The increasing use of transcatheter aortic valve implantation (TAVI) in high-risk patients, especially those with ventricular dysfunction, justifies further evaluation of the selection and the results of the procedure. A database was used to characterize the profile of patients and evaluate TAVI results according to the degree of ventricular dysfunction.
MethodsThis was a longitudinal observational study that included all patients with severe aortic stenosis (AoS) submitted to TAVI between 2009 and 2014, comparing those with left ventricular ejection fraction (LVEF) ≤ 40% vs. > 40%. The safety and efficacy outcomes were evaluated at 30 days and 1 year.
ResultsOf the 172 patients, 20 (11.6%) had LVEF ≤ 40%. These patients were younger, with a higher prevalence of smoking, previous acute myocardial infarction, coronary artery bypass graft surgery, permanent pacemaker, and pulmonary artery hypertension. Higher functional classes were also more often observed in this group. The group with LVEF ≤ 40% had lower mean aortic valve gradient for an equivalent valve area. The procedure success did not differ between groups. There were no differences in mortality in coronary and cerebrovascular events, bleeding, vascular complications, and acute renal failure in the 30 day and 1 year follow-up. In the LVEF ≤ 40% group, the mean LVEF increased from 31.5 to 45.1% 1 year after the procedure (p=0.002).
ConclusionsTAVI in patients with severe AoS and LVEF ≤ 40% does not increase the risk of complications and is associated with LVEF improvement.
A utilização crescente do implante transcateter de prótese valvar aórtica (TAVI) em pacientes de alto risco, em especial naqueles com disfunção ventricular, justifica uma avaliação mais profunda da seleção e dos resultados do procedimento. Utilizamos nosso banco de dados para caracterizar o perfil dos pacientes e avaliar os resultados do TAVI de acordo com o grau de disfunção ventricular.
MétodosEstudo observacional longitudinal no qual foram incluídos todos os pacientes com estenose aórtica (EAo) grave, submetidos ao TAVI entre 2009 e 2014, e comparados àqueles com fração de ejeção do ventrículo esquerdo (FEVE) ≤ 40% vs. > 40%. Foram avaliados os desfechos de segurança e eficácia em 30 dias e 1 ano.
ResultadosDentre os 172 pacientes, 20 (11,6%) apresentavam FEVE ≤ 40%. Esses pacientes eram mais jovens, com maior prevalência de tabagismo, infarto agudo do miocárdio prévio, cirurgia de revascularização miocárdica, marca-passo definitivo e hipertensão arterial pulmonar. Também se observou, nesse grupo, maior frequência de classes funcionais mais elevadas. O grupo com FEVE ≤ 40% apresentou menor gradiente valvar aórtico médio para área valvar equivalente. As taxas de sucesso do procedimento não diferiram entre os grupos. Não foram observadas diferenças na mortalidade, nos eventos coronarianos, cerebrovasculares, sangramentos, complicações vasculares e disfunção renal aguda no acompanhamento de 30 dias e 1 ano. No grupo FEVE ≤ 40%, a média da FEVE elevou-se de 31,5 para 45,1% 1 ano após o procedimento (p=0,002).
ConclusõesO TAVI em pacientes com EAo grave e FEVE ≤ 40% não aumenta o risco de complicações e está associado à melhora da FEVE.
Degenerative aortic stenosis (AoS) is the most common valve disease in adults, whose prevalence increases with age, affecting approximately 4% of individuals older than 80 years. Data from the Instituto Brasileiro de Geografia e Estatística (IBGE) estimates that within 20 years, the country will have 14.5 million elderly individuals older than 75 years, of whom 400,000 to 650,000 might have degenerative AoS.1,2
Patients with severe AoS have formal indication for valve replacement, due to the unfavorable prognosis with clinical treatment.3,4 However, approximately 30% of patients with AoS and surgical indication are not submitted to valve replacement due to advanced age, comorbidities, physician or patient's refusal to conventional surgical treatment.5
In the context of patients with symptomatic severe AoS and high surgical risk or inoperable patients, the transcatheter aortic valve implantation (TAVI) was introduced, and the PARTNER (Placement of AoRtic TraNscathetER Valves) trial was the first randomized study to show that TAVI was an alternative not inferior to surgery in high risk patients (cohort A) and a superior option to clinical treatment in those considered inoperable (cohort B).6 More recently, the CoreValve US Pivotal Trial showed that TAVI may be superior to valve replacement surgery, in terms of reducing mortality in patients with high surgical risk (Society of Thoracic Surgeons Predicted Risk of Mortality - STS PROM ≥ 15%).7
The increasing use of TAVI in high-risk patients, especially those with ventricular dysfunction, justifies further evaluation of procedure selection and outcomes. This institution's database was used to characterize the profile of patients and evaluate TAVI results according to the ventricular dysfunction degree. The authors compared patients with moderate to severe systolic dysfunction of the left ventricle (LV), defined as left ventricular ejection fraction (LVEF) ≤ 40%, with those individuals with LVEF > 40%.
MethodsAn observational and longitudinal study was carried out, which included all patients with severe, symptomatic AoS submitted to TAVI, from January 2009 to June 2014, at Instituto Dante Pazzanese de Cardiologia and Hospital do Coração (HCor), both located in São Paulo (SP), after the project approval by the Institutional Ethics Committee.
Clinical data were obtained by clinical examination and complementary tests (resting electrocardiography, chest X-ray, transthoracic Doppler echocardiography with protocol for aortic complex measurements; computed tomography angiography of the heart and total aorta, and cardiac catheterization with coronary angiography). Transesophageal echocardiography was used to guide the procedure and detect possible complications due to prosthesis implantation.
Data were recorded on appropriate forms, which were developed for the study and stored in electronic spreadsheets.
Patient preparation and type of prosthesis usedAll patients were pretreated with 100mg acetylsalicylic acid and 300mg clopidogrel on the day prior to implantation. In patients with renal dysfunction, intravenous hydration with 0.9% saline solution at a dose of 0.3 to 0.5mL/kg/hour was started 12hours before the procedure. Additionally, aiming to prevent contrast-induced nephropathy, the angiograms required during the procedure were obtained with low-osmolarity contrast, with a 50% dilution.
The self-expanding CoreValve (Medtronic – Minneapolis, USA) and Acurate (Symetis – Ecublens, Switzerland) prostheses were used, as well as the Edwards Sapien (Edwards Lifesciences – Irvine, USA) balloon-expandable prosthesis. The choice of prosthesis was based on device availability and the operators choice.
The patients were admitted to the intensive care unit after the intervention, and on the first day after the procedure, underwent laboratory tests, electrocardiogram, and echocardiogram.
Outcomes and definitionsOutcomes and complications were detailed according to the standardized and updated definitions of the Valve Academic Research Consortium-2 (VARC2).8 Device success was considered a single prosthesis implantated in the appropriate place, with no prosthesis-patient mismatch, obtaining a mean aortic transvalvular gradient < 20mmHg or peak velocity < 3 m/s, and in the absence of aortic regurgitation equal or greater than moderate – according to transesophageal or transthoracic echocardiography definitions.
Procedural success and safety (30 days) and efficacy (1 year) outcomes were assessed. Stroke was determined by the onset of focal or global neurological deficit lasting > 24hours, or the presence of a new cerebral infarction area, or bleeding in neuroimaging methods, regardless of symptom duration. Hemorrhagic complications were divided into: (1) life-threatening bleeding: when fatal or overt bleeding occurred in a vital organ (intracranial, intraocular, and pericardial), or bleeding that resulted in hypovolemic shock or severe hypotension requiring vasopressors or surgery, or overt bleeding with hemoglobin decrease ≥ 5g/dL or that required transfusion of four or more packed red blood cells; (2) major bleeding: overt bleeding with decrease in hemoglobin ≥ 3g/dL or that required transfusion of two or three packed red blood cells, or bleeding that required hospitalization or surgery; (3) minor bleeding: any significant bleeding (e.g., puncture site hematoma) that did not meet the criteria for life-threatening or major bleeding.
Vascular complications were classified as major, according to the following criteria: occurrence of aortic dissection, aorta or aortic annulus rupture, or LV perforation; diagnosis of vascular injury at the femoral puncture site, which resulted in death, major, or life-threatening bleeding; vascular injury that caused visceral ischemia or neurological impairment; non-cerebral distal embolization that required surgery; need for surgical or percutaneous intervention, which led to death, major bleeding, visceral ischemia, or neurological impairment; or any documented ipsilateral ischemia.
Chronic renal failure was determined by the presence of creatinine clearance < 50mL/minute. Acute kidney injury after the procedure was classified according to Acute Kidney Injury Network (AKIN) criteria, being evaluated until the seventh day post-implantation. Kidney injury was categorized as: (a) stage 1: increase of 0.3mg/dL or 150 to 200% increase of baseline serum creatinine level or urine output < 0.5mL/kg/hour for 6hours after the procedure; (b) stage 2: increase > 200 to 300% of baseline serum creatinine and urine output < 0.5mL/kg/hour for > 12hours; (c) stage 3: increase > 300% of baseline serum creatinine or serum creatinine ≥ 4.0mg/dL, associated with an increase of at least 0.5mg/dL of baseline level; urine output < 0.3mL/kg/hour for 24hours or anuria for more than 12hours. The presence of pulmonary hypertension was determined by the pulmonary artery systolic pressure (measured by transthoracic echocardiography) > 55mmHg.
Statistical analysisContinuous variables were described as mean and standard deviation, and categorical variables as absolute number and percentage. Student's t-test (or the Mann-Whitney test) was used to compare continuous variables for independent samples, and the chi-squared test or Fisher's exact test was used for categorical variables, as appropriate. LVEF comparisons during follow-up were performed using Friedman's test for repeated measures. All analyses were performed using Statistical Package for the Social Science (SPSS) software version 19 and R, version 3.1.2. Statistical significance level was set at 5%.
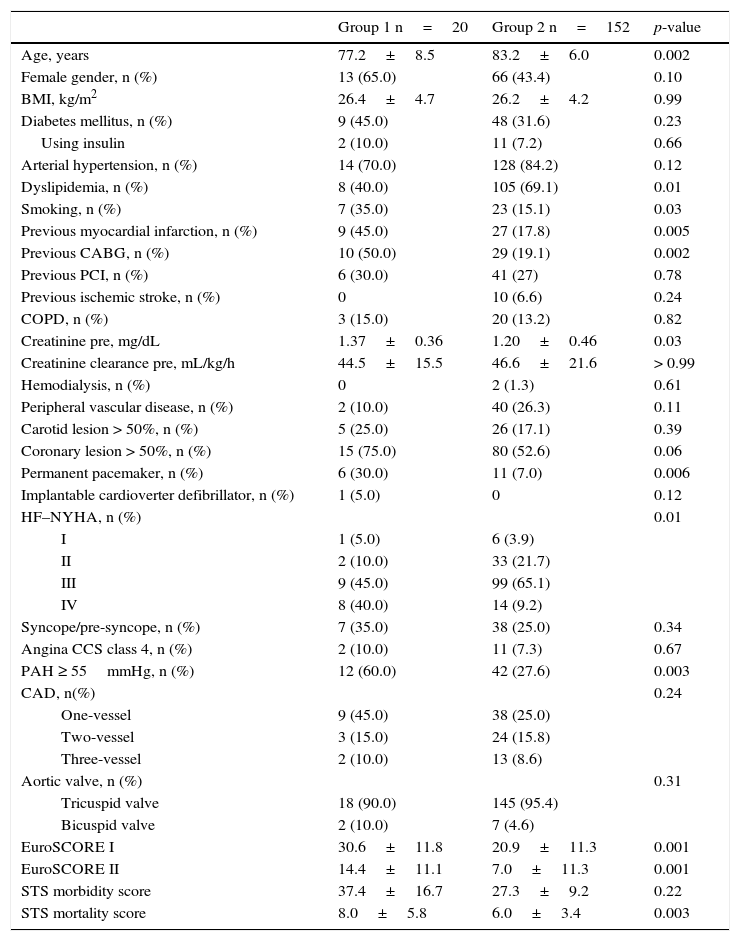
ResultsFrom January 2009 to June 2014, 172 patients were submitted to TAVI, distributed into Group 1, comprising patients with LVEF ≤ 40% (n=20; 11.6%) and Group 2, comprising patients with LVEF > 40% (n=152). Patients with LVEF ≤ 40% were younger (77.2±8.5 vs. 83.2±6.0 years; p=0.002), had higher prevalence of smoking (35.0% vs. 15.1%; p=0.03), previous acute myocardial infarction (45.0% vs. 17.8%; p=0.005), coronary artery bypass grafting surgery (50.0% vs. 19.1%; p=0.002), permanent pacemaker (30.0% vs. 7.0%; p=0.006), and pulmonary artery hypertension (60.0% vs. 27.6%; p=0.003). Group 1 also had increased frequency of higher functional classes and higher serum creatinine levels (1.37±0.36 vs. 1.20±0.46mg/dL; p=0.03). In the pre-TAVI evaluation, EuroSCORE I (30.6%±11.8% vs. 20.9%±11.3%; p < 0.001), EuroSCORE II (14.4%±11.1% vs. 7.0±11.3%, p<0.001), and STS score for mortality (8.0±5.8 vs. 6.0±3.4; p=0.003) were higher for the group with LVEF ≤ 40% (Table 1).
Basal clinical data.
| Group 1 n=20 | Group 2 n=152 | p-value | |
|---|---|---|---|
| Age, years | 77.2±8.5 | 83.2±6.0 | 0.002 |
| Female gender, n (%) | 13 (65.0) | 66 (43.4) | 0.10 |
| BMI, kg/m2 | 26.4±4.7 | 26.2±4.2 | 0.99 |
| Diabetes mellitus, n (%) | 9 (45.0) | 48 (31.6) | 0.23 |
| Using insulin | 2 (10.0) | 11 (7.2) | 0.66 |
| Arterial hypertension, n (%) | 14 (70.0) | 128 (84.2) | 0.12 |
| Dyslipidemia, n (%) | 8 (40.0) | 105 (69.1) | 0.01 |
| Smoking, n (%) | 7 (35.0) | 23 (15.1) | 0.03 |
| Previous myocardial infarction, n (%) | 9 (45.0) | 27 (17.8) | 0.005 |
| Previous CABG, n (%) | 10 (50.0) | 29 (19.1) | 0.002 |
| Previous PCI, n (%) | 6 (30.0) | 41 (27) | 0.78 |
| Previous ischemic stroke, n (%) | 0 | 10 (6.6) | 0.24 |
| COPD, n (%) | 3 (15.0) | 20 (13.2) | 0.82 |
| Creatinine pre, mg/dL | 1.37±0.36 | 1.20±0.46 | 0.03 |
| Creatinine clearance pre, mL/kg/h | 44.5±15.5 | 46.6±21.6 | > 0.99 |
| Hemodialysis, n (%) | 0 | 2 (1.3) | 0.61 |
| Peripheral vascular disease, n (%) | 2 (10.0) | 40 (26.3) | 0.11 |
| Carotid lesion > 50%, n (%) | 5 (25.0) | 26 (17.1) | 0.39 |
| Coronary lesion > 50%, n (%) | 15 (75.0) | 80 (52.6) | 0.06 |
| Permanent pacemaker, n (%) | 6 (30.0) | 11 (7.0) | 0.006 |
| Implantable cardioverter defibrillator, n (%) | 1 (5.0) | 0 | 0.12 |
| HF–NYHA, n (%) | 0.01 | ||
| I | 1 (5.0) | 6 (3.9) | |
| II | 2 (10.0) | 33 (21.7) | |
| III | 9 (45.0) | 99 (65.1) | |
| IV | 8 (40.0) | 14 (9.2) | |
| Syncope/pre-syncope, n (%) | 7 (35.0) | 38 (25.0) | 0.34 |
| Angina CCS class 4, n (%) | 2 (10.0) | 11 (7.3) | 0.67 |
| PAH ≥ 55mmHg, n (%) | 12 (60.0) | 42 (27.6) | 0.003 |
| CAD, n(%) | 0.24 | ||
| One-vessel | 9 (45.0) | 38 (25.0) | |
| Two-vessel | 3 (15.0) | 24 (15.8) | |
| Three-vessel | 2 (10.0) | 13 (8.6) | |
| Aortic valve, n (%) | 0.31 | ||
| Tricuspid valve | 18 (90.0) | 145 (95.4) | |
| Bicuspid valve | 2 (10.0) | 7 (4.6) | |
| EuroSCORE I | 30.6±11.8 | 20.9±11.3 | 0.001 |
| EuroSCORE II | 14.4±11.1 | 7.0±11.3 | 0.001 |
| STS morbidity score | 37.4±16.7 | 27.3±9.2 | 0.22 |
| STS mortality score | 8.0±5.8 | 6.0±3.4 | 0.003 |
Group 1: left ventricular ejection fraction ≤ 40%; Group 2: left ventricular ejection fraction > 40%. BMI: body mass index; CABG: coronary artery bypass graft surgery; PCI: percutaneous coronary intervention; COPD: chronic obstructive pulmonary disease; HF: heart failure; NYHA: New York Heart Association; PAH: pulmonary artery hypertension; CAD: coronary artery disease; STS: Society of Thoracic Surgeons.
The pre-procedure echocardiogram showed mean LVEF of 31.5±3.9% in Group 1 and 61.5±8.4% in Group 2. Group 1 had higher left atrial diameter (48.5±6.3 vs. 44.3±5.0mm; p=0.003) and higher LV end-diastolic diameter (60.8±8.1 vs. 49.3±5.5mm; p<0.001). The valve area evaluation through the continuity equation showed an equivalent area in both groups (0.66±0.13 vs. 0.67±0.15cm2; p=0.75), but lower maximum (67.7±23.0 vs. 90.1±21.3mmHg, p<0.001) and mean systolic transaortic gradients (40.9±14.2 vs. 55.7±14.3mmHg; p<0.001) for the group with LVEF ≤ 40%. The aortic valve annulus measurement and the evaluation of the pre-aortic regurgitation did not differ between the groups (Table 2).
Pre-procedure echocardiographic data.
| Group 1 n=20 | Group 2 n=152 | p-value | |
|---|---|---|---|
| Left atrium, mm | 48.5±6.3 | 44.3±5.0 | 0.003 |
| LVDD, mm | 60.8±8.1 | 49.3±5.5 | <0.001 |
| PASP, mmHg | 54.0±12.1 | 47.1±14.2 | 0.02 |
| Maximum LV-Ao gradient, mmHg | 67.7±23.1 | 90.1±21.3 | <0.001 |
| Mean LV-Ao gradient, mmHg | 40.9±14.2 | 55.7±14.3 | < 0.001 |
| Aortic valve area, cm2 | 0.66±0.13 | 0.67±0.15 | 0.75 |
| Aortic annulus, cm2 | 23.1±1.8 | 22.4±1.9 | 0.08 |
| Aortic regurgitation, n (%) | 0.48 | ||
| Absent/minimum | 1 (5.0) | 15 (9.9) | |
| Mild | 16 (80.0) | 125 (82.2) | |
| Moderate | 3 (15.0) | 12 (7.9) |
Group 1: left ventricular ejection fraction ≤ 40%; Group 2: left ventricular ejection fraction > 40%. LVDD: left ventricular diastolic diameter; PASP:pulmonary artery systolic pressure; LV: left ventricle; Ao: aorta.
A total of 84 CoreValve prostheses, 52 Edwards Sapien prostheses, and 36 Acurate prostheses were implanted. There was a significant decrease in transvalvular gradients in both groups. Procedural success was similar in both groups (90.0% vs. 93.4%; p=0.57). In Group 1, all the procedures were performed using the femoral access, while in Group 2, the femoral (86.8%), transaortic (6.6%), transapical (5.3%), subclavian artery (0.7%), and iliac artery accesses (0.7%) were used (Table 3).
Procedure data.
| Group 1 n=20 | Group 2 n=152 | p-value | |
|---|---|---|---|
| Vascular access, n (%) | 0.56 | ||
| Femoral | 20 (100) | 132 (86.8) | |
| Transaortic | 0 | 10 (6.6) | |
| Transapical | 0 | 8 (5.3) | |
| Subclavian | 0 | 1 (0.7) | |
| Iliac | 0 | 1 (0.7) | |
| Type of prosthesis, n (%) | 0.39 | ||
| CoreValveTM | 12 (60.0) | 72 (47.4) | |
| Sapien | 6 (30.0) | 46 (30.3) | |
| Acurate | 2 (10.0) | 34 (22.4) | |
| Mean systolic gradient prea, mmHg | 44.7±21.0 | 69.9±29.4 | 0.003 |
| Mean systolic gradient posta, mmHg | 4.7±6.2 | 3.9±6.2 | 0.40 |
| Device success, n (%) | 18 (90.0) | 144 (94.7) | 0.57 |
| Clinical success, n (%) | 18 (90.0) | 143 (93.4) | 0.40 |
| Aortic regurgitation post | 0.16 | ||
| Absent/minimum | 8 (40.0) | 80 (52.6) | |
| Mild | 7 (35.0) | 66 (43.4) | |
| Moderate | 5 (25.0) | 6 (3.9) |
Group 1: left ventricular ejection fraction ≤ 40%; Group 2: left ventricular ejection fraction > 40%.
At 30 days and 1 year, the cumulative mortality rates were 12.8 and 16.9%, respectively; for stroke, they were 2.9 and 2.9%; for major vascular complications, 7.0 and 7.6%; for major or life-threatening bleeding, 24.4 and 25.6%, showing no significant differences between the two groups. The outcomes of emergency surgery, acute renal failure, acute respiratory failure, atrioventricular block, conduction disorders, permanent pacemaker, infections, and endocarditis at 30 days and 1 year, were also similar between groups (Table 4).
Cumulative 30-day and 1-year outcomes after the procedure.
| 30-day outcomes | 1-year outcomes | |||||
|---|---|---|---|---|---|---|
| Group 1 n=20 | Group 2 n=152 | p-value | Group 1 n=20 | Group 2 n=152 | p-value | |
| Death, n (%) | 1 (5.0) | 21 (13.8) | 0.27 | 1 (5.0) | 28 (18.4) | 0.13 |
| Myocardial infarction, n (%) | 1 (5.0) | 3 (2.0) | 0.40 | 1 (5.0) | 4 (2.7) | 0.47 |
| Stroke, n (%) | 1 (5.0) | 4 (2.6) | 0.55 | 1 (5.0) | 4 (2.6) | 0.55 |
| TIA, n (%) | 1 (5.0) | 2 (1.3) | 0.24 | 2 (10.0) | 2 (1.3) | 0.02 |
| Bleeding, n (%) | 8 (40.0) | 66 (43.4) | 0.77 | 8 (40.0) | 68 (44.7) | 0.70 |
| Minor | 4 (20.0) | 28 (18.4) | 4 (20.0) | 28 (18.4) | ||
| Major | 4 (20.0) | 30 (19.7) | 4 (20.0) | 30 (19.7) | ||
| Life-threatening | 0 (0) | 8 (5.3) | 0 (0) | 10 (6.6) | ||
| ARF, n (%) | 3 (15.0) | 38 (25.0) | 0.73 | 4 (20.0) | 40 (26.3) | 0.86 |
| AKIN 1 | 2 (10.0) | 24 (15.8) | 2 (10.0) | 24 (15.8) | ||
| AKIN 2 | 0 | 5 (3.3) | 1 (5.0) | 5 (3.3) | ||
| AKIN 3 | 1 (5.0) | 9 (5.9) | 1 (5.0) | 11 (7.2) | ||
| Vascular complications, n (%) | 4 (20.0) | 28 (18.4) | 0.23 | 4 (20.0) | 29 (19.1) | 0.82 |
| Minor | 1 (5.0) | 19 (12.5) | 1 (5.0) | 19 (12.5) | ||
| Major | 3 (15.0) | 9 (5.9) | 3 (15.0) | 10 (6.6) | ||
| Infection, n (%) | 1 (5.0) | 31 (20.4) | 0.38 | 3 (15.0) | 36 (23.7) | 0.76 |
| Respiratory | 1 (5.0) | 15 (9.9) | 1 (5.0) | 18 (12.8) | ||
| Vascular | 0 | 2 (1.3) | 0 | 2 (1.3) | ||
| Other | 0 | 14 (9.2) | 2 (10.0) | 16 (10.5) | ||
| Acute respiratory failure, n (%) | 1 (5.0) | 9 (5.9) | 0.87 | 1 (5.0) | 10 (6.6) | 0.79 |
| Atrioventricular block, n (%) | 1 (5.0) | 26 (17.1) | 0.16 | 1 (5.0) | 28 (18.4) | 0.13 |
| Conduction disorder, n (%) | 5 (25.0) | 53 (34.9) | 0.38 | 6 (30.0) | 53 (34.9) | 0.67 |
| Permanent pacemaker, n (%) | 1 (5.0) | 19 (12.5) | 0.33 | 2 (10.0) | 23 (15.0) | 0.54 |
| Endocarditis, n (%) | 0 | 1 (0.7) | 0.72 | 0 | 1 (0.7) | 0.72 |
| Emergency surgery, n (%) | 0 | 1 (0.7) | 0.72 | 0 | 1 (0.7) | 0.72 |
TIA: transient ischemic attack; ARF: acute renal failure; AKIN: Acute Kidney Injury Network.
The LVEF in Group 1 significantly improved, increasing from 31.5% pre-procedure to 35.8, 41.8, and 45.1% at 1, 6, and 12 months post-procedure, respectively (p=0.002; Fig. 1).
DiscussionThis study showed that left ventricular dysfunction had no significant effect on procedural success or the outcomes at 30 days and 1 year post-TAVI. Data from a Canadian registry (n=339) also failed to demonstrate an adverse impact of left ventricular dysfunction on short- and long-term post-procedural clinical outcomes.9 The British registry, with a larger number of patients (n=2,535), showed that neither the low LVEF, nor a low gradient affected TAVI success or 30 day mortality. Long-term survival, however, was reduced in patients with low LVEF and low gradient, but not in those with low LVEF and high gradient, or normal LVEF and paradoxically low gradient.10 Conversely, a German registry (n=1,302) found increased risk of death at 30 days and 1 year for patients with low LVEF associated with low flow and low gradient post-TAVI.11
In the present study, patients with LVEF ≤ 40% had favorable results in the short and medium term, which may reflect the selection of patients with preserved cardiac reserve and consequent LVEF recovery after TAVI, an effect that has been previously reported.12 Indeed, the most relevant fact of this study was the significant LVEF improvement during the course of 1 year. A recent publication showed that patients with significant reduction in ventricular function (LVEF ≤ 30%) submitted to TAVI showed rapid improvement in left ventricular function, as well as functional class improvement.13 Similarly, the study by Zhao et al. showed that, after removal of the left ventricular outflow tract obstruction, patients submitted to TAVI showed early recovery of the parameters that evaluated the systolic and diastolic functions of the left ventricle, particularly those with left ventricular dysfunction. Among patients with LVEF<50%, LVEF increased from 46±5.7% to 57±4.5% (p=0.02). Better septal performance was also observed, which can be crucial for those patients with coronary artery disease, in which the septum has an important role in maintaining stroke volume.14
Data from studies with surgical patient population and LV dysfunction (LVEF ≤ 30% or NYHA III or IV) demonstrate that the predictors of systolic function recovery, defined as gain of more than 10% in LVEF on the seventh day post-intervention, were the cardiothoracic index and mean systolic transaortic gradient; the latter can be considered an indirect indicator of ventricular function assessment in these patients. Complementarily, patients with LV dysfunction secondary to valvular heart disease (afterload mismatch) benefited more than those with primary myocardial dysfunction unrelated to AoS.15
A study published in 2012 suggests a similar situation, in which the LV systolic function improves promptly after TAVI, also including patients with preserved LVEF. This favorable evolution is demonstrated by echocardiography through more sensitive methods, such myocardial deformation analysis (strain and speckle tracking). Improvement occurs predominantly in the basal and medial LV segments.16
Current surgical outcomes demonstrate that patients with low-flow, low-gradient AoS have better evolution when submitted to surgical aortic valve replacement, compared to those managed clinically, after appropriate selection. The mortality found in a recent series was estimated at 10%, and the long-term prognosis was much better in those submitted to aortic valve replacement. In these patients, the percutaneous valve replacement strategy could have similar results, or even superior, by preventing the use of extracorporeal circulation and prolonged ventilation.17 In this sense, Bauer et al. demonstrated that, when compared to the surgical treatment, TAVI showed better recovery of LVEF on the seventh day after surgery in patients with left ventricular dysfunction (LVEF<45%).18 In addition to the improvement in systolic function parameters, diastolic function improvement after TAVI can be observed.19
Study limitationsFirstly, this study reflects the experience of a limited number of patients treated at two centers. Secondly, the absence of systolic volume measurements, LV dimensions, and aortic valve impedance prevented the categorization of patients with low flow. Finally, these results are limited to the 30 day and 1 year follow-up.
ConclusionsTranscatheter aortic valve implantation showed to be effective and safe in patients with moderate to severe left ventricular dysfunction, which may reflect the choice of patients with preserved cardiac reserve and post-procedure ejection fraction recovery. Further studies are required to confirm the results found in this challenging group of patients with severe aortic stenosis and left ventricular dysfunction.
Funding sourceNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.