Transcatheter occlusion of ostium secundum atrial septal defects using commercial available devices is a safe and effective procedure. We present our experience with two new generation coated nitinol wire devices.
MethodsWe report device characteristics, implantation technique and outcomes of patients with atrial septal defects treated with the Lifetech CERA™ ASD Occluder and the Cocoon Septal Occluder.
ResultsProcedures were performed in 49 patients, 37 were female. Ages ranged from 7 to 68 years and body weight from 17 to 90kg. The aortic rim was present in 34.7% and atrial septal aneurysms in 14.3% of the patients. Mean diameter was 13±7mm whereas the balloon stretched diameter was 22±7mm. Implantation was possible in all cases and 55 devices were used. Forty-five CERA™ and four Cocoon devices were used. Three patients required occlusion of a second orifice. During the procedures the first device had to be replaced by a larger one in two cases, and in the third case it was replaced due to a tuliplike malformation of the left atrial disc. Immediate occlusion occurred in 91.9% and in 95.9% at 6 months. There were no deaths or other significant complications.
ConclusionsThe use of both CERA™ and Cocoon devices was simple and reproducible in experienced hands. Short-term occlusion rates were similar to the ones obtained with the Amplatzer™ device. More studies and long-term follow-up are required to determine the actual advantages of coated nitinol wire devices.
Oclusão Percutânea das Comunicações InteratriaisTipo Ostium Secundum com Próteses de Nitinol Revestidas de Última Geração
IntroduçãoA oclusão percutânea das comunicações interatriais tipo ostium secundum com as próteses disponíveis comercialmente é procedimento seguro e eficaz. Apresentamos a experiência com duas próteses de nova geração, com fios de nitinol revestidos.
MétodosDescrevemos as características das próteses, a técnica de implante e os resultados de pacientes portadores de comunicações interatriais, tratados com as próteses Lifetech CERA® ASD Occluder e Cocoon Septal Occluder.
ResultadosForam realizados procedimentos em 49 pacientes, sendo 37 do sexo feminino. A idade variou de 7 a 68 anos, e o peso de 17 a 90kg. Estavam presentes borda aórtica em 34,7% e aneurisma de septo atrial em 14,3% dos pacientes. Os diâmetros estáticos dos orifícios principais foram de 13±7mm e os diâmetros estirados de 22±7mm. O implante foi possível em todos os casos, sendo empregados 55 dispositivos. Foram utilizadas próteses CERA® em 45 pacientes e Cocoon nos demais. Três pacientes necessitaram ocluir um segundo orifício. Durante os procedimentos, foi necessária a substituição da primeira prótese por outra de maior diâmetro em dois casos e por deformidade da prótese (configuração em tulipa) no terceiro caso. A oclusão imediata ocorreu em 91,9% e em 95,9% no 6o mês. Não ocorreram óbitos ou outras complicações significativas.
ConclusõesO manuseio das próteses CERA® e Cocoon foi simples e reprodutível nas mãos de operadores experientes. As taxas de oclusão imediata foram semelhantes às obtidas com as próteses Amplatzer®. Mais estudos e seguimento de longo prazo se fazem necessários para determinar as reais vantagens do revestimento dos fios de nitinol.
The percutaneous closure of ostium secundum-type interatrial communications (osASD), initially described by King and Mills in 1976,1 is, at present, the therapeutic method of choice universally accepted at all major centers. This procedure has proven safe and effective, making it an excellent alternative to conventional surgical treatment.
Despite excellent results in the short and medium term obtained with the Amplatzer Septal Occluder® prosthesis (ASO; AGA Medical Corp., Golden Valley, Minnesota, United States), new devices have been developed, aiming to correct deficiencies or suggesting modifications in the design of the prostheses, the amount and thickness of nitinol wires, the outer coating type to prevent the release of nickel into the bloodstream, and in different delivery mechanisms. Unfortunately, the number of publications in the literature on these new devices is scarce, perhaps because they are not available in the United States.2−4
For each of these new devices, there are advantages and disadvantages with respect to the technical aspects and to the profile of the prostheses. Prostheses registered by the Brazilian National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA) and available in Brazil offer a choice of an interesting treatment with safety, efficacy, and reproducibility, so that the professional can choose the device that best suits his/her purposes.
This study aimed to present this group’s experience with two last generation prostheses, with coated nitinol wires, calling attention to its features, the implantation technique, and the results.
METHODSStudy designThis was a single-center, single-arm, retrospective review on patients with osASD who underwent percutaneous closure with Lifetech CERA® ASD Occluder (Lifetech Scientific Corporation, Shenzhen, China) and Cocoon Septal Occluder (Vascular Innovations Co. Ltd., Bangtanai, Pakkret, Thailand) prostheses, between July 2010 and December 2013. Due to the relatively small number of cases, no comparisons were made between devices.
Patient selectionIn the mentioned period, patients with osASD with hemodynamic repercussion and without fixed pulmonary hypertension, subjects>6 years of age and weighing at least 15kg underwent percutaneous occlusion. The diagnosis of the defects was established by transthoracic echocardiography (TTE) and trans-esophageal echocardiography (TEE), for morphological analysis of the defect, identification of rims, and for obtaining the static diameter (D), confirming the indication of intervention.
DevicesLifetech CERA® ASD OccluderThis is a new self-expandable prosthesis, comprising a mesh of nitinol (nickel-titanium alloy) composed of two disks and a central portion that promote occlusion of the defect, similar to the design of the ASO prosthesis. The nitinol wires are coated with a titanium nitride compound, which prevents the release of nickel into the bloodstream and allow for a quicker and more homogeneous endothelial growth with antithrombotic properties. Inside the device, a polyester membrane is sutured in order to reduce the occurrence of residual shunts, accelerating the time to defect occlusion (Figure 1).
This is a very flexible device that conforms well to defects and has a satisfactory profile. The central portion is 4mm long and is manufactured in diameters from 6mm to 42mm, in consecutive increments of 2mm. The left disk measures 12mm more than the center portion in devices up to 10mm, 14mm more in devices up to 32mm, and 16mm more in other sizes. The right disc measures 4mm less than the left disk in devices up to size 32mm, and 6mm less in other sizes.
For its loading, the graft is threaded to a metal cable and delivered through long 7-14F sheaths.
Cocoon Septal OccluderThis prosthesis presents a very similar design to the previous device. It is also a self-expanding prosthesis comprising two discs with a central connector, composed of nitinol wires coated with platinum by nanofusion, which increases the radiopacity of the device, facilitating and enhancing its fluoroscopic visualization and positioning. The platinum coating also prevents the release of nickel into the bloodstream, preventing nitinol corrosion and increasing the longterm biocompatibility of the occluder (Figure 2). The Coccoon Septal Occluder has satisfactory flexibility, with an appropriate conformation to defects.
The length of the central connector is 3mm in 8mm and 10mm prostheses and 4mm in the other devices. The device has available diameters from 8mm to 40mm in consecutive increments of 2mm. The left disc is 12mm larger than the central portion in prostheses up to 10mm, and 14mm larger in the other devices. The right disc is 2mm smaller than the left disk in prostheses up to 10mm, and 4mm smaller in the other devices.
In its interior, the device has a polypropylene membrane sutured in order to minimize the possibility of residual shunts, shortening the time to defect occlusion. The device is loaded by a thread system and released by means of a long 6-12F sheath.
ProcedureAll patients received general anesthesia and orotracheal intubation after a minimum 8-hour fasting. The subjects underwent right and left heart catheterization via femoral venipuncture and were monitored by TEE (three-dimensional, where available). Angiograms were not performed.
Heparin was administered in doses of 100IU/kg for children and 5,000 to 10,000IU in adults, after having obtained venous access and with a trans-esophageal probe positioned. Cefazolin at doses of 50mg/kg in children or 2g in adults was administered to all patients before and 6 hours after the procedure.
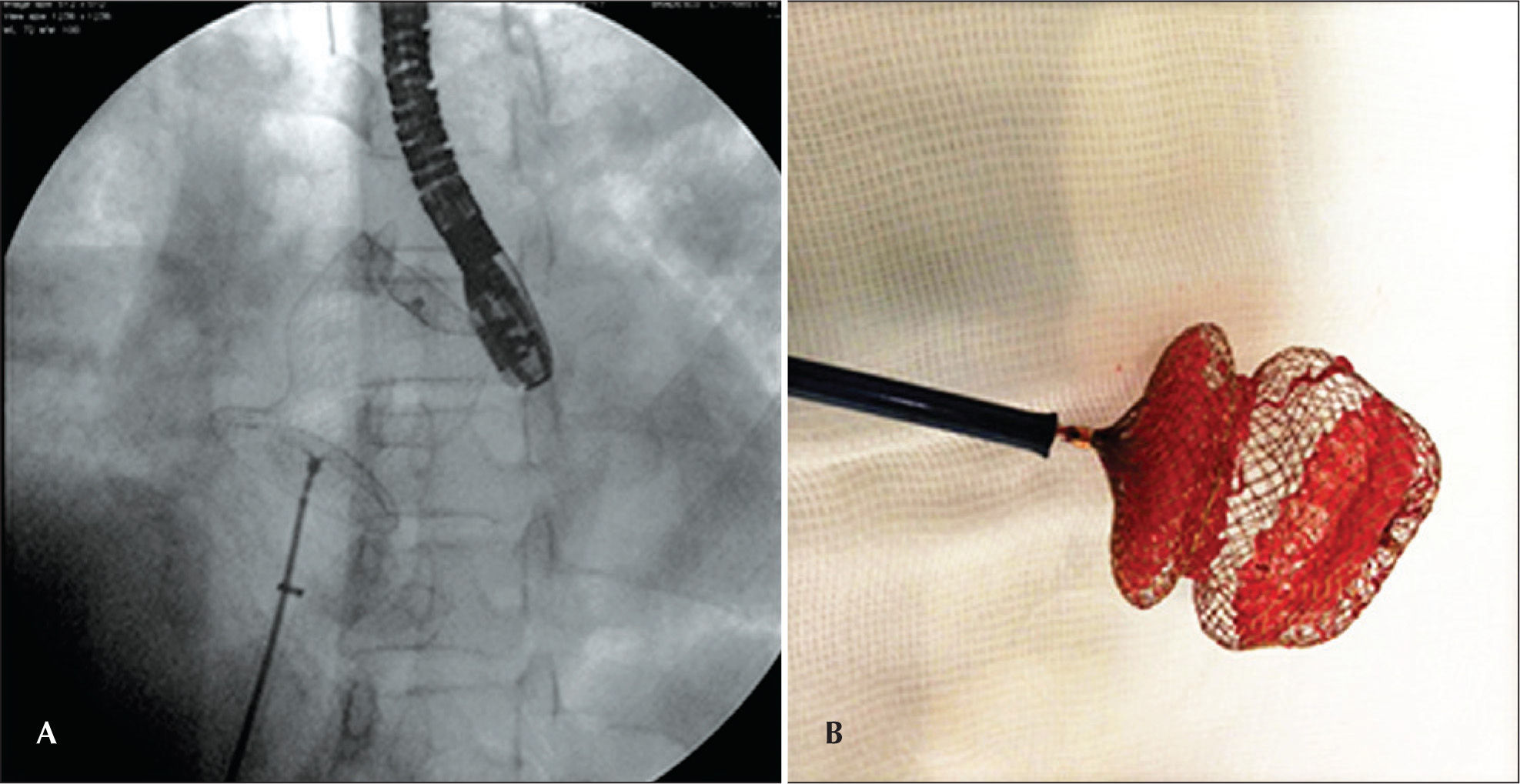
Stretched diameter (SD) measures, with an appropriate balloon provided by the retailer company, were performed in all cases. The choice of the prosthesis was based on SD, with the use of prostheses with sizes ranging from the SD up to 4-6mm larger in cases with very thin and defective edges. The prosthesis was loaded on the long sheath with a compatible caliber, according to the specifications of the manufacturer, and released with previously detailed standard techniques.5 The position of the device was confirmed by TEE; when considered satisfactory, the device was detached from the delivery system (Figure 3).
Hemostasis was obtained by compression, and all patients were extubated in the hemodynamics laboratory and transferred to a closed unit until the following day.
Follow-upTTEs were performed in the day following the procedure.
All patients were instructed to use acetylsalicylic acid (3 to 5mg/kg/day in children and 200mg/day in adults) for six months in association with 75mg of clopidogrel bisulfate, in adults, for three months. Adherence to procedures for infective endocarditis prophylaxis was recommended for 6 months.
TTEs were performed at one, three, and 12 months, and then annually. TEE was performed after six months.
Statistical analysisContinuous variables were expressed as mean and standard deviation and categorical variables as numbers and percentages.
RESULTSAs of July 2010, 49 patients underwent percutaneous osASD occlusion with use of the prostheses previously mentioned; 37 of them were female. The ages ranged from 7 to 68 years (32±19 years), and the weights from 17 to 90kg (66±18kg).
Two patients had suffered ischemic strokes and, at that time, were misdiagnosed as having a patent foramen ovale in pre-procedure TTEs. A 16-year-old female exhibited transposition of great vessels, having been submitted to balloon atrioseptostomy (AS), with subsequent arterial switch surgery (Jatene procedure) in the neonatal stage. The osASD caused by AS had been left open and needed closure. Two months before the osASD occlusion, the patient presented some episodes of ventricular tachycardia with syncope and received an implantable cardioverter defibrillator. Another patient complained of migraine with visual changes before implantation. After the procedure, the episodes improved, but recurred after the first three months. After the implants, migraine was not identified in any other patient.
On TEE, an aortic rim was present in 17 cases (34.7%) and absent in the others. Atrial septal aneurrysm was identified in seven cases (14.3%): five were of hypermobile type, swaying to the right/left atrium, according to the cardiac cycle (type C), and two bulged fixedly towards the right atrium (type A).6
Two patients had associated defects, occluded in the same procedure with specific prostheses: a perimembranous interventricular communication and a patent ductus arteriosus. Seven patients had additional orifices with multiple fenestrations in addition to the main orifice, and three of them required a second prosthesis to completely occlude the orifices.
The static diameter of the major orifices ranged from 4 to 32mm (13±7mm) and with SDs from 10 to 36mm (22±7mm). The SD obtained with the use of the measuring balloon was 33.3% higher than the static diameters measured during TEE.
Implant was possible in all cases; 55 devices were used in 49 patients with a mean of 1.1 prosthesis by patient. CERA® prostheses were used in 45 patients, and Cocoon prostheses in four. The diameters of the devices implanted in the main orifices ranged from 8 to 38mm (24±8mm), and the most widely used diameter was that of 18mm. Prostheses of 8, 14, and 16mm were implanted in three patients who required a simultaneous occlusion of the smaller orifices.
During the procedures, it was necessary to replace the first prosthesis in three patients. In two cases, the initial prosthesis was removed still connected to the release cable, and was replaced by another of a larger diameter, since the defect suffered dilation during the maneuvers of the implant. In the third case, the first prosthesis presented a concave shape of the left disk during implantation (tuliplike or wine goblet configuration), 7 failing to properly adapt to the orifice; it was thus withdrawn. SD remeasuring was performed, and the prosthesis was replaced by a larger device, which was implanted without difficulty.
Immediate (minor) complications were identified in four cases. TEE detected filiform thrombi in the exchange guide of a patient and in the distal end of the long sheath in another patient. All had been heparinized, and the procedure did not last longer than usual. An additional dose of heparin was applied, and a careful removal of affected materials was performed. Then, the materials were washed and reintroduced, allowing for the implantation with the traditional technique in both cases. No patient had sequelae resulting from thrombi, which were not visualized by TEE after removal of the long sheath and guide. Two patients had transient arrhythmias: the first showed an exacerbated sinus bradycardia for several minutes after completion of the procedure during anesthesia reversal, recovering without the need for any intervention. Another patient had junctional rhythm by manipulating the catheter in the right atrium without compromising his cardiac output, also with spontaneous resolution.
Mean follow-up was 21 months (1 to 41 months). The patient who had junctional rhythm during the procedure was hospitalized with atrial fibrillation, with rapid ventricular response one month after implantation. Currently, this patient is on sinus rhythm and in use of antiarrhythmic medication. There were no deaths in this series. The immediate occlusion rate of 91.9% to 95.9% at six months was not different from the results obtained with the ASO device.5,8
In the TTE study conducted in the first month, minimal residual shunt was detected in four cases (8.1%), three of whom had multiple orifices. One patient, who had a very thin septum, required two prostheses. In the TTE for control after one month, a significant residual shunt was noticed between the two prostheses, which were stable and maintained their original positions in the interatrial septum. This patient was electively referred for surgery, with good results. After the sixth month of follow-up, only a trivial leak persisted in one patient; another patient, who still had not reached the sixth month of follow-up, showed minimal residual shunt.
DISCUSSIONThis article focused on the use, handling, and results of two new prosthetic coated-nitinol wire prostheses about which there is little reference in the contemporary literature.3
The first potential advantage of the coated wires is to prevent the release of nickel into the bloodstream after implantation. In nitinol prosthesis, the release of nickel from the device starts immediately after the procedure and reaches a maximum after one month, and may cause allergic and toxic reactions in sensitive patients.9 While this has not proved a problem of great magnitude, some cases of occurrence or of worsening of migraine in patients undergoing ASO prosthesis implantation have been attributed to an allergic reaction to nickel,10 which makes a coating of nitinol wire desirable. Another advantage is the increased biocompatibility. Coated nitinol wires are more biocompatible and less thrombogenic, although the nitinol prostheses already have a lower rate of thrombosis compared to other metallic devices.11,12
Erosions caused by the prosthesis are rare, but potentially fatal. These situations are extremely risky to patients, unpredictable, and may be of late occurrence. Although its mechanism is still unknown, the erosion appears to be related to the abrasion of cardiac tissue adjacent to the metallic prosthesis, which can have some facilitators, for instance, associated pathologies (Marfan syndrome), changes in tissue thickness and composition related to age, and variations in tissue resistance and in the ability to withstand abrasive forces.13
The oversizing of the device can lead to a contact of the edges of the prosthesis with aortic or atrial walls, and its movement in relation to the tissue resembles a circular saw.14 With respect to the time of appearance, erosions may occur days after implantation or even six years later. The mortality rate caused by erosion is 0.004% to 0.015%.15,16 In 2004, the estimated incidence of erosion cases in the United States, with the use of ASO prosthesis, was 0.1%.12 Other publications report erosion rates ranging between 0.07% and 0.11% in the United States, and between 0.04% and 0.17% in the rest of the world, based on the number of ASO sold or implanted.17 Clearly, these numbers are only estimates, since many patients do not have their implants registered, and many authors do not usually report their complications; however, the incidence of this complication is fortunately low. Whether due to the fewer implants that have been performed, or to the smaller number of reports in the literature, or to the greater flexibility of the new devices, the fact is that no cases of erosion with CERA® and Cocoon prostheses have been reported.
No essential differences were observed with respect to older prostheses with the same design of those of the present study. Both prostheses are easy to handle, of simple release, and can be captured and repositioned while attached to the delivery system.
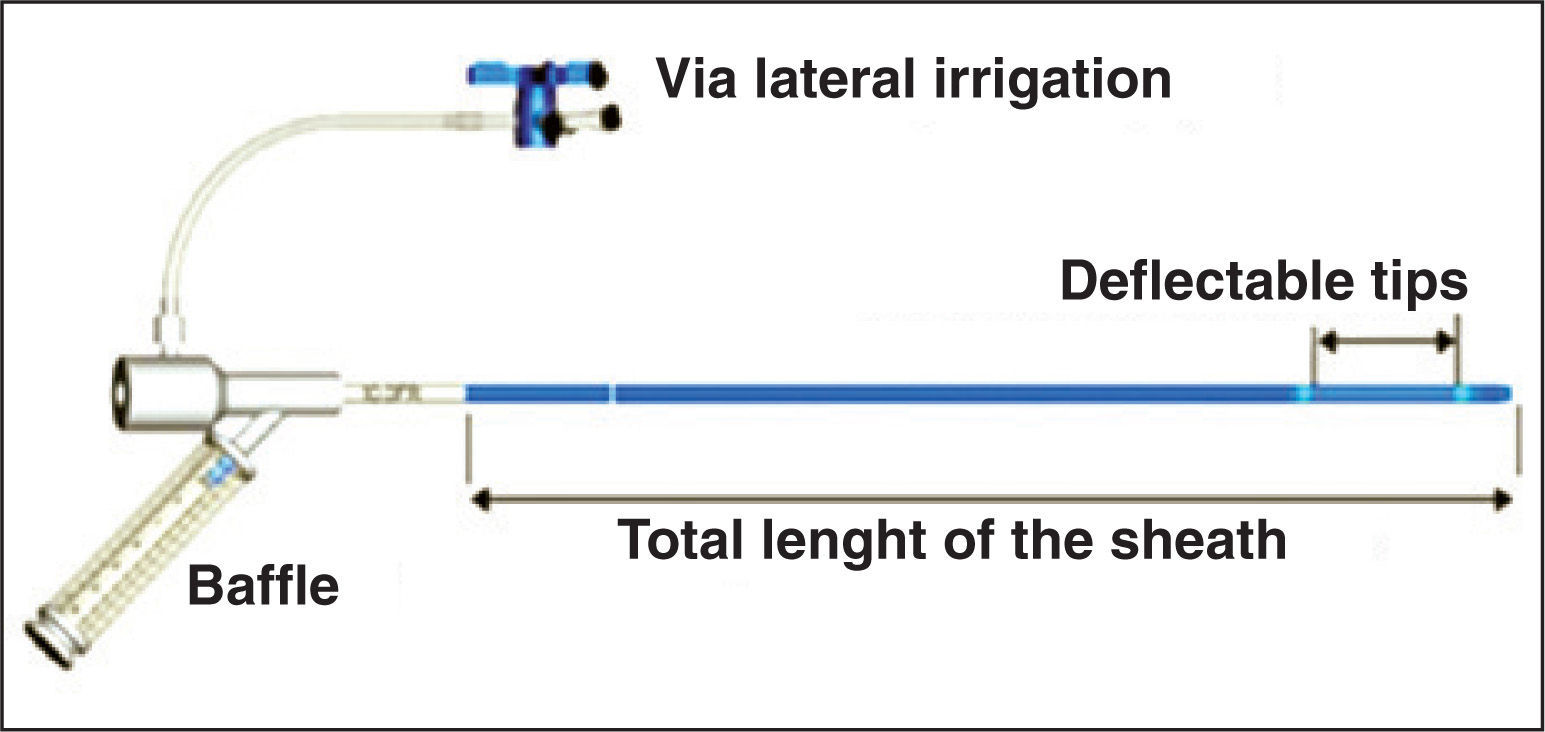
The long sheaths of the two devices are more rigid and less flexible than desired. In some cases, this causes the prosthesis to be positioned perpendicularly to the defect, making its implantation difficult. Conversely, these devices respond reasonably well to the rotation maneuvers that are necessary in such cases. The use of the Mullins sheath (Cook Medical Inc., Bloomington, In, United States) or of the new FuStar® sheath (Lifetech Scientific Corporation) are excellent alternatives for those situations. FuStar® sheaths are long (55, 70, 80, and 90mm) and flexible, with deflectable tips of 3 or 5cm, which can be curved up to a 160° angle by manipulating a cylindrical control in its proximal section, providing a better alignment of the prosthesis with respect to the orifice (Figure 4).
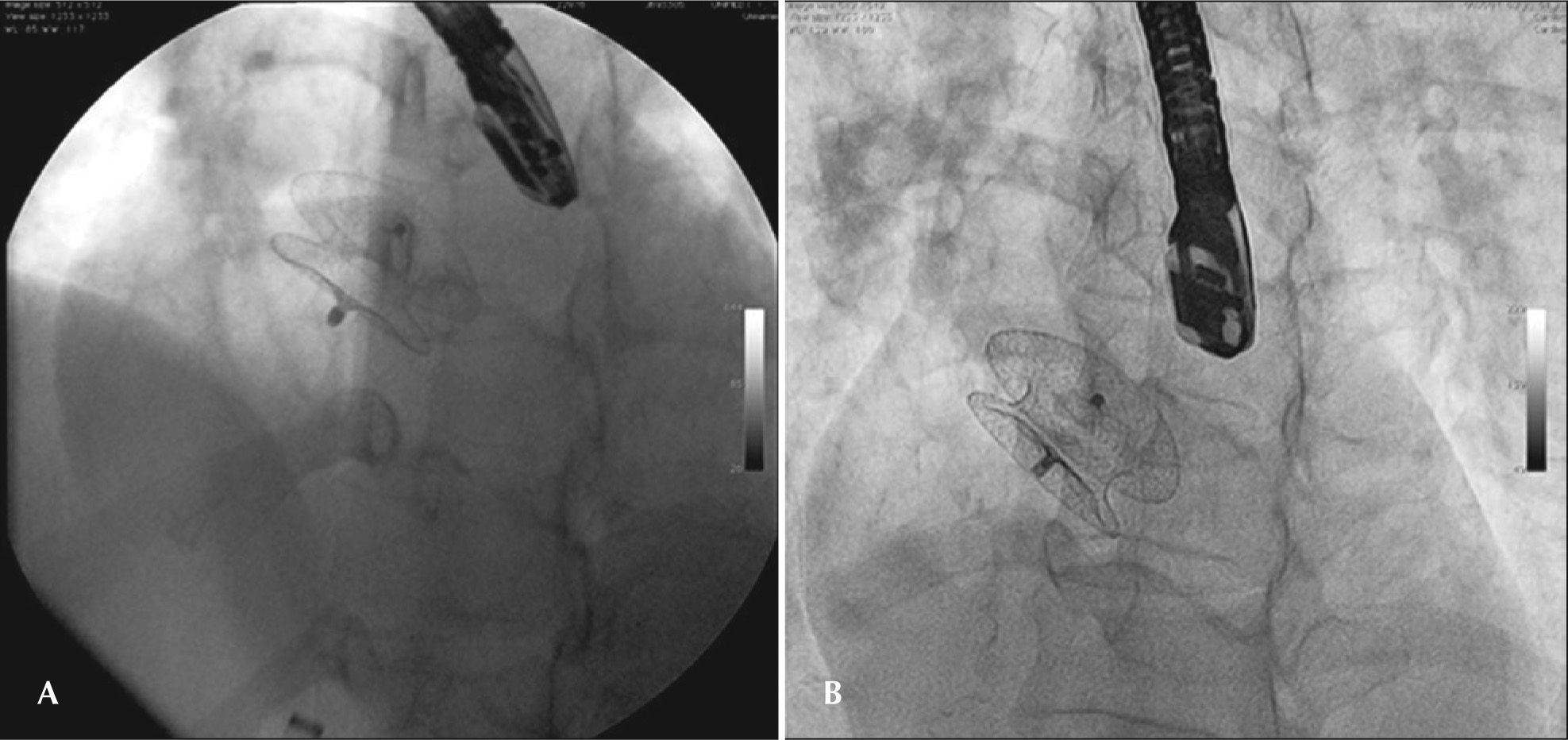
The malformation “in tulip”, described for CERA® prostheses, appears to be exclusive to this device, although the authors managed to reproduce in a bench test a similar malformation in a Figulla prosthesis (Occlutech, Prague, Czech Republic). The concave shape of the left disk prevents a proper fit of the device to the septum and prevents its reintroduction into the sheath. This supposedly happens in larger devices, where, to configure the left disk, it is pushed against the posterior wall of the left atrium. Another factor that appears to contribute to this malformation is the polyester fabric, stuck to the circumference of the device, and which has additional sutures in the more distal portion (ceiling) of the left disk. If the device takes an abnormal shape, it should not be recaptured. Rather, it must be completely externalized, noting that it is entirely free within a heart chamber (usually the left atrium), and only then trying to recapture it. Vigorous attempts to pull the left disc inside the sheath will change the nitinol properties, making its entry into the long sheath without the aid of a loop catheter impossible (Figure 5). It is suggested that, in order to avoid this kind of malformation, the left disc pin should be held by a forceps and pulled, stretching the device while loading it.
– Malformation in tulip: In the left panel, the prosthesis with the concave configuration of the left disk, completely externalized in the left atrium, still attached to the delivery system. On the right, the prosthesis on the bench, after its removal, showing the malformation that prevented its use.
The handling of the prostheses CERA® and Cocoon proved to be a simple, safe, and easily reproducible task in the hands of experienced surgeons for the closure of osASD. Both grafts appeared flexible and with a satisfactory profile. Immediate occlusion rates reproduced those in the literature with the Amplatzer® Septal Occluder prosthesis, and CERA® and Cocoon prostheses proved to be an excellent alternative to traditional prostheses. Further studies and long-term follow-up of patients are needed in order to determine the real advantages of the coating of nitinol wire.
CONFLICTS OF INTERESTFrancisco Chamié is a consultant and proctor for Boynton, Neomex, Bioassist, and AbbMed companies. The other authors declare no conflicts of interest.
SOURCE OF FINANCINGNone.