Studies on the cost-effectiveness ratio of drug-eluting stents (DES) are rare. Our objective was to evaluate the results and compare costs (incremental cost-effectiveness ratio – ICER) per restenosis avoided between DES and bare metal stents (BMS) using the propensity score.
METHODSTwo hundred and twenty consecutive patients were included in the study, of which 111 were treated with DES and 109 with BMS. The propensity score was used to adjust the effect of the intervention, by means of matching, stratification and weighing.
RESULTSMost patients were male (67.7% vs. 66.9%; P = 0.53), with a mean age of 65.9 years. Patients treated with the DES had a higher rate of diabetes (54% vs. 17.4%; P < 0.001), three-vessel disease (18.9% vs. 10.1%; P = 0.029) and poor ventricular function (54.1% vs. 22%; P < 0.0001). The diameter of stents was 2.76 ± 0.35mm vs. 2.91 ± 0.47mm (P = 0.006) and the sum of the lengths of stents was 37.6 ± 23mm vs. 24.8 ± 15.8mm (P < 0.0001). Restenosis was observed on 6.3% vs. 12.8% of the patients (P = 0.099) and in 4.1% vs. 9.8% of the lesions (P = 0.048). There was an incremental cost of R$ 9,500.00 and the ICER was R$ 147,538.00 per restenosis avoided (above the World Health Organization threshold). However, when the propensity score was used, the variables that best classified patients for DES and had a maximum ICER of R$ 4,776.96 were age > 72 years, diabetes and lesions with diameter < 3.2mm and length > 18mm.
CONCLUSIONSAlthough DES were not cost-effective in the overall population, the propensity score showed that in elderly patients, diabetics and patients with long lesions or small vessels, the use of DES was cost-effective.
Uso do Escore de Propensão na Análise de Custo-Efetividade com Utilização Seletiva deStents Farmacológicos e Não Farmacológicos
IntroduçãoOs estudos sobre a razão de custo-efetividade dos stents farmacológicos (SFs) são escassos. Nosso objetivo foi avaliar os resultados e comparar os custos (razão custo-efetividade incremental – RCEI) por reestenose evitada entre SFs e stents não farmacológicos (SNFs) utilizando o escore de propensão.
MétodosIncluímos na análise 220 pacientes tratados consecutivamente, dos quais 111 com SFs e 109 com SNFs. O escore de propensão foi usado para ajustar o efeito da intervenção por meio de pareamento, estratificação e ponderação.
ResultadosPredominaram pacientes do sexo masculino (67,7% vs. 66,9%; P = 0,53), com média de idade de 65,9 anos. Pacientes tratados com SFs apresentaram maior frequência de diabetes (54% vs. 17,4%; P < 0,001) e doença triarterial (18,9% vs. 10,1%; P = 0,029) e pior função ventricular (54,1% vs. 22%; P < 0,0001). O diâmetro dos stents foi de 2,76 ± 0,35mm vs. 2,91 ± 0,47mm (P = 0,006) e a soma do comprimento dos stents foi de 37,6 ± 23mm vs. 24,8 ± 15,8mm (P < 0,0001). Reestenose ocorreu em 6,3% vs. 12,8% dos pacientes (P = 0,099) e em 4,1% vs. 9,8% das lesões (P = 0,048). Houve incremento de custo de R$ 9.590,00 e a RCEI foi de R$ 147.538,00 por reestenose evitada (acima do limiar da Organização Mundial da Saúde). Entretanto, utilizando o escore de propensão, as variáveis que melhor classificaram os pacientes para SFs e apresentam RCEI máxima de R$ 4.776,96 foram idade > 72 anos, diabetes e lesões com diâmetro < 3,2mm e comprimento > 18mm.
ConclusõesApesar de os SFs não terem sido custo-efetivos na população em geral, o escore de propensão demonstrou que em idosos, diabéticos e pacientes com lesões longas ou vasos de fino calibre o uso de SFs foi custo-efetivo.
Brazilian national clinical practice shows that although the use of drug-eluting stents (DES) significantly contributes to the treatment of coronary artery disease, it is still exclusive to wealthy patients or to those who have prepaid health plans.1 In Brazil, few studies have mentioned the implications of the cost-effectiveness of DES.2–5 Although the use of DES has not been cost-effective in the populations evaluated, none of these studies has shown the real financial impact of its use, as they were non-randomised studies.
Despite the natural selection bias when more complex patients are treated with DES the results are higher than those found with bare-metal stents (BMS). Propensity score matching enables the correction or at least minimisation of the selection bias between DES and BMS.
The propensity score is defined as the probability of exposure to a treatment, according to each variable used in the matching. The score is usually obtained from logistic regression and varies from 0 to 1, reflecting each individual’s probability (based on their characteristics) of receiving the treatment of interest. Therefore, individuals with the same score have equal chances of receiving treatment even though they may not present the same characteristics, A ‘virtual randomisation’, in which comparable patients may be separated between exposed and non-exposed patients, may be obtained by analysing similar propensity scores.
This study aimed to compare the clinical results and the medium-term incremental cost-effectiveness ratio (ICER) between patients with DES and those with BMS. Due to the impossibility of randomisation, a propensity score was used to calculate the ICER in order to adjust the intervention effect by means of matching, stratification, and weighting between the groups, and thus classify the patients who most benefited from the use of DES.
METHODSA prospective cohort, consecutive, non-randomised study was conducted with 220 patients undergoing percutaneous coronary intervention (PCI) with implantation of one or more stents in private healthcare institutions in Rio de Janeiro, Brazil. The patients were divided into two groups: one group of 111 patients treated with DES, who used one or more Taxus® stents (Boston Scientific – Natick, MA, USA), and another group of 109 patients treated with BMS, who used one or more Liberté® stents (Boston Scientific – Natick, MA, USA).
Patients with stable angina or acute coronary syndrome without ST-segment elevation were included. Patients with acute myocardial infarction and cardiogenic shock were excluded. Procedures were conducted by six professionals from three institutions. BMS implants were mostly suggested for less complex lesions, when the long-term use of thienopyridines was not possible or due to financial issues limiting the use of DES.
While the patients were in hospital, success, death, myocardial infarction, target lesion revascularisation, stent thrombosis and direct costs were evaluated. The follow-up with all patients was conducted either via telephone or via appointments with a physician. Clinical restenosis occurred when symptom recurrence was observed. Only patients who developed restenosis underwent cardiac catheterisation. The dual antiplatelet therapy was maintained for three months following BMS, and for at least one year following DES.
Statistical analysisThe chi-squared test or Fisher’s exact test were used, and the 95% confidence interval (CI) and the mean ± standard deviation of all variables were calculated. In the survival analysis and in the event-free survival, the Kaplan-Meier method and the log rank test were used. The Cox method was used in the multivariate analysis. STATISTICA 8 (StatSoft Inc., Houston, TX, USA) was used in the analysis. The results were considered statistically significant when P < 0.05.
Cost analysisA decision-analytic model was developed to estimate the probabilities of outcomes and costs of DES vs. BMS. These models are developed through the chronological sequence of problem identification, problem structuring (decision tree) and parameterisation of model analysis (cost estimates, outcomes and risks).6 Effectiveness data were obtained from the study cohort. The costs due to intervention were calculated as direct costs: hospital stays, complementary examinations, stent prices, medication and professional fees. Costs were calculated in Brazilian reals, based on the currency value in 2005. The currency exchange rate was established on December 31, 2005 (US$ 1.00 = R$ 2.34). The prices of the medications were calculated based on the Brasíndice table on June 30, 2005 (maximum consumer price [preço máximo ao consumidor – PMC]).7 The price of the complementary examinations was obtained from the Brazilian Hierarchical Classification of Medical Procedures (Classificação Brasileira de Hierarquização de Procedimentos Médicos – CBHPM, 2003).8 Concerning the stents, the price of the purchases by the participant institutions was considered, and the unit price for each device was R$ 4,200.00 for the BMS and R$ 11,762.00 for the DES.
The ICER9 was calculated by dividing the difference of direct costs between the DES and BMS by the effectiveness difference (one-year event-free survival for restenosis). Figure 1 shows the formula used for calculating the ICER between the implants with DES vs. BMS. The incremental cost suggested by the World Health Organization (WHO) is up to three times the gross domestic product (GDP) per capita per year of life saved. In this period, for the calculus base suggested by the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística – IBGE, the total amount was US$ 20,313.00.10 The statistical analysis of the decision model was obtained by the TreeAge Pro Healthcare Module, version 2005 (TreeAge Software Inc. – Williamstown, MA, USA).
– Formula to calculate the incremental cost-effectiveness ratio between drug-eluting stent implantation and bare-metal stent implantation. Costs = hospital stays, complementary examinations, percutaneous procedure, professional fees, and stent prices (drug-eluting stents and bare-metal-stents); effectiveness = one-year event-free survival for restenosis; ICER = incremental cost-effectiveness ratio; DES = drug-eluting stent; BMS = bare-metal stent.
The matching procedure with balanced scores was used, and the score was calculated based on a logistic regression model that varied from 0 to 1, describing the probability of each individual receiving the necessary treatment according to their characteristics. Logarithmic odds were specifically used. A logit model was estimated by using all predictor variables in order to obtain the probability and calculate the logarithmic odds ratio for each observation in the sample of each individual group.11,12
The propensity score was developed to predict with maximum distinction the probability that a patient would receive a BMS, and it was considered an overfitted logistic model. In addition, by optimising the variance in the entrance of each clinical feature, it was guaranteed that for each probability produced by the model there would be representatives of both stent types. Finally, six strata with probability zones were generated to guarantee the described purpose. The ICER was analysed after the calculation of the propensity score to estimate the subgroups classified to receive the DES.
Ethical aspectsThe study protocol was approved by the Research Ethics Committee of the Universidade do Estado do Rio de Janeiro (PROJECT 681 A) and was established according to the present scientific guidelines.13
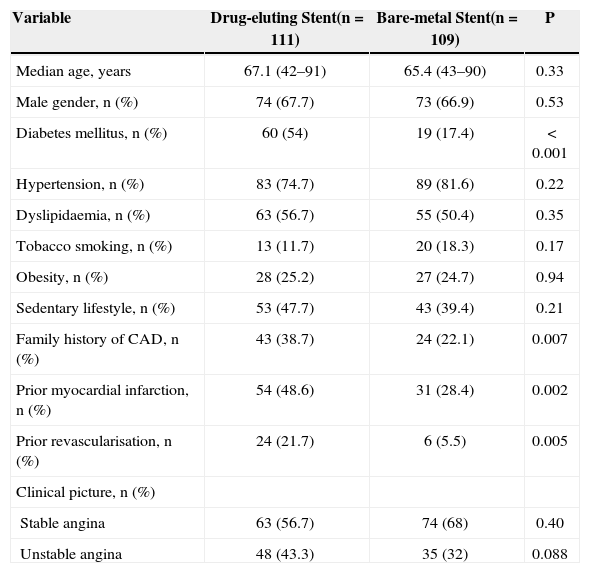
RESULTSMost patients were male (67.7% vs. 66.9%; P = 0.53), with a median age of 65.9 years (42 to 91 years). Patients treated with DES had higher rates of diabetes (54% vs. 17.4%; P < 0.001), prior myocardial infarction (48.6% vs. 28.4%; P = 0.002) and prior revascularisation procedures than the BMS group (21.7% vs. 5.5%; P = 0.005) (Table 1).
Clinical Features and Comorbidities in the Study Population
| Variable | Drug-eluting Stent(n = 111) | Bare-metal Stent(n = 109) | P |
|---|---|---|---|
| Median age, years | 67.1 (42–91) | 65.4 (43–90) | 0.33 |
| Male gender, n (%) | 74 (67.7) | 73 (66.9) | 0.53 |
| Diabetes mellitus, n (%) | 60 (54) | 19 (17.4) | < 0.001 |
| Hypertension, n (%) | 83 (74.7) | 89 (81.6) | 0.22 |
| Dyslipidaemia, n (%) | 63 (56.7) | 55 (50.4) | 0.35 |
| Tobacco smoking, n (%) | 13 (11.7) | 20 (18.3) | 0.17 |
| Obesity, n (%) | 28 (25.2) | 27 (24.7) | 0.94 |
| Sedentary lifestyle, n (%) | 53 (47.7) | 43 (39.4) | 0.21 |
| Family history of CAD, n (%) | 43 (38.7) | 24 (22.1) | 0.007 |
| Prior myocardial infarction, n (%) | 54 (48.6) | 31 (28.4) | 0.002 |
| Prior revascularisation, n (%) | 24 (21.7) | 6 (5.5) | 0.005 |
| Clinical picture, n (%) | |||
| Stable angina | 63 (56.7) | 74 (68) | 0.40 |
| Unstable angina | 48 (43.3) | 35 (32) | 0.088 |
CAD = coronary artery disease; n = number of patients.
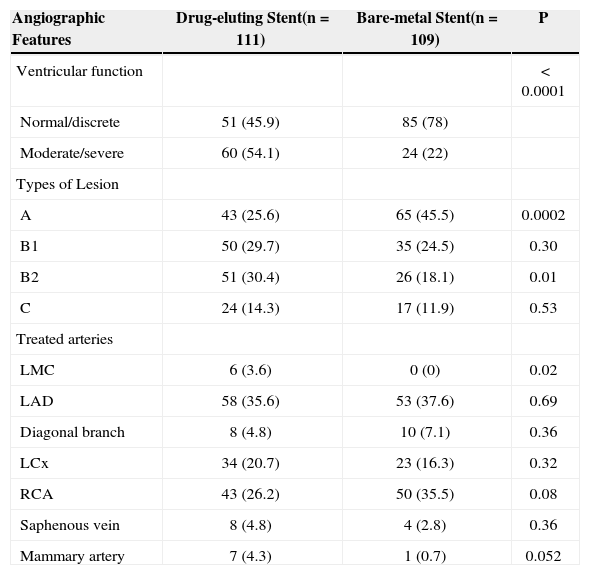
Concerning the angiographic features, patients treated with DES presented a higher rate of three-vessel disease (18.9% vs. 10.1%; P = 0.029) and poor ventricular function (54.1% vs. 22%; P < 0.0001). There was no difference regarding the number of lesions treated per patient (1.51 vs. 1.31; P = 0.18), but the group treated with DES showed a higher incidence of type B2 complex lesions (30.4% vs. 18.1%; P = 0.01). The most treated artery was the left anterior descending artery (35.6% vs. 37.6%; P = 0.69). Mammary grafts (4.3% vs. 0.7%; P = 0.052) and lesions of the left main coronary artery (3.6% vs. 0; P = 0.02) were frequently treated with DES (Table 2).
Angiographic Profile and Ventricular Function of the Study Population
| Angiographic Features | Drug-eluting Stent(n = 111) | Bare-metal Stent(n = 109) | P |
|---|---|---|---|
| Ventricular function | < 0.0001 | ||
| Normal/discrete | 51 (45.9) | 85 (78) | |
| Moderate/severe | 60 (54.1) | 24 (22) | |
| Types of Lesion | |||
| A | 43 (25.6) | 65 (45.5) | 0.0002 |
| B1 | 50 (29.7) | 35 (24.5) | 0.30 |
| B2 | 51 (30.4) | 26 (18.1) | 0.01 |
| C | 24 (14.3) | 17 (11.9) | 0.53 |
| Treated arteries | |||
| LMC | 6 (3.6) | 0 (0) | 0.02 |
| LAD | 58 (35.6) | 53 (37.6) | 0.69 |
| Diagonal branch | 8 (4.8) | 10 (7.1) | 0.36 |
| LCx | 34 (20.7) | 23 (16.3) | 0.32 |
| RCA | 43 (26.2) | 50 (35.5) | 0.08 |
| Saphenous vein | 8 (4.8) | 4 (2.8) | 0.36 |
| Mammary artery | 7 (4.3) | 1 (0.7) | 0.052 |
RCA = right coronary artery; LCx = left circumflex artery; LAD= left anterior descending artery; n = number of patients; LMC= left main coronary artery.
Concerning the procedure, the diameter of DES vs. BMS was 2.76 ± 0.35mm vs. 2.91 ± 0.47mm (P = 0.006), and the sum of the lengths of stents was 37.6 ± 23mm vs. 24.8 ± 15.8mm (P < 0.0001). The average hospital stay was similar in both groups (1.75 days vs. 1.49 days; P = 0.21).
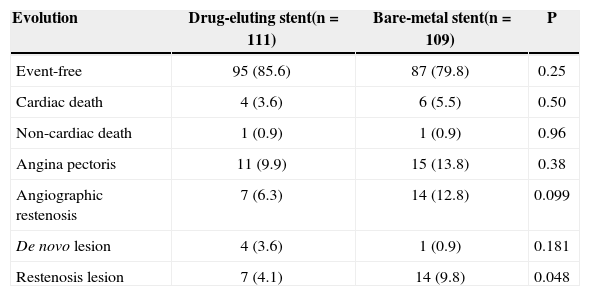
In the late follow-up (average of 17 months), the frequency of asymptomatic patients was 85.6% vs. 79.8% (P = 0.25) in the DES and BMS groups, respectively. The number of cardiac (4 vs. 6; P = 0.50) and non-cardiac deaths (1 vs. 1; P = 0.96) was similar between the groups (Table 3). Among the four cardiac deaths within the group treated with DES, one was due to restenosis with myocardial infarction treated with primary angioplasty, and three were due to congestive heart failure. Among the six deaths of patients treated with BMS, one presented sudden death, and the others presented acute coronary syndrome without ST-segment elevation.
Follow-up of the Study Population
| Evolution | Drug-eluting stent(n = 111) | Bare-metal stent(n = 109) | P |
|---|---|---|---|
| Event-free | 95 (85.6) | 87 (79.8) | 0.25 |
| Cardiac death | 4 (3.6) | 6 (5.5) | 0.50 |
| Non-cardiac death | 1 (0.9) | 1 (0.9) | 0.96 |
| Angina pectoris | 11 (9.9) | 15 (13.8) | 0.38 |
| Angiographic restenosis | 7 (6.3) | 14 (12.8) | 0.099 |
| De novo lesion | 4 (3.6) | 1 (0.9) | 0.181 |
| Restenosis lesion | 7 (4.1) | 14 (9.8) | 0.048 |
n = number of patients.
Restenosis was observed in 6.3% vs. 12.8% of the patients (P = 0.099) and in 4.1% vs. 9.8% of the lesions (P = 0.048). There were 11 revascularisations in the DES group, seven to treat restenosis and four to treat de novo lesions. Among the restenoses, two were treated with a new PCI, four with coronary artery bypass graft surgery and one in a conservative manner. In the BMS group, there were 15 new revascularisations, 14 to treat restenosis and one to treat de novo lesions. Among the 14 restenoses, seven were clinically treated, six through PCI and one through surgery.
The two-year survival rates were 96.2% in the DES group and 89.3% in the BMS group (P = 0.76).
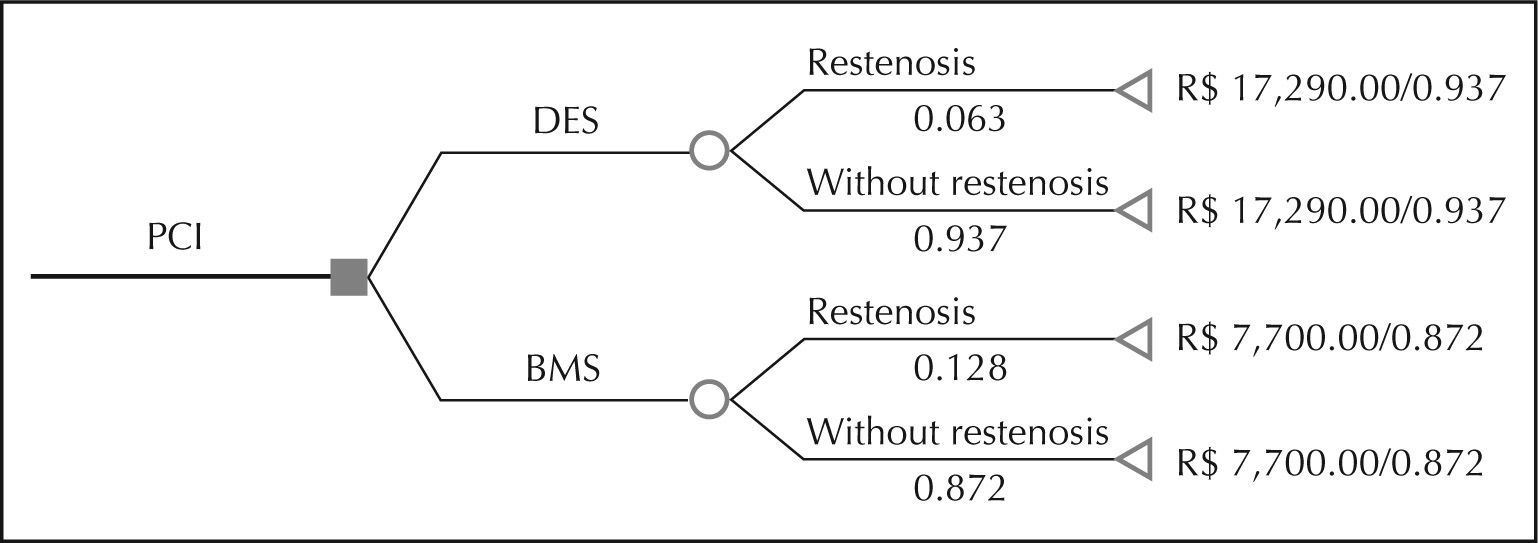
Concerning costs, the decision tree was modelled in the restenosis of the DES group (6.3%) vs. the BMS group (12.8%) over the average follow-up of 17 months. Figure 2 shows the decision tree with the rates and costs related to both groups and presents the relationship between cost and effectiveness.
The net benefit of the DES implant was 6.3% restenosis reduction, and there was an incremental cost of R$ 9,590.00. The ICER was R$ 147,538.00 per restenosis prevented, whose incremental price was above the threshold suggested by the World Health Organization (R$ 47,532.00).10 Therefore, the DES implant proved to be a non-cost-effective treatment (Table 4).
Incremental Cost-effectiveness Ratio between Drug-eluting Stent Implantation and Bare-metal Stent Implantation
| Drug-eluting stent | Bare-metal stent | |
|---|---|---|
| Cost | R$ 17,290.00 | R$ 7,700.00 |
| Incremental cost | R$ 9,590.00 | |
| Effectiveness | 0.937 | 0.872 |
| Incremental effectiveness | 0.065 | |
| Cost-effectiveness | R$ 18,452.00* | R$ 8,830.00* |
| ICER | R$ 147,538.00 |
ICER = incremental cost-effectiveness ratio.
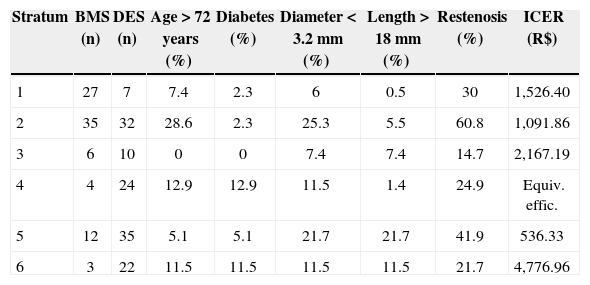
Considering restenosis (Table 5) and survival (Table 6) separately, the analysis of the propensity score for ICER showed that patients who were > 72 years old, patients with diabetes, and patients who had lesions < 3.2mm in diameter and > 18mm in length were the best-qualified subgroups to receive DES. These subgroups represented strata in which ICER varied from R$ 536.33 to R$ 4,776.96, within the threshold suggested by the WHO.
Propensity Score for Incremental Cost-effectiveness Ratio and One-year Restenosis
| Stratum | BMS (n) | DES (n) | Age > 72 years (%) | Diabetes (%) | Diameter < 3.2mm (%) | Length > 18mm (%) | Restenosis (%) | ICER (R$) |
|---|---|---|---|---|---|---|---|---|
| 1 | 27 | 7 | 7.4 | 2.3 | 6 | 0.5 | 30 | 1,526.40 |
| 2 | 35 | 32 | 28.6 | 2.3 | 25.3 | 5.5 | 60.8 | 1,091.86 |
| 3 | 6 | 10 | 0 | 0 | 7.4 | 7.4 | 14.7 | 2,167.19 |
| 4 | 4 | 24 | 12.9 | 12.9 | 11.5 | 1.4 | 24.9 | Equiv. effic. |
| 5 | 12 | 35 | 5.1 | 5.1 | 21.7 | 21.7 | 41.9 | 536.33 |
| 6 | 3 | 22 | 11.5 | 11.5 | 11.5 | 11.5 | 21.7 | 4,776.96 |
ICER = incremental cost-effectiveness ratio; DES = drug-eluting stent; BMS = bare-metal stent; R$ = Brazilian reals.
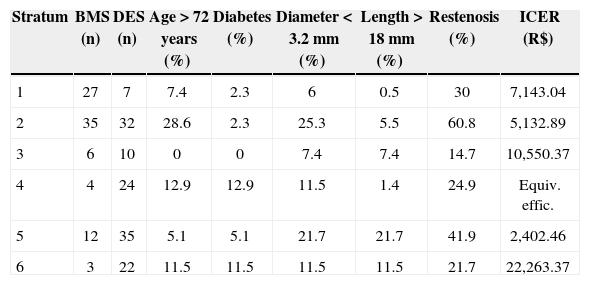
Propensity Score for Incremental Cost-effectiveness Ratio and One-year Survival
| Stratum | BMS (n) | DES (n) | Age > 72 years (%) | Diabetes (%) | Diameter < 3.2mm (%) | Length > 18mm (%) | Restenosis (%) | ICER (R$) |
|---|---|---|---|---|---|---|---|---|
| 1 | 27 | 7 | 7.4 | 2.3 | 6 | 0.5 | 30 | 7,143.04 |
| 2 | 35 | 32 | 28.6 | 2.3 | 25.3 | 5.5 | 60.8 | 5,132.89 |
| 3 | 6 | 10 | 0 | 0 | 7.4 | 7.4 | 14.7 | 10,550.37 |
| 4 | 4 | 24 | 12.9 | 12.9 | 11.5 | 1.4 | 24.9 | Equiv. effic. |
| 5 | 12 | 35 | 5.1 | 5.1 | 21.7 | 21.7 | 41.9 | 2,402.46 |
| 6 | 3 | 22 | 11.5 | 11.5 | 11.5 | 11.5 | 21.7 | 22,263.37 |
ICER = incremental cost-effectiveness ratio; DES = drug-eluting stent; BMS = bare-metal stent; R$ = Brazilian reals.
In a real-world analysis comparing DES to BMS, this study has shown that the patients from the DES group presented more complex clinical and angio-graphic features, but similar clinical events, compared with the BMS group. The incidence of clinical restenosis did not show significant differences, nor did the survival curves. The rate of restenosis due to the lesion was higher in the group treated with BMS, but this group’s results exceeded expectations, probably due to the sensible selection of patients with a less favourable profile. Therefore, the comparison of the ICER of the two groups showed that DES are not cost-effective compared to the BMS. The propensity score used demonstrated that the variables age > 72 years, diabetes and < 3.2-mm-diameter and > 18-mm-length lesions were the best factors to classify the groups, leading to a cost-effective ICER.
Despite the fact that the propensity score is a tool to understand the differences between groups, this ‘fictitious randomisation’ does not replace the main advantage of randomisation, which is to generate homogeneous groups in relation to unknown variables.
Although the development of new technologies and devices related to intervention helps to reduce costs, the financial burden necessary to implant these devices is still very high, and this remains the physician’s major challenge.
The different results between the groups are the consequence of a better late evolution in patients receiving DES. The limited use of DES, whether in Brazil or in developed countries, is exclusively related to costs,1–5 which are still very high. The perspective of better results in more severe patients compared with patients in less severe conditions is the largest factor contributing to the use of DES.14,15 Therefore, this article aimed to investigate the societal cost of the use of DES in a complex clinical scenario.
The WHO suggests that the incremental cost of the treatment present a threshold equivalent to three times the per capita GDP. According to IBGE, in 2005 the per capita GDP was US$ 6,771.00, which generates a threshold of US$ 20,313.00 (R$ 47,532.00) for the treatment’s incremental cost.10
In this clinical case, the incremental cost of R$ 9,590.00 (US$ 4,098.00) for each DES corresponds to a cost-effectiveness of R$ 18,452.00 (US$ 7,885.00) per restenosis prevented. The cost-effectiveness of R$ 8,830.00 (US$ 3,773.00) per restenosis prevented by using DES corresponds to an ICER of R$ 147,538.00 (US$ 63,050.00), which is above the acceptable threshold of R$ 47,532.00. Therefore, the DES implant proved to be a non-cost-effective treatment strategy.
The decision tree of the cost-effectiveness analysis shows that the DES provided a net benefit of 6.3% restenosis reduction. Nevertheless, the cost increment per stent, cost per restenosis prevented, as well as the ICER factors,confirm the hypothesis that DES are not cost-effective when compared with BMS in private health systems.
Besides the additional cost of DES, the low rates of restenosis and of new interventions among patients treated with BMS contributed to cost reduction in these patients. This positive response may be explained by the good performance of BMS and by the selective indication of patients. Consequently, differences in costs are important factors in the non-cost-effectiveness of DES compared with BMS.
The treatment of an extremely heterogeneous population, in which the events in the BMS group were below expectations, provides the best explanation for the failure to achieve an ICER that favoured the use of DES. Additionally, the results were influenced by the fact that a control angiographic protocol was not elaborated. The decision model in which the economic calculations were based is strongly effectiveness-dependent and considers restenosis rates of 30% in patients with BMS and 6% in patients with DES.
Previous studies have shown unequal results concerning the analysed population and the DES cost. In Brazil, the model developed to compare sirolimus-eluting stents with standard stents in patients with the same type of lesion has shown that the costs of sirolimus stents are partially compensated within the first year, especially in high-risk subgroups. However, the additional costs considered acceptable for certain clinical intervention benefits have not been established in Brazil.2–5
In the real world, DES are less cost-effective than in controlled studies. These stents must be used particularly in high-risk patients. Randomised studies16–21 have shown that the use of DES was cost-effective. In the Basel Stent Kosten-Effektivitats Trial (BASKET), Kaiser et al.22 included 826 patients and demonstrated that the high cost of DES was not compensated by cost reduction during the follow-up. However, DES were cost-effective in the elderly and in high-risk patients.
The cost outcomes demonstrated in randomised studies do not reflect the experience and records of the real world, especially due to different rates of new revascularisations between the two study methodologies.
In the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) study, Lemos et al.23 evaluated the use of BMS (450 patients) vs. the use of DES (508 patients) in a real world setting. In a one-year period, the event rate was 14.8% in patients with BMS and 9.7% in patients with DES, revealing that the use of DES was not cost-effective. Ferreira et al.24 followed-up 217 patients with single-vessel coronary artery disease treated with only one stent, and observed an ICER of R$ 131,647.84 per restenosis that was prevented. The present study’s findings are in agreement with the ICERs found by Lemos et al.23 and by Ferreira et al.,24 which confirms that non-randomised studies are not cost-effective.
Cost-effective estimates are sensitive to several variables. It is still a challenge to establish the real impact of DES in clinical practice. Regional factors, market price and the number of stents per patient may also influence cost-effectiveness. The decision to limit the use of DES to high-risk patients who are likely to develop restenosis can improve its cost-effectiveness, but it will be necessary to find evidence to compare the absolute benefits among the patient groups. This study was relevant since, by means of the propensity score, the benefits of DES use resulting from its cost-effectiveness in certain subgroups of a cohort were clearly shown for the first time. The follow-up of all patients and the use of propensity score demonstrated that the use of DES in specific subgroups is cost-effective in spite of the impossibility of randomisation.
Limitations of the studyAs this study used records provided by private institutions, it was not possible to perform an appropriate randomisation with a control group. Because the physicians decided the follow-up of the patients, total angiographic control was not possible, especially in the asymptomatic patients in this study. Therefore, it is not possible to say that no patient died of clinically silent restenosis. The number of patients from this population was limited, but it allowed for inference of the evolution of these patients during the study period.
CONCLUSIONSThis study demonstrated that, in national clinical practice, patients treated with DES presented a more complex clinical and angiographic profile than those treated with BMS, but both presented similar clinical events. Although the rates of restenosis due to lesions were higher in the group treated with BMS, the ICER showed that DES are not cost-effective in the total population. The use of propensity score to reduce the differences between the groups showed that DES are cost-effective in the subgroups of the elderly, diabetics and patients with long lesions or small vessels.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
This article is part of the doctoral research of the first author at the Universidade do Estado do Rio de Janeiro (UERJ). It would not have been possible without the invaluable help of the directors, physicians, and staff of the Mario Lioni (Amil Par), Prontocor, and Clínica Status Cor Hospitals, and of the clinical cardiologists who provided data or allowed the authors to contact their patients. Special thanks to Bernardo Tura for statistical guidance.