Based on the hypothesis that the neointima found in drug-eluting stents (DES) with biodegradable polymers at 28days is not a definitive neointima and that optical coherence tomography (OCT) is an effective method for sequential neointimal evaluation, we aim, in this experimental study, to compare OCT findings at 28 and 90days, in two different DES with biodegradable polymers: the sirolimus-eluting stent (Inspiron®, Scitech) and the biolimus A9-eluting stent (Biomatrix®, Biosensors International).
MethodsOverall, 6 non-atherosclerotic pigs were submitted to the implantation of 6 Inspiron® stents and 6 Biomatrix® stents. Each pig received both stent types, one in each coronary artery (left anterior descending artery and circumflex artery) and after 28 and 90 days qualitative in-stent OCT analyses were performed at 1-millimeter intervals.
ResultsQualitative assessment was performed in-stent pairing millimeter by millimeter. Heterogeneous neointimal tissue was evidenced in 39% at 28days and in 0% at 90days, the presence of intraluminal tissue in 18% at 28days and in 0% at 90days, luminal irregularity in 62% at 28days and in 2% at 90days (P < 0.005). There was no difference between groups regarding the quality of the neointima over time (P > 0.05).
ConclusionsThe OCT findings corroborate the hypothesis that the neointima found in DES with biode gradable polymers at 28days is not a definitive neointima. The most significant experimental evidence is the change in the neointimal characteristics observed at sequential OCT.
Evolução Temporal da Proliferação Neointimal após Implante de Dois Tipos de Stent Farmacológico com Polímeros Biodegradáveis em Modelo Porcino:Avaliação Qualitativa por Tomografia de Coerência Óptica Sequencial
IntroduçãoBaseados na hipótese de que a neoíntima encontrada em stents farmacológicos (SFs) com polímeros biodegradáveis aos 28 dias não é a neoíntima definitiva e de que a tomogra fia de coerência óptica (TCO) é um método eficaz para a avaliação sequencial da neoíntima, objetivamos, neste estudo experimental, comparar os achados da TCO aos 28 dias e aos 90 dias em dois tipos de SF com polímeros biodegradáveis: o stent liberador de sirolimus (Inspiron®, Scitech) e o stent liberador de biolimus A9 (Biomatrix®, Biosensors International).
MétodosNo total, 6 porcos não-ateroscleróticos foram submetidos a implante de 6 stents Inspiron® e de 6 stents Biomatrix®. Cada porco recebeu os dois tipos de stent, um em cada artéria coronária (descendente anterior e circunflexa) e após 28 dias e 90 dias foram rea lizadas avaliações qualitativas intrastent a cada milímetro com TCO.
ResultadosA avaliação qualitativa, feita por pareamento milímetro a milímetro intrastent, evidenciou neoíntima heterogênea em 39% aos 28 dias e em 0% aos 90 dias, presença de tecido intraluminal em 18% aos 28 dias e em 0% aos 90 dias, irregularidade luminal em 62% aos 28 dias e em 2% aos 90 dias (P < 0,005). Não houve diferença entre os grupos quanto à qualidade da neoíntima ao longo do tempo (P > 0,05).
ConclusõesOs achados à TCO corroboram a hipótese de que a neoíntima encontrada em SFs com polímeros biodegradáveis aos 28 dias não é a neoíntima definitiva. A evidência experimental mais significativa é a mudança das características da neoíntima observada à TCO sequencial.
Current knowledge of the vascular response and the resulting neointimal hyperplasia related to the implantation of drug-eluting stents (DES) is largely based on preclinical studies in porcine coronary arteries. 1-4 In animals, peak neointimal growth with bare-metal stents (BMS) occurs at 28days; with DES, studies in animals have shown favourable results for re-endothelialization at 28days, albeit incomplete. 5-8
Vascular healing in response to DES implantation is dynamic and depends on the type of drug used, which in turn depends on its release kinetics from durable or biodegradable polymers. 9-16 Evaluation through histology does not allow for time-dependent analyses, and there are additional limitations, such as tissue loss related to the technique employed for material preparation (non-satisfactory cutting or colouring, thickness, and angle of the cut).
Due to its high resolution, optical coherence tomography (OCT) may be a promising intravascular imaging tool in the sequential evaluation of neointimal formation. Compared to histology, OCT allows for the evaluation of the vascular healing process in vivo at different stages, providing qualitative and quantitative information along the full length of the stent. 17-20 By enabling analysis at multiple time points, it is possible to compare the same intrastent region at different times and thereby assess temporal neointima maturation, as well as to evaluate each intrastent millimetre strut by strut.
Based on the hypothesis that the neointima found in DES with biodegradable polymers at 28days are not definitive, and that OCT is an effective method for the sequential evaluation of neointima, this experimental study aimed to compare the qualitative findings of OCT at 28days and 90days in two types of DES with biodegradable polymers: the sirolimus-eluting stent (Inspiron®, Scitech Medical Products Ltda. – Goiânia, Brazil) and the biolimus-A9 eluting stent (Biomatrix®, Biosensors International – Singapore).
METHODSAll procedures followed the standards for laboratory animal protection and care established by the Ethical Principles in Animal Experimentation of the Research Support and Animal Experimentation Service of the Instituto do Coração da Faculdade de Medicina da Universidade de São Paulo, as well as the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences and National Research Council, National Academy Press, Washington, DC, 1996) and the Ethical Principles in Animal Experimentation of the Brazilian College of Animal Experimentation (COBEA).
Subjects and stent implantationSix domestic juvenile, non-atherosclerotic pigs, weighing between 18kg and 23kg, were obtained from a commercial breeding farm.
One day before the procedure, the pigs received 600mg of acetylsalicylic acid and 150mg of clopidogrel orally. The anaesthesia protocol consisted of premedication (ketamine 3mg/kg and midazolam 0.5mg/kg, intramuscular) followed by intravenous infusion of thiopental, endotracheal intubation, and the start of mechanical ventilation; the level of anaesthesia was maintained with isoflurane.
A 6F vascular sheath was introduced under direct vision after dissection of the common femoral artery. A dose of 10,000IU unfractionated heparin was administered and the left coronary artery was selectively catheterised under fluoroscopy using a Judkins right (JR) 4 6F guide catheter. Then, nitroglycerin was injected through the catheter at a dose of 200μg, and an Inspiron® or Biomatrix® stent was randomly implanted in each anterior descending or circumflex artery of each pig, trying to maintain a stent-releasing balloon/artery ratio of 1.1/1. The Inspiron® stents were 2.5mm or 3mm in diameter and 16mm in length. All implanted Biomatrix® stents were 3mm in diameter and 14mm in length.
Optical coherence tomographyAn M2 OCT Imaging System (Imaging LightLab, Inc., Westford, USA) was used with ImageWire® automated catheter pullback (LightLab Imaging, Inc.) at a speed of 1mm/s and 2mm/s after proximal occlusion of the arterial segment under study with a Helios® balloon (LightLab Imaging, Inc.). Specific software from the OCT equipment was used to make measurements at 1mm intervals starting from the most distal part of the stent and moving towards the proximal end.
Morphometric AnalysisThe first stent image analysed was the one that allowed for the drawing of a complete circle using struts in the distal border. The stent and luminal areas were measured by manual tracing (mm2). The neointimal area (mm2) was calculated as the lumen area minus the stent area. The neointimal area percentage (%) was calculated as the ratio between the neointimal area and the stent area (neointimal area percentage=area of the stent – lumen area/stent area). For the analysis of neointimal thickness (mm), the distance from the luminal surface of the stent strut reflection (called the “blooming artifact”) to the luminal contour was measured. Since the blooming artefact was systematically included in the measurements, to determine strut apposition it was necessary to take into account its total thickness (thickness of the metal strut+thickness of the polymer+drug) plus a correction factor of 20μm, which corrects for the actual location of the strut surface by discounting half of the blooming. 18,19 The distance between the luminal surface of blooming produced by the struts and the contour of the vascular lumen was analysed individually for each strut. For the stents in this study, if the distance was greater than the sum of the thicknesses of the struts (Biomatrix®, 112μm; Inspiron®, 75μm) plus the thicknesses of the polymers (Biomatrix®, 10μm; Inspiron®, 5μm) plus 20μm (correction factor), the strut would be considered malpositioned.
Characterisation of neointimal tissueThe qualitative analysis evaluated transverse sections at each millimetre along the stent at 28 and 90days. Features such as the presence of neointima with luminal irregularities, the presence of intraluminal tissue, and the heterogeneous aspects of neointima were detected and described.
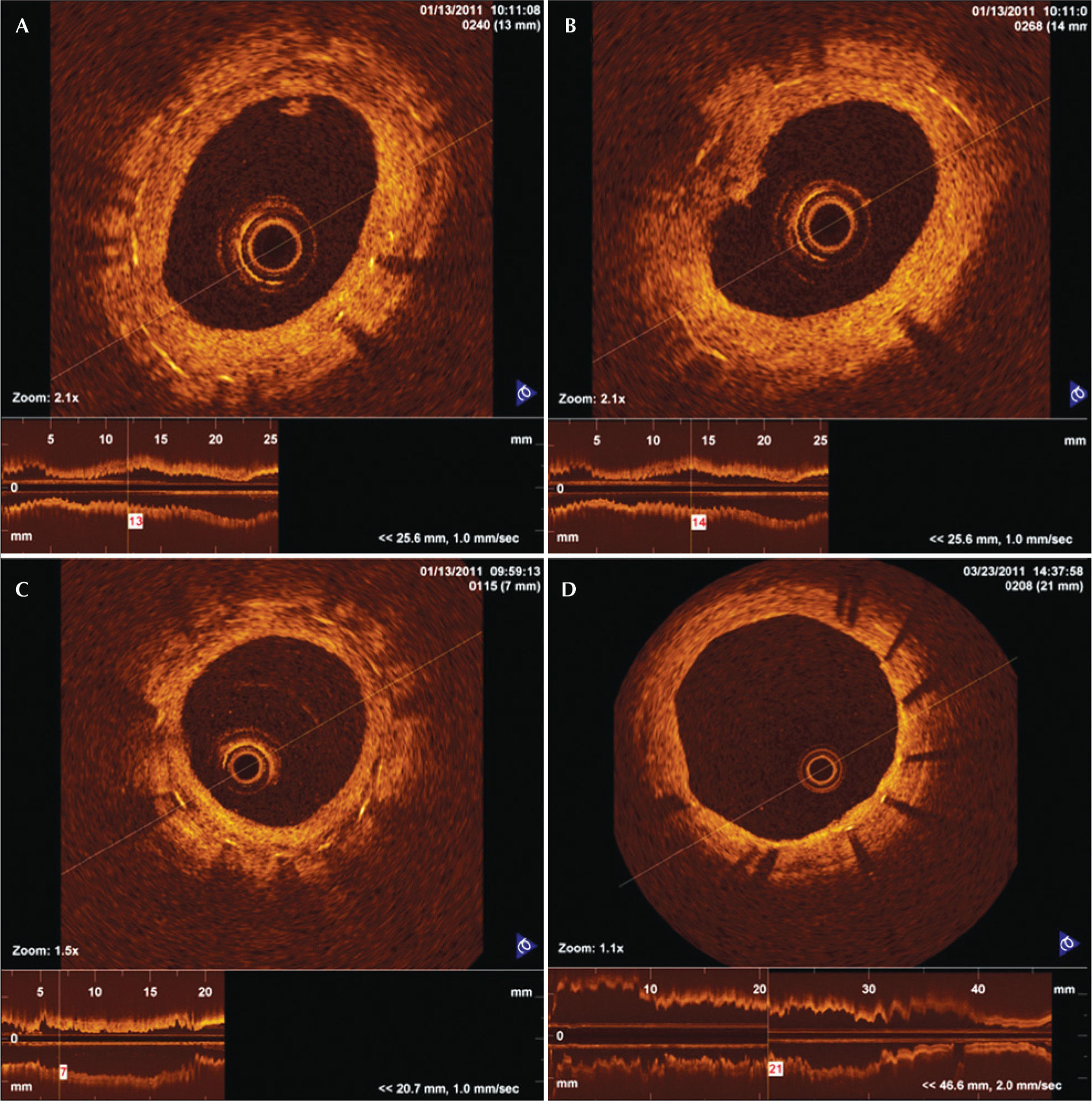
Neointimal tissue with irregularities was defined by the presence of indistinct and irregular contours on the luminal surface of the vessel and by the presence or absence of discontinuous neointima. Intraluminal tissue was identified as material protruding into the vessel lumen, which may or may not adhere to the neointima. Heterogeneous aspects of neointimal tissue were identified when an image with low light intensity was observed in the OCT of neointimal tissue without signal attenuation behind the area (Figure 1).
Optical coherence tomography images of a cross-section of porcine coronaries with DES. In A, intraluminal tissue, which is material that is located in the vessel lumen and may or may not adhere to the neointimal tissue. In B, neointimal tissue with irregularities, showing the presence of irregular and indistinct borders of the luminal surface of the vessel with the presence or absence of discontinuous neointima. In C, heterogeneous aspects of neointimal tissue are depicted in the image showing low light intensity of neointimal tissue near the stent struts. In D, homogeneous aspects of neointimal tissue with light intensity compatible with normal neointima are shown.
These events were compared in cross-sectional OCT images taken after 28 and 90days and evaluated regarding their progression or resolution.
Statistical AnalysisData are shown as the mean±SD or as numbers and percentages. The comparison between days 28 and 90, as well as the interaction of the effects of the type of stent and the progression/regression of the temporal neointima over time were analysed with a general linear model with repeated measures. The significance level was set at 5%. Analyses were performed using SPSS 20.0 (IBM SPSS – Chicago, USA).
RESULTSAll six pigs survived the index procedure and underwent angiographic analysis and OCT at 28days and at 90days after implantation.
In total, 2,685 stent struts along 350mm of total stent length were analysed (1,366 struts at 28days and 1,319 struts at 90days); 338 paired transverse sections were analysed at 28days and at 90days (Inspiron®=90 and Biomatrix®=79 transverse sections analysed at each time point) (Figure 2). Twelve transverse sections were excluded from the analysis due to image artifacts. No malpositioned struts were observed, as all struts analysed were covered by neointimal tissue.
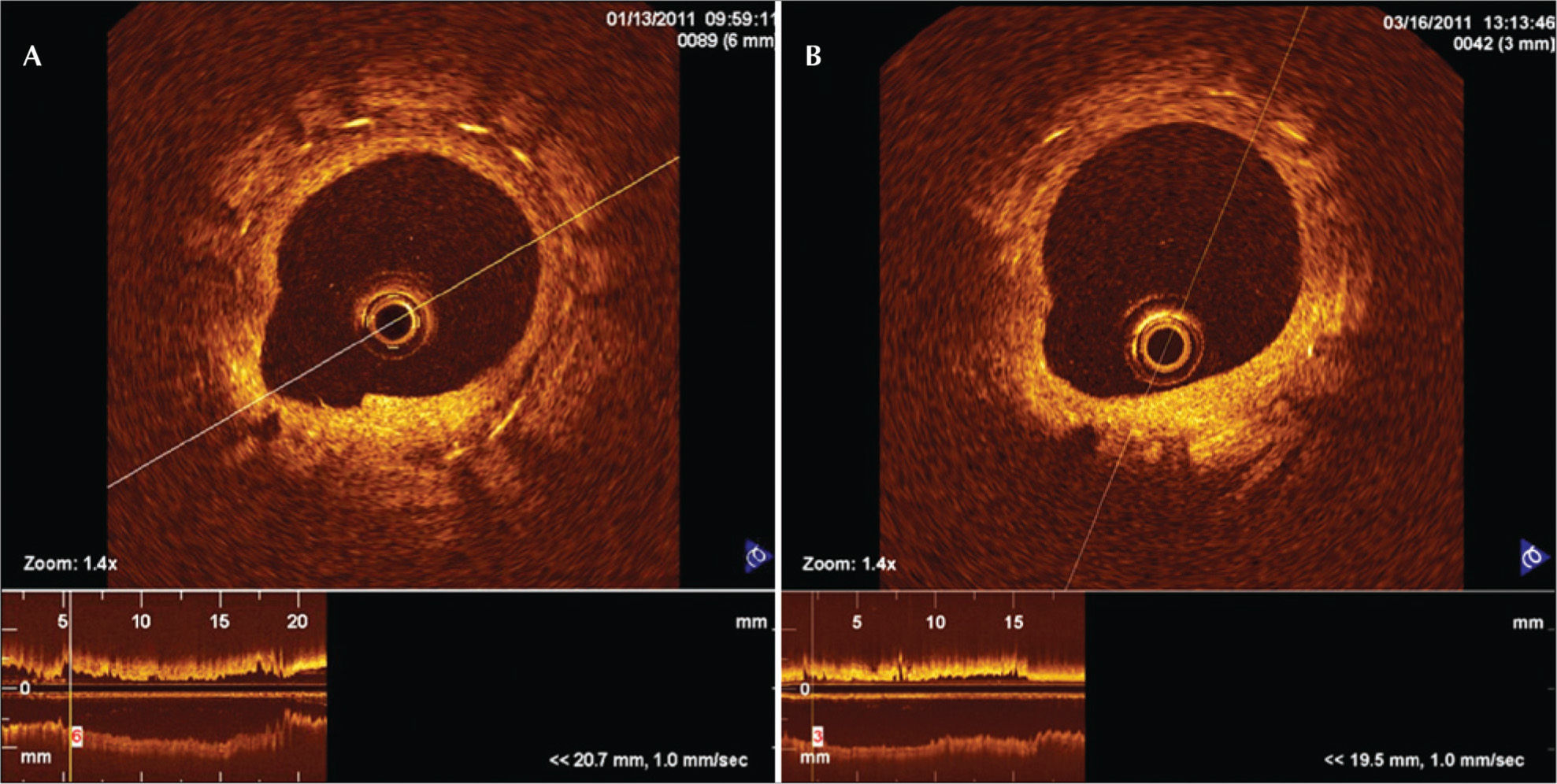
Paired optical coherence tomography images of a cross-section of the Inspiron® stent implanted in the left anterior descending artery at 28days (A) and 90days (B). At 28days, the heterogeneous aspects of the neointimal tissue can be observed, which are replaced by homogeneous aspects at 90days.
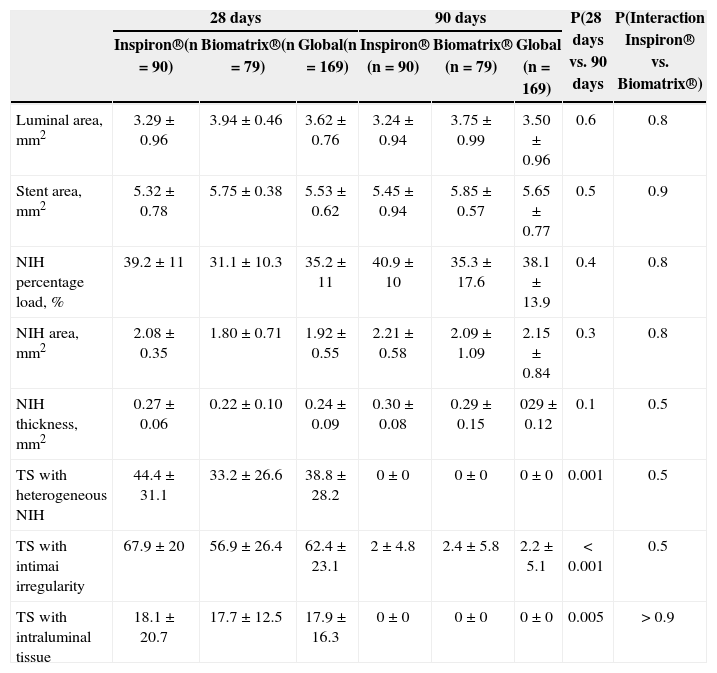
Events such as the presence of luminal irregularities, heterogeneous aspects of neointimal tissue, and the presence of intraluminal tissue were evaluated in the 12 stents at 28days. The presence of heterogeneous neointimal tissue, irregular lumen, and luminal tissue in the transverse sections was higher at 28days than at 90days (38.8±28.2% vs. 0%; P=0.001; 62.4±23.1% vs. 2.2±5.1%; P < 0.001; 17.9±16.3% vs. 0%; P=0.005, respectively). This behaviour was observed for both stents analysed (Table 1).
Quantitative and qualitative findings from sequential optical coherence tomography images taken at 28 and 90days after Inspiron® and Biomatrix® stent implantation in porcine coronary arteries
| 28days | 90days | P(28days vs. 90 days | P(Interaction Inspiron® vs. Biomatrix®) | |||||
|---|---|---|---|---|---|---|---|---|
| Inspiron®(n=90) | Biomatrix®(n=79) | Global(n=169) | Inspiron® (n=90) | Biomatrix® (n=79) | Global (n=169) | |||
| Luminal area, mm2 | 3.29±0.96 | 3.94±0.46 | 3.62±0.76 | 3.24±0.94 | 3.75±0.99 | 3.50±0.96 | 0.6 | 0.8 |
| Stent area, mm2 | 5.32±0.78 | 5.75±0.38 | 5.53±0.62 | 5.45±0.94 | 5.85±0.57 | 5.65±0.77 | 0.5 | 0.9 |
| NIH percentage load, % | 39.2±11 | 31.1±10.3 | 35.2±11 | 40.9±10 | 35.3±17.6 | 38.1±13.9 | 0.4 | 0.8 |
| NIH area, mm2 | 2.08±0.35 | 1.80±0.71 | 1.92±0.55 | 2.21±0.58 | 2.09±1.09 | 2.15±0.84 | 0.3 | 0.8 |
| NIH thickness, mm2 | 0.27±0.06 | 0.22±0.10 | 0.24±0.09 | 0.30±0.08 | 0.29±0.15 | 029±0.12 | 0.1 | 0.5 |
| TS with heterogeneous NIH | 44.4±31.1 | 33.2±26.6 | 38.8±28.2 | 0±0 | 0±0 | 0±0 | 0.001 | 0.5 |
| TS with intimai irregularity | 67.9±20 | 56.9±26.4 | 62.4±23.1 | 2±4.8 | 2.4±5.8 | 2.2±5.1 | < 0.001 | 0.5 |
| TS with intraluminal tissue | 18.1±20.7 | 17.7±12.5 | 17.9±16.3 | 0±0 | 0±0 | 0±0 | 0.005 | > 0.9 |
NIH, neointima hyperplasia; TS, transverse sections.
The incidence of qualitative events in the transverse sections analysed was similar at 28days for both stents, and each showed heterogeneous neointimal tissue (44.4%±31.1 and 33.2±26.6% for the Inspiron® and Biomatrix® stents, respectively; P=0.5), intraluminal tissue (18.1±20.7% and 17.7±12.5% for Inspiron® and Biomatrix® stents, respectively; P > 0.9) and luminal irregularities (67.9±20% and 56.9±26.4% for Biomatrix® and Inspiron® stents, respectively; P=0.5) (Table 1).
DISCUSSIONSeveral studies in animal models and previous clinical trials showed delayed endothelialisation of DES when compared to BMS, 21-23 and observed the frequent presence of struts covered by thin layers of neointimal tissue, with a thickness well below the limits of detection by intracoronary ultrasound. This delayed endothelialisation may play an important role in the pathogenesis of DES thrombosis. 23,24 Additionally, autopsy studies have identified the presence of bare struts as a more robust morphological predictor for the occurrence of thrombosis in first-generation DES. 25
The present study with sequential OCT, despite not showing bare struts, did provide images that indicate heterogeneity in the healing process after 28days. This result suggests that this is a step in the neointimal tissue maturation process for both stents studied, since, at 90days, no heterogeneous neointimal tissue, luminal irregularity, and intraluminal tissue were observed.
It should be noted that at both 28 and 90days, although the process of stent endothelialisation is faster in pigs than in humans, 7 polymers from both Inspiron® and Biomatrix® stents are still present and undergoing degradation and their respective antiproliferative drugs are still present. 12,26 A study with a later evaluation time point (> six months) may be needed to analyse DES in order to evaluate the inflammatory and chronic biological responses after drug elution and polymer degradation.
It will be important to correlate the OCT finding with histological data to identify and understand the neointima maturation process. Teramoto et al. 27 correlateimages with low light intensity signal visualised in OCT with histological findings, and observed areas of low cellularity and the presence of fibrin and proteoglycans.
Second-generation OCT systems, which are already available for both pre-clinical and clinical use (Fouriedomain optical coherence tomography [FDOCT]), arcapable of acquiring images at higher speeds with fewer artefacts and without the need for proximal occlusion of the coronary artery. Recent FDOCT studies compared the optical density of neointimal tissue longitudinally after stent implantation to differentiate fibrin from neointimal tissue. 28,29
These findings corroborate the results of the present study, as they demonstrated neointimal maturation over time and showed a progressive reduction in the amount of fibrin and its replacement by neointimal tissue, which was maintained after 28days.
Further evaluations are needed to better understand the possible roles of these different regions in neointimal tissue in the arterial healing process after implantation of DES.
Study limitationsQualitative analyses were performed by a single investigator; therefore, the assessment of the qualitative characteristics of the neointima could be biased despite the best efforts to remain objective.
The DES were implanted in porcine non-atherosclerotic coronary arteries and the vascular response was evaluated in the context of normal porcine coronary arteries. Preclinical studies with these stents in the arteries of animals with experimental atherosclerosis (minipigs, rabbits) can mora accurately answer the questions regarding the effectiveness of these stents. 21
CONCLUSIONSThe OCT results corroborate the hypothesis that the neointima found in DES with biodegradable polymers at 28days is not the definitive neointima. The most significant experimental evidence is the change in characteristics of the neointima observed in OCT images taken after 90days. This fundamental tool is useful in the preclinical analysis of stents, allowing the sequential assessment of neointimal tissue in vivo.
FINANCIAL SUPPORTThe present study is part of the National Development Program of Vascular Stents (PDNS), initiated in 2004-2005. It is supported by the Secretariat of Science, Technology and Strategic Inputs (SCTIE)/Department of Science and Technology (DECIT), the Brazilian Ministry of Health, the National Council for Scientific and Technological Development (CNPq) and the Financier of Studies and Projects (FINEP) of the Brazilian Ministry of Science and Technology. The development of the laser cutting of stents was supported by the Foundation for Research Support of the State of São Paulo (FAPESP; Support type: Technological Innovation/Innovative Research in Small and Micro Enterprises [PIPE]).
CONFLICT OF INTERESTCelso K. Takimura and Francisco R. M. Laurindo are scientific consultants at Scitech Medical Products. Over the past 36 months, Pedro A. Lemos has been a scientific consultant to Scitech Medical Products and has delivered national and international lectures sponsored by Boston Scientific and Braun; he is also a member of Boston Scientific Latin America SCIMAB (Scientific Medical Advisory Board). The remaining authors declare to have no conflicts of interest.