, and it has been shown that the number of procedural complications decreases as the team becomes familiar with the occlusion technique.22,23
The ACP consists of a lobe and a disk connected by a flexible pin that articulates and allows angulation between them. The lobe, which is implanted in the LAA body, has 12 small hooks for device stabilization. It is of utmost importance that, to prevent embolization of the prosthesis, the lobe undergoes some degree of compression, to promote contact between the hooks and the LAA walls. For this purpose, it is important to use the largest measurements obtained, choosing devices that are 4 to 6mm larger than the landing zone. However, the use of prosthetic devices much larger than the implantation region causes device migration, as seen in case 5. An excessively oversized device deforms the lobe, modifying the positioning of the hooks, retracting them and causing loss of their contact with the LAA wall. The same occurs if the selected device is too small, smaller than the landing zone, a situation in which also there is no contact of the hooks with the LAA wall.
The implantation of the prosthesis should promote appendage body occlusion, isolating its entire trabecular portion, whose uneven surface creates conditions for the formation of thrombi found in AF. The presence of the disc, positioned aiming to obliterate the LAA ostium, acts as a second occluder element, increasing procedural safety. It is important for the disk to maintain a concave shape and to adapt perfectly to the ostium, preventing spaces that can be a source of thrombi or even the origin of periprosthetic regurgitation.
It is presumed that periprosthetic residual flow may be the cause of new cerebral ischemic strokes and, therefore, should be prevented, although there is some controversy in the literature.24,25 Currently, residual flows with diameter ≥ 5mm are considered as significant,26 which, when present, should be occluded with a second device. The use of more than one device for LAA occlusion has been successfully performed and appears to be associated with favorable results in patient follow-up.27
ConclusionsThe occlusion of the left atrial appendage in this small number of cases showed to be safe and effective, in spite of the complexity and comorbidities of the patients included in this study.
The initial results are encouraging and, despite the short-term follow-up of these patients, they confirm that percutaneous occlusion of the left atrial appendage can become a valid alternative to oral anticoagulation in patients who cannot use it.
Funding sourceNone declared.
Conflicts of interestEnio Guérios is a consultant and proctor of St. Jude Medical.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.