Drug-eluting stents have been used since 2002 in different patient populations aiming to achieve high success rates with low clinical and angiographic restenosis rates. With the late thrombosis adverse events associated to the first generation sirolimus and paclitaxel-eluting stents, second-generation everolimus and zotarolimus-eluting stents has been recently developed.
METHODSThe POLAR registry is a prospective, non-randomized, multicenter study, which included 988 patients, totaling 1,362 lesions treated with the everolimus-eluting stent Promus®. In order to represent the clinical practice, almost all subtypes of patients and lesions were included in this registry. Clinical follow-up was planned to be performed 1, 6, 12 and 24 months after the procedure.
RESULTSMost patients were male (69.8%), with mean age of 64.9±9.4years, 35.2% were diabetics and 55% had been treated for acute coronary syndrome. Vessel diameter was 2.95±0.43mm and lesion extension was 20.5±5.6mm. A total of 1.14±0.38 stent/patient were implanted and the procedural success rate was 96.6%. Major adverse cardiac events occurred in 4.5% of patients, and stent thrombosis was observed in 5 patients (0.5%) after a clinical follow-up of 12 months.
CONCLUSIONSThe present registry suggests that everolimus-eluting stents are safe and effective in daily clinical practice patients, with a low rate of major adverse cardiac events at the end of the first year of follow-up.
Resultados Clínicos de Um Ano do Registro POLAR(PrOmus eluting stent registry in Latin AmeRica)
IntroduçãoDesde 2002, os stents farmacológicos são utilizados em diversas populações de pacientes objetivando alcançar elevados índices de sucesso, com baixas taxas de reestenose angiográfica e clínica. Com os resultados adversos em relação à trombose tardia associados aos stents farmacológicos de primeira geração eluidores de sirolimus e paclitaxel, surgiram recentemente os stents farmacológicos de segunda geração eluidores de zotarolimus e everolimus.
MétodosO registro POLAR é um registro prospectivo, não randomizado, multicêntrico, que incluiu 988 pacientes totalizando 1.362 lesões tratadas com o stent Promus®. Objetivando representar a prática clínica, praticamente todos os subtipos de pacientes e lesões foram incluídos neste registro. O seguimento clínico foi planejado para ser realizado 1 mês, 6 meses, 12 meses e 24 meses após o procedimento.
ResultadosA maioria dos pacientes era do sexo masculino (69,8%), com média de idade de 64,9 ± 9,4 anos, 35,2% eram diabéticos e 55% tinham sido tratados na vigência de síndrome coronária aguda. O diâmetro do vaso foi de 2,95 ± 0,43mm e a extensão da lesão, de 20,5 ± 5,6mm. Foi implantado 1,14 ± 0,38 stent/paciente e o sucesso do procedimento foi alcançado em 96,6% dos casos. Eventos cardíacos adversos maiores ocorreram em 4,1% dos pacientes, e trombose de stent esteve presente em 5 pacientes (0,5%) após o seguimento clínico de 12 meses.
ConclusõesO presente registro sugere que os stents farmacológicos eluidores de everolimus são seguros e eficazes em pacientes da prática clínica diária, com baixas taxas de eventos cardíacos adversos maiores ao término do primeiro ano de seguimento.
Drug-eluting stents have been a therapeutic approach for coronary artery disease throughout the last decade, when the European regulatory agency approved the first two drug-eluting stents, the sirolimus (Cypher®, Cordis Corporation - Warren, United States) and paclitaxel-eluting (Taxus®, Boston Scientific – Natick, United States) stents. These stents were approved after study results that compared these devices with conventional stents.1,2
Despite initial encouraging results suggesting that first-generation drug-eluting stent restenosis incidence was reduced compared with that of conventional stents, there has been great concern after evidence showed increased rates of late and very late thrombosis with these new devices.3 Since then, developing new drug-eluting stents with similar efficacy to firstgeneration stents and increased safety has become a priority. Second-generation stents were developed with reduced polymeric load and/or more biocompatible polymers. Among these new stents, the everolimus- eluting stents have demonstrated promising initial results in controlled.4
The Promus® stent (Boston Scientific – Santa Clara, CA, USA) releases the antiproliferative drug everolimus from a stable, non-adhesive, thin biocompatible polymer layer, which covers the thin strut cobalt-chrome platform.
The aim of the POLAR registry was to clinically evaluate the Promus® stent in patients in clinical practice and, therefore, not considered in initial controlled studies.
METHODCasuistry and clinical study designThe POLAR registry was a prospective, non-randomised, multicentre study conducted after Promus® stent commercialisation to evaluate patient safety and clinical effectiveness of the Promus® stent in patients in current clinical practice.
From November of 2008 to July of 2010, 1,121 patients with coronary lesions were treated with the Promus® stent in 40 Latin American centres. Of these patients, 133 were excluded for having received a nonstudy stent in addition to the Promus® stent.
Since the objective was to represent daily clinical practice, the inclusion criteria were not strict and included>18-year-old patients who underwent routine or emergency angioplasty. Patients with a life expectancy<24 months were excluded from the study because it would not be possible to perform follow-up studies. Additionally, those with unfavourable coronary anatomy for percutaneous treatment and those who had to maintain dual antiplatelet therapy during the 12 months were also excluded.
The Ethics Committees of all participant centres approved the registry protocol, and all of the patients signed an informed consent.
Coronary intervention procedure and antiaggregation protocolThe antiplatelet protocol consisted of using acetylsalicylic acid at a 300mg loading dose and a 100mg/day maintenance dose, and clopidogrel at a 300–600mg/day loading dose and a 75mg/day maintenance dose before stent implantation. After the procedure, acetylsalicylic acid treatment was recommended indefinitely, and clopidogrel treatment was recommended for a 12-month-period, following current clinical practice.
Pre-dilatation the number of stents implanted were left to the operator’s discretion. Post-dilatation was recommended when there was≥30% residual stenosis.
Objectives and definitionsThe primary objective of this registry was to evaluate major adverse cardiac event (MACE) incidence, which is defined as the combined occurrence of cardiac death, non-fatal myocardial infarction, or target-lesion revascularisation after 12 months of clinical follow- up. The secondary objectives were: rate of MACE six months post-procedure; technical success, defined as implantation associated with residual stenosis≤30% of Promus® stent in the target lesion; procedural success, defined as technical success with no occurrence of MACE during hospitalization; and all deaths during hospitalization and after one month, six months, 12 months, and 24 months.
The following definitions were applied to the aforementioned cardiac events. Cardiac death was any death that could not be attributed to a non-cardiac cause. Myocardial infarction occurred when at least one of the following criteria was present: increased creatine phosphokinase (CPK) levels of more than two times the normal upper limit with an altered creatine phosphokinase-MB fraction (CPK-MB); increased CPK levels greater than five times the normal upper limit for surgical myocardial revascularisation cases; > 1ng/ mL increase of Troponin T or>0.5ng/mL AccuTnl; or electrocardiography results demonstrating new pathological Q-waves longer than≥0.04seconds in at least two derivations with borderline altered CPK-MB. Target vessel revascularisation was defined as a new percutaneous intervention procedure or myocardial revascularisation surgery in the same epicardial vessel treated in the index procedure. Stent thrombosis definitions were based on those from the Academic Research Consortium (ARC),5 and were divided into definite stent thrombosis (angiographic or pathological confirmation), probable stent thrombosis (any death that could not be explained within 30 days or target vessel myocardial infarction without angiographic thrombosis confirmation) and possible stent thrombosis (any death that could not be explained within 30 days).
Clinical follow-up was performed via phone interview or physician appointment at one month, six months, 12 months, and 24 months after Promus® stent implantation.
Data collection and managementThe Cardiovascular Research Centre (São Paulo, SP, Brazil) was in charge of elaborating and implementing the registry, performing the study, and monitoring the data.
Categorical variables were expressed as absolute frequencies and percentages. Continuous variables were expressed as mean±standard deviation.
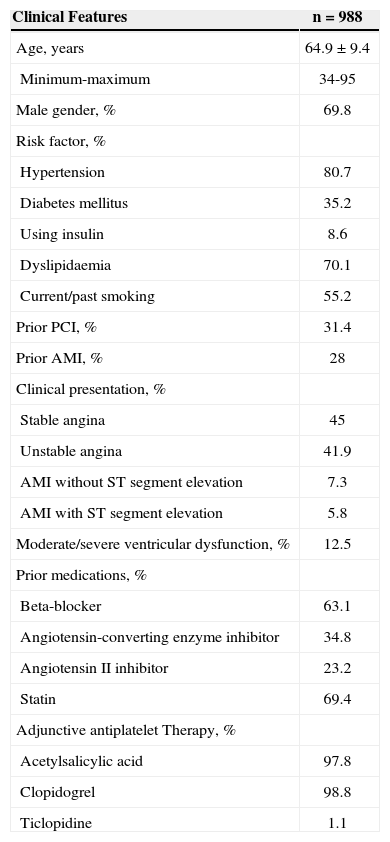
RESULTSTable 1 demonstrates the base clinical and demographic features of the 988 treated patients. Most of the patients were men (69.8%) whose mean age was 64.9±9.4years, and 35.2% were diabetic. Over half of the treated group had acute coronary syndrome (55%), most of the patients were using acetylsalicylic acid (97.8%), and one patient was using thienopyridines (100%).
Demographic Data, Clinical Presentation, and Risk Factors
| Clinical Features | n=988 |
|---|---|
| Age, years | 64.9±9.4 |
| Minimum-maximum | 34-95 |
| Male gender, % | 69.8 |
| Risk factor, % | |
| Hypertension | 80.7 |
| Diabetes mellitus | 35.2 |
| Using insulin | 8.6 |
| Dyslipidaemia | 70.1 |
| Current/past smoking | 55.2 |
| Prior PCI, % | 31.4 |
| Prior AMI, % | 28 |
| Clinical presentation, % | |
| Stable angina | 45 |
| Unstable angina | 41.9 |
| AMI without ST segment elevation | 7.3 |
| AMI with ST segment elevation | 5.8 |
| Moderate/severe ventricular dysfunction, % | 12.5 |
| Prior medications, % | |
| Beta-blocker | 63.1 |
| Angiotensin-converting enzyme inhibitor | 34.8 |
| Angiotensin II inhibitor | 23.2 |
| Statin | 69.4 |
| Adjunctive antiplatelet Therapy, % | |
| Acetylsalicylic acid | 97.8 |
| Clopidogrel | 98.8 |
| Ticlopidine | 1.1 |
AMI=acute myocardial infarction; PCI=percutaneous coronary intervention; N=number of patients.
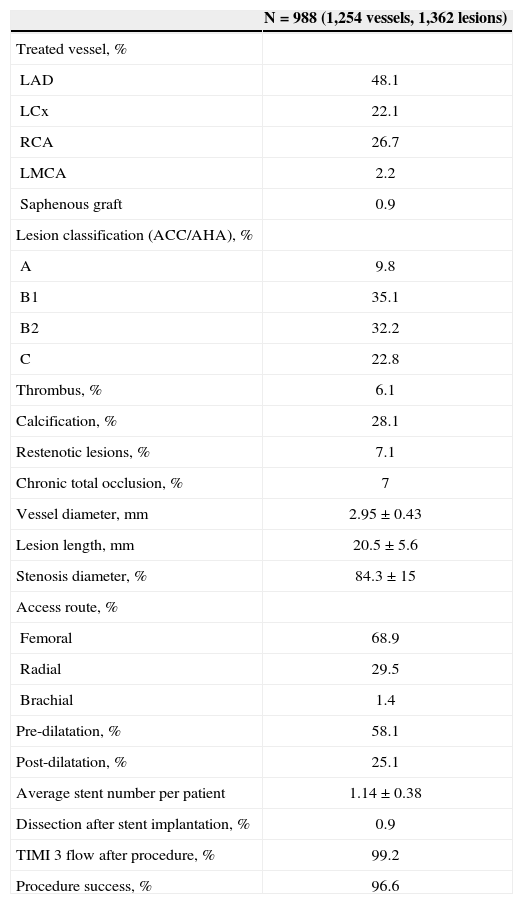
The left anterior descending artery was the most frequently treated vessel, and 55% of the lesions were B2/C type lesions (Table 2). The mean vessel diameter was 2.95±0.43mm, and the mean lesion length was 20.5±5.6mm. The patients received a mean of 1.14±0.38 stents, and the procedures were successful in 96.6% of cases.
Angiographic and Procedure Features
| N=988 (1,254 vessels, 1,362 lesions) | |
|---|---|
| Treated vessel, % | |
| LAD | 48.1 |
| LCx | 22.1 |
| RCA | 26.7 |
| LMCA | 2.2 |
| Saphenous graft | 0.9 |
| Lesion classification (ACC/AHA), % | |
| A | 9.8 |
| B1 | 35.1 |
| B2 | 32.2 |
| C | 22.8 |
| Thrombus, % | 6.1 |
| Calcification, % | 28.1 |
| Restenotic lesions, % | 7.1 |
| Chronic total occlusion, % | 7 |
| Vessel diameter,mm | 2.95±0.43 |
| Lesion length,mm | 20.5±5.6 |
| Stenosis diameter, % | 84.3±15 |
| Access route, % | |
| Femoral | 68.9 |
| Radial | 29.5 |
| Brachial | 1.4 |
| Pre-dilatation, % | 58.1 |
| Post-dilatation, % | 25.1 |
| Average stent number per patient | 1.14±0.38 |
| Dissection after stent implantation, % | 0.9 |
| TIMI 3 flow after procedure, % | 99.2 |
| Procedure success, % | 96.6 |
ACC/AHA=American College of Cardiology/American Heart Association; LAD=left anterior; LCx=left circumflex artery; RCA=right coronary artery; LMCA=left main coronary artery.
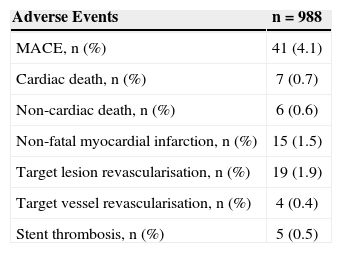
Data regarding 12-month clinical follow-up after the index procedure was obtained from 90.5% of the patients via outpatient or telephone appointment (Table 3). Cardiac death occurred in 0.7% of the patients, non-fatal myocardial infarction occurred in 1.5%, and target lesion revascularisation occurred in 1.9%, totalling a MACE rate of 4.1%. Five cases of stent thrombosis (0.5%) were verified. These cases occurred within the first six months after the procedure. No additional cases were detected through 12 months of follow-up.
Adverse Events in 12 Months
| Adverse Events | n=988 |
|---|---|
| MACE, n (%) | 41 (4.1) |
| Cardiac death, n (%) | 7 (0.7) |
| Non-cardiac death, n (%) | 6 (0.6) |
| Non-fatal myocardial infarction, n (%) | 15 (1.5) |
| Target lesion revascularisation, n (%) | 19 (1.9) |
| Target vessel revascularisation, n (%) | 4 (0.4) |
| Stent thrombosis, n (%) | 5 (0.5) |
MACE=major adverse cardiac events (cardiac death, non-fatal acute myocardial infarction, or target vessel revascularisation; n=number of patients.
Data reported in this article were derived from the results obtained with the Promus® everolimus-eluting stent, and confirm previously published results for these stents.4–7
The SPIRIT Clinical Trial Program presented various clinical trials that sought to test the clinical and angiographic effectiveness of the aforementioned stents. SPIRIT I5, included 26 randomised patients who had received everolimus-eluting stents. However, this initial publication already demonstrated that these stents had superior performance compared with first-generation stents.
In the SPIRIT II trial,6 300 patients were randomised 3:1 to test the effectiveness of the everolimus-stent compared with the Taxus® paclitaxel-eluting stent. In this non-inferiority trial that featured late loss as a primary endpoint, the everolimus-eluting stent was superior to the paclitaxel-eluting stent (late lumen loss of 0.11±0.27mm vs. 0.36±0.39mm at 6 months; P<0.0001).
In the SPIRIT IV trial,7 3,687 patients with stable or unstable angina were randomised 2:1 for treatment with the everolimus-eluting stent (n=2,458) or the paclitaxel-eluting stent (n=1,229). After 12 months of follow-up, researchers observed superiority of the everolimus-eluting stent regarding target vessel failure (3.9% vs. 6.6%, P=0.0008) and target lesion revascularisation (2.5% vs. 4.6%, P (non-inferiority)<0.0001 and P (superiority)=0.09). Probable and definite thrombosis rates were also significantly lower with these stents (0.29% vs. 1.06%, P=0.003).
In addition to the SPIRIT program, everolimus-eluting stents were also tested in the COMPARE,8 BASKET-PROVE,9 ISAR-TEST 410, and SORT OUT IV11 studies. All of these studies demonstrated positive results for the new everolimus-eluting stents.
A recent meta-analysis12 evaluated everolimus- eluting stent safety in relation to thrombosis. In total, 17,101 patients were included, with an average follow-up period of 21.7months. These devices demonstrated significant reductions in stent thrombosis (risk rate [RR]=0.55, 95% confidence interval [95% CI] 0.38-0.78, P=0.001) target vessel revascularisation (RR=0.78, 95% CI 0.64-0.92, P=0.004) and myocardial infarction (RR=0.78, 95% CI 0.64-0.96, P=0.02) compared with the sirolimus-, paclitaxel- and zotarolimus-eluting stents.
The results of the present study are in aggreement with those from previous studies. With a large patient population and a 12-month follow-up period for more than 90% of the patients, it was possible to verify reduced event incidence for 12 months (4.1%), with a low rate of target vessel (0.4%) and target lesion revascularisation (1.9%). Moreover, this patient population was representative of real-world interventional cardioIogy, since the mean patient age was 64.9±9.4years, 55% of the patients had acute coronary syndrome and 35.2% were diabetic.
The results shown here have relevant similarities to those from previously published registries. The first generation stent registries e-Cypher13 (n=15,157), e- SELECT14 (n=15,147), and WISDOM15 (n=778) had similar MACE (5.8%, 4.8%, and 5.2%, respectively) and stent thrombosis (0.88%, 1.3%, and 0.6%, respectively) results after 12 months of follow-up. The results from the second-generation stent studies RESOLUTE16 and BEACON17 were similar (4.1% and 6.5%, respectively, for MACE; and 1.2% and 0%, respectively, for stent thrombosis) after 12 months of follow-up.
Study limitationsThis study’s main limitation is that it was not randomised. Furthermore, because patient data for some treatment centres during the inclusion period was not available, a possible selection bias could not be excluded. Finally, strict adhesion to dual anticoagulant therapies could not be assured during patient follow-up.
CONCLUSIONSThe present study evaluated the safety and effectiveness of the Promus® everolimus-eluting stent in a minimally selected patient population. After a 12-month follow-up period, these devices were proven to be effective and safe with low rates of major adverse cardiac events and stent thrombosis.
CONFLICTS OF INTERESTRogério Sarmento-Leite was a lecturer for events or activities that were related to this registry, and was sponsored by Boston Scientific. The other authors declare to have no conflicts of interest.