Transfemoral access is the preferred approach for transcatheter aortic valve implantation. However, some situations, such as the presence of peripheral vascular disease, preclude the use of such access. In these cases, subclavian access is an alternative approach for this procedure. This study aimed at evaluating the Brazilian experience using the subclavian approach for transcatheter CoreValve® prosthesis implantation.
MethodsAortic valve area<1cm2, aortic valve ring≥20mm and≤27mm (26mm and 29mm CoreValve®), ascending aorta≤43mm and subclavian artery with a diameter≥6mm, without significant obstructive lesions, marked tortuosity and excess calcification were requisites for the procedure. The access through the subclavian artery was obtained by surgical dissection and, under direct vision, a subclavian artery puncture was performed. Once artery access was obtained, the standard technique was used.
ResultsBetween January 2008 and April 2012, 8 patients with peripheral vascular disease underwent CoreValve® prosthesis implantation through the subclavian artery in 4 institutions. The procedure was successful in all cases with reduction of the mean transvalvular pressure gradient from 46.4±17.5mmHg to 9.3±3.6mmHg (P=0.0018) and improvement of symptoms. At 30 days and after 275±231 days of follow-up, 87.5% and 62.5% of the patients, respectively, were free from major adverse events (death, myocardial infarction, stroke and urgent cardiac suregery).
ConclusionsIn the Brazilian experience, the subclavian access was a safe and effective alternative for transcatheter CoreValve® implantation.
Acesso pela Artéria Subclávia paraImplante por Cateter da Bioprótese Valvar Aórtica CoreValve®: Dados do Registro Brasileiro
IntroduçãoA via de acesso transfemoral é preferencial para o implante por cateter de bioprótese valvar aórtica. Entretanto, algumas situações, como a presença de doença vascular periférica, impossibilitam a utilização desse acesso. Nesses casos, o acesso por dissecção da artéria subclávia é uma alternativa para a realização do procedimento. Nosso objetivo foi avaliar a experiência brasileira com a utilização da artéria subclávia como via de acesso para o implante por cateter da bioprótese CoreValve®.
MétodosForam requisitos para o procedimento área valvar aórtica<1cm², ânulo valvar aórtico>20mm e<27mm (CoreValve® de 26mm e 29mm), aorta ascendente<43mm e artéria subclávia com diâmetro>6mm, isenta de lesões obstrutivas significativas, tortuosidade acentuada e calcificação excessiva. O acesso pela artéria subclávia foi obtido por dissecção cirúrgica e, sob visão direta, punção da artéria subclávia. Obtido o acesso arterial, empregou-se a técnica padrão.
ResultadosEntre janeiro de 2008 e abril de 2012, 8 pacientes com doença vascular periférica foram submetidos a implante de prótese CoreValve® pela artéria subclávia em 4 instituições. O procedimento foi realizado com sucesso em todos os casos, com redução do gradiente transvalvar aórtico médio de 46,4+17,5mmHg para 9,3+3,6mmHg (P=0,0018) e melhora dos sintomas. Aos 30 dias e no seguimento de 275+231 dias, 87,5% e 62,5% dos pacientes, respectivamente, apresentavam-se livres de complicações maiores (óbito, infarto do miocárdio, acidente vascular cerebral e cirurgia cardíaca de urgência).
ConclusõesNa experiência brasileira, o acesso pela artéria subclávia mostrou-se seguro e eficaz como via alternativa para o implante por cateter da bioprótese CoreValve®.
Transcatheter aortic valve implantation is a safe and effective procedure for the treatment of patients with symptomatic aortic stenosis that is either inoperable or that carries a high surgical risk. 1,2 For this procedure, the femoral approach is preferred, since it is less invasive. However, some situations, such as the presence of atheromatous disease, tortuosity, and calcification in the territories of the femoral and iliac arteries, prevent the procedure from being performed through the femoral artery. In these cases, alternative access routes can be used, such as the transapical and transaortic routes.1,3,4 However, complications arising from the thoracotomy and the incision in the left ventricular apex are not uncommon with these techniques.5,6 Access through dissection of the subclavian artery has been described as a less invasive option and therefore more attractive for the implantation of the CoreValve® bioprosthesis (CoreValve® Revalving System, Medtronic, Inc. – Minneapolis, USA).7−11 In this study, the Brazilian experience with the use of the subclavian artery as an access route for CoreValve® bioprosthesis implantation is reported, with data obtained from the Brazilian Registry of Transcatheter Aortic Valve Bioprostheses Implantation.12
METHODSPatient selectionPatients in whom the subclavian artery was used as an access for CoreValve® bioprosthesis implantation were selected for this study from the Brazilian registry. The subclavian artery was used only when there were contraindications to femoral access, and whenever possible, the left subclavian access was chosen. Patients considered to be suitable for the procedure were those with aortic valve area<1cm2, annular aortic valve≥20mm and≤27mm (CoreValve® of 26mm and 29mm), ascending aorta≤43mm, and subclavian artery diameter≥6mm, free of significant obstructive lesions, severe tortuosity, and excessive parietal calcification.
The EuroSCORE and STS scores were used to estimate the risk of surgical mortality in this series of patients.13,14
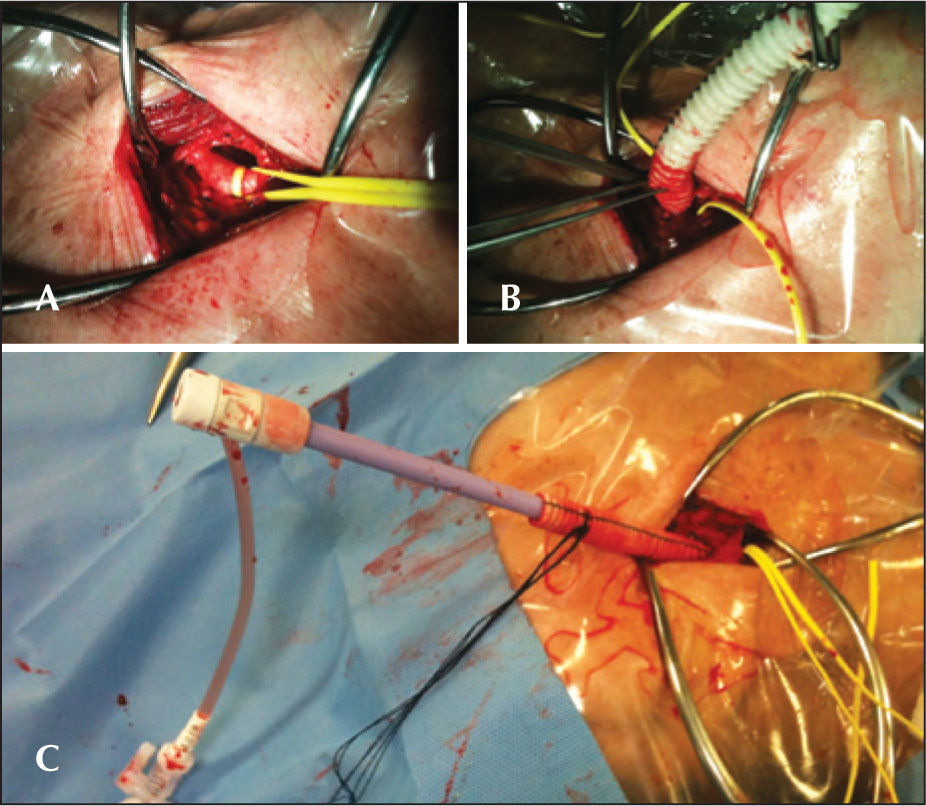
ProcedurePreparation for the procedure consisted of antibiotic and antiplatelet therapy with aspirin and/or clopidogrel. The valve implantations were performed under general anaesthesia. Alternative access through the subclavian artery was obtained by surgical dissection. For the dissection, an infraclavicular incision was used. Under direct vision, the subclavian artery was punctured for the positioning of the 18F sheath or, when the artery was deep, a Dacron graft was anastomosed to the artery and used for insertion of the 18F sheath (Figure 1). After obtaining arterial access, the standard technique (Figure 2) was used for the CoreValve® bioprosthesis implantation, which consists of three porcine pericardium leaflets, mounted and sutured into an auto-expansible nitinol stent 5cm in length. At the end of the procedure, the 18F sheath was removed, and the subclavian artery was sutured.
– In A, aortography and placement of a guidewire inside the left ventricle. In B, introduction of the CoreValve® prosthesis for the left subclavian artery. In C, a prosthesis positioned in the aortic annulus. In D, aortography demonstrating a well-positioned and competent CoreValve® prosthesis.
Clinical data and information on complementary exams were collected during medical appointments or by telephone contact and entered into an electronic spreadsheet developed for the Brazilian Registry. All outcomes and complications followed the criteria established by the Valve Academic Research Consortium Consensus on Definition Event Event (VARC).15
Statistical analysisContinuous variables are shown as the mean±standard deviation, and categorical variables are shown as frequencies (number and percentage). For the comparative analysis of categorical variables, the chi-squared or Fisher’s exact tests were used. For the sequential analysis of continuous variables in the same patient, a paired t-test was used. The significance level was set at 5% (P<0.05).
RESULTSBetween January of 2008 and April of 2012, 277 patients with aortic valve stenosis and either contraindications for or high-risk for conventional surgical treatment underwent transcatheter CoreValve® bioprosthesis implantation in 12 centres, and were included in the Brazilian Registry. At four of these institutions, the subclavian access route was used for the treatment of eight (2.9%) patients: two (25%) through the right subclavian artery and six (75%) through the left subclavian artery.
The basal demographic and clinical characteristics of the eight patients receiving the CoreValve® bioprosthesis implant through the subclavian artery are described in Table 1. The high surgical risk in this group of patients was demonstrated by the EuroSCORE and STS risk scores, which were both above 30%. The contraindication for femoral access was the presence of peripheral vascular disease, and three (37.5%) of the patients also had an abdominal aortic aneurysm.
Basal Demographic and Clinical Data
| Characteristics | n=8 |
|---|---|
| Age, years (SD) | 84 (7.3) |
| Male gender, n (%) | 5 (62.5) |
| Logistic EUROSCORE, % (SD) | 32 (16.4) |
| STS score, % (SD) | 30.9 (23.4) |
| Functional Class (NYHA), n (%) | |
| I or II | 0 (0) |
| III or IV | 8 (100) |
| Diabetes, n (%) | 3 (37.5) |
| Arterial hypertension, n (%) | 5 (62.5) |
| Renal failure*, n (%) | 6 (75) |
| Coronary artery disease, n (%) | 5 (62.5) |
| Previous percutaneous coronary intervention | 2 (25) |
| CABG | 2 (25) |
| Cerebrovascular disease, n (%) | 1 (12.5) |
| Peripheral vascular disease, n (%) | 7 (87.5) |
| Chronic obstructive pulmonary disease, n (%) | 2 (25) |
| Definitive pacemaker, n (%) | 1 (12.5) |
SD=standard deviation; n=number of patients; NYHA=New York Heart Association; CABG=coronary artery bypass graft.
The mean hospital stay was 14±12.9days (one to 43 days). The mean follow-up of patients was 275±231 days (one to 679 days). The one-month, oneyear, and two-year follow-up data were available for seven (87.5%) patients, five (62.5%) patients, and one (12.5%) patient, respectively. There was no loss of clinical follow-up in any case.
ProcedureData from the procedure are described in Table 2. Successful CoreValve® bioprosthesis implantation was achieved in 100% of the cases. In two (25%) cases it was necessary to perform post-dilation to properly expand the bioprosthesis and to reduce the intensity of the perivalvular insufficiency. Echocardiography detected the reduction of the mean and peak aortic transvalvular pressure gradients (Table 3). At the end of the procedure, there was mild periprosthetic aortic regurgitation in six (75%) cases.
Procedure Data
| Characteristics | n=8 |
|---|---|
| Proctor follow-up, n (%) | 3 (37.5) |
| Transesophageal echocardiogram, n (%) | 5 (62.5) |
| Anaesthesia, n (%) | |
| General | 8 (100) |
| Sedation | 0 (0) |
| Access route, n (%) | |
| Percutaneous | 0 (0) |
| Surgical | 8 (100) |
| Valvuloplasty, n (%) | 5 (62.5) |
| Bioprosthesis, n (%) | |
| CoreValve® 26mm | 3 (37.5) |
| CoreValve® 29mm | 5 (62.5) |
| Post-dilation, n (%) | 2 (25) |
| Device success, n (%) | 8 (100) |
n=number of patients
Echocardiographic Data
| Basal(n=8) | Post-implantation(n=7) | P | |
|---|---|---|---|
| Aortic valve area, cm3 (SD) | 0.7 (0.2) | NA | |
| Peak gradient, mmHg (SD) | 73.6 (27.8) | 17.8 (7.5) | < 0.001 |
| Mean gradient, mmHg (SD) | 46.4 (17.5) | 9.3 (3.6) | 0.0018 |
| LVEF, % (SD) | 58.7 (9.2) | 63.4 (12.4) | 0.175 |
SD=standard deviation; LVEF=left ventricular ejection fraction; n=number of patients; NA=not available.
The transcatheter treatment of aortic valve stenosis was effective at alleviating the symptoms of heart failure. After 30 days and during follow-up, 85.7% of patients achieved functional class status I or II of the New York Heart Association (NYHA) criteria (P=0.0007 vs. baseline).
Table 4 illustrates the complications that occurred within 30 days and during follow-up. During the procedure, one case of subclavian artery dissection occurred and was corrected with stenting, and one patient had bleeding that required a transfusion. One death from cardiovascular causes occurred one day after the procedure in a patient who developed refractory cardiogenic shock after valve implantation. Thus, mortality at 30 days was 12.5%. Two other deaths from non-cardiovascular causes occurred 43 and 679 days after the CoreValve® bioprosthesis implantation.
Adverse Event
| 30days | 275±231days | |
|---|---|---|
| Death, n (%) | 1 (12.5) | 3 (37.5) |
| Cardiovascular death | 1 (12.5) | 1 (12.5) |
| Stroke, n (%) | 0 | 0 |
| Myocardial infarction, n (%) | 0 | 0 |
| Acute renal failure, n (%) | 2 (25) | 2 (25) |
| Stage 1 | 1 (12.5) | 1 (12.5) |
| Stage 2 | 0 | 0 |
| Stage 3 | 1 (12.5) | 1 (12.5) |
| Haemorrhagic complication, n (%) | 1 (12.5) | 1 (12.5) |
| Life-threatening, n (%) | 0 | 0 |
| Major, n (%) | 1 (12.5) | 1 (12.5) |
| Minor, n (%) | 0 | 0 |
| Vascular complication, n (%) | 1 (12.5) | 0 |
| Major, n (%) | 1 (12.5) | 0 |
| Minor, n (%) | 0 | 0 |
| Permanent pacemaker*, n (%) | 2 (33.3) | 2 (33.3) |
| Major-complication free**, n (%) | 7 (87.5) | 5 (62.5) |
| Complication-free***, n (%) | 5 (62.5) | 4 (50) |
Two patients had renal failure after the procedure, one of which was associated with refractory cardiogenic shock and death, as previously described. None of the cases had a stroke, acute myocardial infarction (AMI), or the need for urgent heart surgery; thus, throughout the 275±231 days of follow-up, five (62.5%) patients were free of major complications. In this series, excluding the patient who died one day after the procedure and one patient who was already using a pacemaker, two (33.3%) underwent implantation of a permanent pacemaker for advanced atrioventricular conduction disturbances.
DISCUSSIONThe current versions of both devices available for clinical use, CoreValve® and Edwards SAPIEN (Edwards Lifesciences – Irvine, USA), with 18F delivery systems (6mm), allow the procedure to be performed through the femoral artery, as long as the arterial lumen diameter is at least 6mm. However, in this population of patients of advanced age and with multiple comorbidities, it is not uncommon to find severe atheromatosis or excessive tortuosity, which prevent the procedure from being performed by this access route. In this case, access through the subclavian artery for CoreValve® bioprosthesis aortic implantation is a feasible, safe, and effective procedure, as demonstrated by several series and also by the present study with data from the Brazilian registry. 9−11 In international records, subclavian access is employed in approximately 5% of cases, and the patients have, in general, a higher surgical risk than patients in whom femoral access was used. 9−11,16−19 In the Brazilian Registry, only 2.9% of cases were treated using the subclavian artery, which is most likely due to the lower experience of centres with this alternative access route. As with the international series, patients from the Brazilian Registry treated through subclavian access had a very high surgical mortality risk, over 30%, demonstrating that the presence of peripheral vascular disease is also a marker of higher clinical and anatomic complexity. 20
As a positive point regarding access through the subclavian artery, it should be emphasized that it is easier to control the stent at the time of its release in the valvular annulus when compared with femoral access, as the shorter distance and less tortuous path allow better transmission of force to the distal system portion, allowing for a more accurate positioning. Moreover, in general, the subclavian arteries are less affected by atheromatosis than the iliac and femoral arteries.
When selecting access through the subclavian artery, there is a preference for the left artery because, intuitively, there would be less risk of stroke than when using the right subclavian artery, considering that the presence and manipulation of the prosthesis delivery device in the brachiocephalic trunk could cause embolization and limit flow into the right carotid. In this case, access through the right artery is feasible only when the diameter of the brachiocephalic trunk artery is>7mm and free of significant atheromatosis.
Another point in favor of using the left subclavian artery as the access route is the more favorable orientation of the bioprosthesis in the valve annulus at the implantation, similar to the positioning obtained by a femoral approach. Therefore, the right subclavian artery should be considered as a good alternative only in cases where the presence of atheromatous disease or tortuosity prevent implantation through the left subclavian artery, or in cases where the diameter of the left subclavian is<7mm in the presence of a patent internal thoracic artery graft to a coronary artery of major anatomical importance, due to the incapacity of accommodating the prosthesis-releasing device (6mm) and maintaining blood flow to the graft. In the present study, following this recommendation, the right subclavian artery access was used in only 25% of cases.
More recently, the use of the subclavian artery for CoreValve® bioprosthesis implantation by totally percutaneous access and hemostasis obtained after the procedure with Prostar™ haemostatic devices (Abbott Vascular – Abbott Park, USA) or ProGlide™ (Abbott Vascular – Abbott Park, USA) has been reported.21 However, validation of this approach also depends on the confirmation of its safety, since severe bleeding complications may result from the failure of the device to perform hemostasis.
CONCLUSIONSIn the Brazilian experience, transcatheter CoreValve® bioprosthesis implantation through the subclavian artery route was safe and effective for use in selected patients in whom femoral access was not feasible. This alternative access route allows for a larger number of patients to benefit from aortic valve bioprosthesis transcatheter implantation.
CONFLICT OF INTERESTSSee Appendix.
Conflict of interest
| Nome | Participation in study1 | Lecturer2 | Consultant3 | Normative committee4 | Personal/ institutional aid5 | Sponsored scientific texts6 | Shareholder7 |
|---|---|---|---|---|---|---|---|
| Dimytri Siqueira | No | No | No | No | No | No | No |
| Eberhard Grube | No | No | Yes | No | No | No | No |
| Fábio Sândoli de Brito Júnior | No | Yes | No | No | No | No | No |
| J. Eduardo Sousa | No | No | No | No | No | No | No |
| João Carlos Dias | No | No | No | No | No | No | No |
| Jose Armando Mangione | No | Yes | No | No | No | No | No |
| Luiz Antonio Carvalho | No | Yes | No | No | No | No | No |
| Marco Antonio Perin | No | Yes | Yes | No | No | No | No |
| Pedro Alves Lemos Neto | No | No | No | No | No | No | No |
| Rogério Sarmento-Leite | No | Yes | No | No | Yes | No | No |
| Teresa Cristina Nascimento | No | No | No | No | No | No | No |
The author participated in clinical and /or experimental studies subsidised by the pharmaceutical industry or equipment related to the registry;
The author was (is) a member of the advisory board of directors of pharmaceutical or equipment industries related to the registry;