Historically, intravascular pressure recording has contributed to the development of interventional cardiology. Although new imaging resources have gained much attention, accurate intravascular pressure measurement remains essential for the diagnosis and evaluation of interventional therapy methods. We describe the use of the miniature manometric system (pressure-wire) to obtain pressure curves in congenital and acquired structural heart diseases.
MethodsThe Radi Analyzer® Xpress (St. Jude Medical Inc., St. Paul, MN, USA) and PressureWire® Certus systems were used in procedures with 5F catheters under general anesthesia and ventilatory support in children. Manometric tracings were obtained simultaneously from pressure-wire and the 5F catheter in patients whose therapy strategies were dependent on the analysis of intravascular pressures, and in whom it was not possible to obtain them accurately by conventional methods.
ResultsPressure-wire was used to obtain pulmonary intravascular pressures in patients with systemic-pulmonary collaterals with or without angiographically detected stenosis, and with different structural heart diseases, in the evaluation of pulmonary branch stenosis, in the localization of surgical conduit stenosis (e.g. after Rastelli surgery), surgical shunts (such as Blalock-Taussig) and in the preoperative evaluation of cavopulmonary shunts. The procedure was performed safely, and manometric tracings were obtained with an adequate quality.
ConclusionsThe miniature manometric system is well accepted as a complementary diagnostic modality for the functional assessment of coronary lesions in interventional cardiology. It is also a complementary diagnostic method in different structural, congenital and acquired heart diseases.
Uso do Sistema Manométrico MiniaturizadoPressure-Wire em Cardiopatias Estruturais Congênitas e Adquiridas
IntroduçãoHistoricamente, o registro de pressões intravasculares contribuiu para o desenvolvimento da cardiologia intervencionista. Embora atualmente novos recursos de imagem sejam preponderantes, a medida acurada de pressões intravasculares ainda é essencial em muitos contextos diagnósticos e de aferição de métodos terapêuticos intervencionistas. Descrevemos a utilização do sistema manométrico miniaturizado (pressure-wire) para obtenção de curvas pressóricas em cardiopatias estruturais congênitas e adquiridas.
MétodosForam utilizados os sistemas RADI Analyzer® Xpress (St. Jude Medical Inc., St. Paul, Minnesota, Estados Unidos) e PressureWire® Certus durante procedimentos com cateteres diagnósticos 5F realizados sob anestesia geral e suporte ventilatório em crianças. Traçados manométricos foram obtidos simultaneamente com o pressure-wire e o cateter 5F em pacientes para os quais era imperativa a decisão terapêutica com base em análise das pressões intravasculares e nos quais não era possível obtê-las acuradamente pelos métodos convencionais.
ResultadosO pressure-wire foi utilizado para obtenção de pressões intravasculares pulmonares em pacientes com colaterais sistêmico-pulmonares, com estenoses angiográficas ou não, e com variadas cardiopatias estruturais, na avaliação de estenoses de ramos pulmonares, na localização de estenoses de condutos cirúrgicos (por exemplo: após cirurgia de Rastelli), shunts cirúrgicos (como o de Blalock-Taussig) e na avaliação pré-operatória de derivações cavo pulmonares. O procedimento foi realizado com segurança, tendo sido obtidos traçados manométricos com qualidade adequada.
ConclusõesO sistema manométrico miniaturizado está consagrado no cenário da cardiologia intervencionista, enquanto modalidade diagnóstica complementar para avaliação funcional de lesões coronárias. Esse sistema também constitui método diagnóstico complementar em diversas cardiopatias estruturais, congênitas e adquiridas.
The recording of pressure curves to explain the physical manifestations of the cardiovascular system was one of the key boosters for the development of modern hemodynamics, as applied to the study of human physiology and pathophysiology. The pioneering studies of Claude Bernard, cited by Buzzi in 1959,1 led to the catheterization of cardiac chambers in 1847, with the blood pressure recording of the right ventricle (RV) in experimental animals.
In a little less than a century later, following the self-experience of Forssman in 1929, the subsequent work of André Cournand and several other researchers decisively boosted the knowledge of the pathophysiology of congenital and acquired diseases by means of pressure records and manometry of the right heart chamber and pulmonary circulation. In parallel, methods of oximetry and analysis of dilution curves of several indicators were developed to measure cardiac output, and for the detection and quantification of leads or communications (shunts) between systemic and pulmonary circulations. Through the association of manometric data to these methods, it was possible to measure vascular resistances and also at the level of the heart valves, in normal conditions and in the presence of structural, congenital, and acquired cardiovascular defects.2,3
In more recent decades, there has been great interest in radiological contrast angiography and other methods of cardiovascular imaging during cardiac catheterization, such as intravascular ultrasound (IVUS) and optical coherence tomography. Unfortunately, the practical use of such technological advances has occurred simultaneously with a certain lack of interest, on the part of interventional cardiologists, in the classical methods of hemodynamics – including blood pressure records, blood flow measurements, and vascular and valve orifice resistance calculations.
In the case of congenital heart diseases, the determination of intravascular and cardiac chamber pressures is of particular importance, since they are defining factors for the modality of treatment to be followed, because these pressures influence the choice of surgical technique, occasionally contraindicating or conditioning the palliative therapy. In the scenario of some of these diseases, sometimes with a very reserved prognosis, the documentation of reliable hemodynamic data represents a fundamental aspect of diagnosis.
Manometry is conventionally performed by diagnostic catheters and systems for measuring pressure, based on a liquid column and an oscillometric diaphragm, which transmit the mechanical drive detected by the catheter tip, with its conversion into an electrical signal, which, in turn, is transduced into a graphic recording with electronic typing. This transduction system of physical signs is subject to artifacts that exacerbate and/or attenuate the output, resulting in erroneous pressure values, as they are inherently influenced and warped by the dynamic frequency response of the system as a whole. As part of the relative neglect to which the method of manometry during cardiac catheterization is currently relegated, generally there is no attention given to a periodic verification of the ideal conditions of dynamic response of the intravascular pressure transduction system.
The miniaturized manometric method is a system for measuring intravascular pressure that adapts a pressure sensor directly to a 0.014, guide-wire, similar to those commonly used in coronary angioplasty, which allows for the recording of simultaneous and comparative pressure graphics between the device and the catheterguide. Since 1996, from the clinical use by Pijls and De Bruyne in the functional evaluation of coronary stenoses, the method was consolidated in the practice of interventional cardiology in various applications related to the measurement of coronary fractional flow reserve and coronary vasodilator reserve.4
The purpose of this article was to describe the pioneering use, in this center, of the pressure-wire system in obtaining reliable pressure curves in patients with congenital and acquired structural heart diseases, when reliable manometric records of the pulmonary circulation, transvalvar gradients, and stenoses of surgical or native vascular conduits are needed.
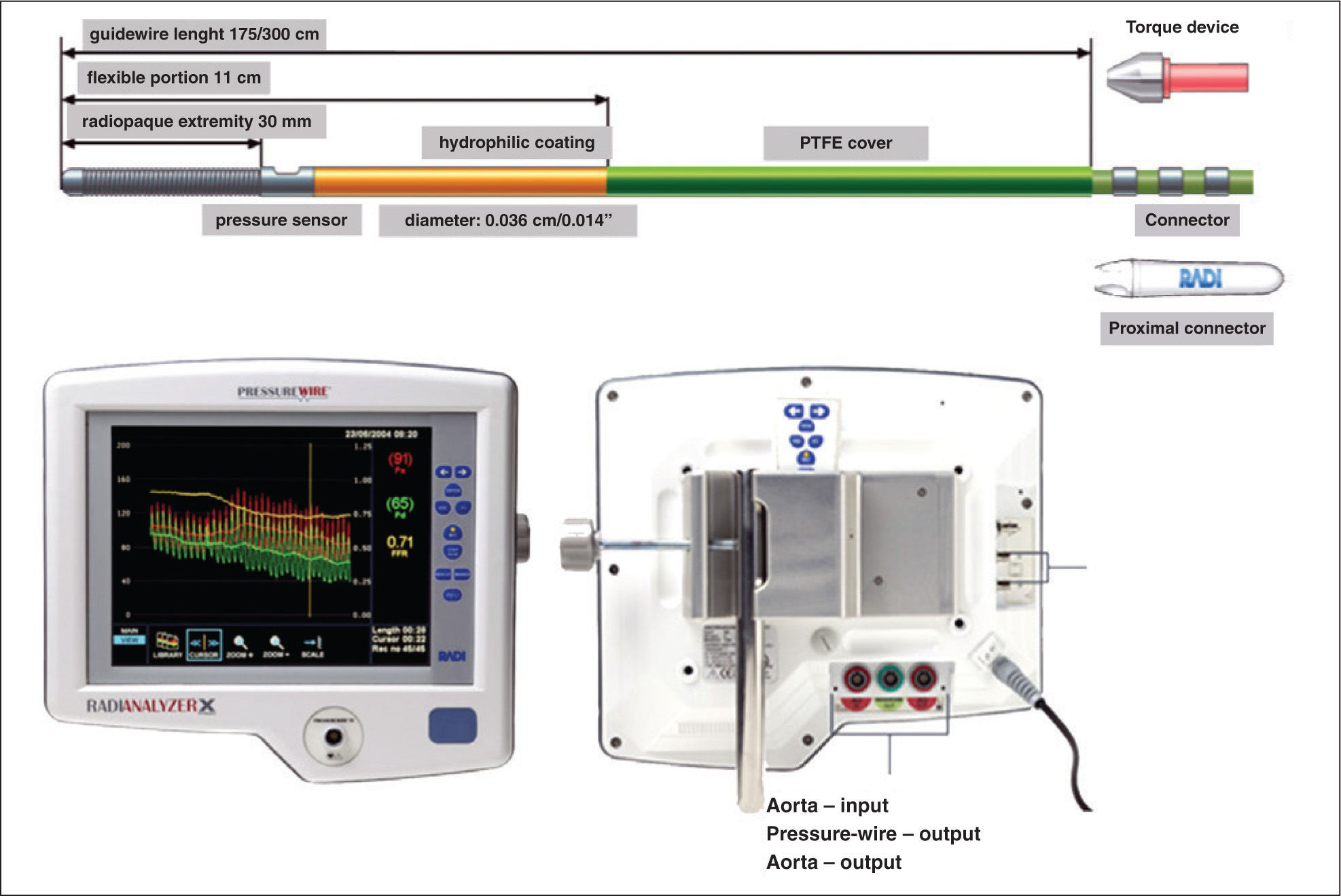
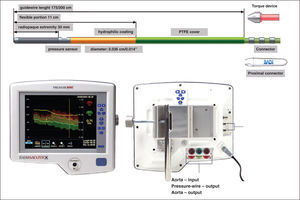
METHODSDescription of devicesThe description in this article refers to the RADI Analyzer® Xpress and PressureWire® Certus (St. Jude Medical Inc. – St. Paul, Minnesota, United States), systems in use in this interventional cardiology laboratory since 2007. In the modalities in which the authors have used these tools, the manometry devices are 0.014, guide-wires with floppy-type tip, similar to those used in coronary angioplasty, with a pressure sensor located at the junction between the more radiopaque distal end and the wire body, approximately 3cm from the tip. The guides are constructed in the core-to-tip format, with a torque transmission at a nearly 1:1 ratio. In the domestic market, the hydrophilic PressureWire® Certus (Figure 1) is available.
The pressure-wire is connected to the RADI Analyzer® Xpress console for data transmission via an adapter at the proximal portion of the guide-wire. The system allows for the measurement of pressures between −30 and 300mmHg, with an accuracy of±1% mmHg plus±1% of the values obtained between −30 and 50mmHg; and of±3% of the values obtained between 5 and 300mmHg. The response frequency of the system is 25Hz, which allows obtaining a signal with high accuracy, even at high heart rates.
The RADI Analyzer® console has dimensions of 29×12×31.5cm, weighs approximately 4.4kg (with the power transformer), and operates at a voltage range of 100-240 volts. Ideally, the device should be operated in air-conditioned environments at temperatures between 15° and 30°C, and with an ambient humidity between 30% and 75%.
Description of the procedureCardiac catheterization patients, for whom manometry of cardiac chambers or systemic or pulmonary vessels constituted very important data for the therapeutic decision, and without the possibility of obtaining information by a habitual modality through the use of diagnostic catheters were selected for inclusion in this study. These subjects were not individually described, but were grouped according to the indication of cardiac catheterization and to the underlying disease.
The procedures were performed using conventional diagnostic catheters, differently than in the context of coronary stenosis evaluation, in which guide-catheters are chosen in view of the therapeutic perspective of a coronary angioplasty. There was no impairment of the quality of the tracings thus obtained.
When using the pressure-wire, the console remains interposed between the conventional pressure transducer system mounted on the examination table and the polygraph. There are four ports on the monitor console: one on the front and three on the back of the machine, which are respectively connected to the pressure-wire via an adapter on the front side; the pressure signal input captured by the diagnostic catheter and the liquid column; the pressure output signal transmitted through the catheter (polygraph – P1); and the pressure output signal transmitted through the pressure-wire (polygraph – P2) on its dorsal surface. After checking the connections, the console is turned on, and the conventional pressure transducer is exposed to atmospheric pressure and zeroed at this level for the polygraph and the console. This step is followed by its exposure to atmospheric pressure and by calibration of the pressure-wire after immersion in a shallow saline film, still into the wrapping system in which it is provided.
The sequence of steps is shown on the console monitor. After the calibration of the device for use, the pressure-wire is inserted into the catheter via a Y-connector and positioned so that the pressure sensor is located at the distal end of the catheter, for equalization of pressures. The equalization of pressures must be performed after removing the introducer needle (needle passer) of the Y-connector and smoothly closing the valve, with no contrast or blood remaining in the catheter. Any minimum pressure difference between catheter and pressure-wire is electronically removed (equalization). After the completion of the pressure measurements, when removing the pressure-wire, a new equalization check should be performed, to verify the quality of data obtained.
The oscillations of pressures obtained according to the respiratory cycle are usually minimized by requesting the patient to perform an expiratory pause; or by request to the anesthetist, when the procedure is performed under sedation, and the patient is on invasive respiratory support, as occurred in the present study.
RESULTSPatients with pulmonary atresia with systemic-pulmonary collateralsIn pulmonary atresia with ventricular septal defect (PAVSD), the pulmonary flow is maintained through the ductus arteriosus, which remains patent, or through systemic-pulmonary collaterals. For surgical planning, the documentation of collateral vessels and their intravascular pressures have fundamental importance, since the presence of a hyperflow through non-stenotic collateral vessels, or of lung segments with double irrigation by pulmonary artery collaterals and their branches can cause high pressure regimens and contraindicate or predict adverse outcomes, according to the surgical strategy adopted.5 The exact documentation of intravascular pressures in these arterial routes is important, both for patients in whom the surgical planning indicates the univentricular repair, and for those intended for a biventricular repair.
The systemic-pulmonary collateral vessels are often tortuous and small-caliber, sometimes with stenoses that are not angiographically demonstrable due to vascular overlapping and to the routine practice of monoplanar angiography. In such circumstances, the pressure-wire method allows for a quite reliable documentation of collateral vessels with protective proximal stenoses, as well as lack thereof, thereby reducing the risks associated with the selective catheterization of these vessels. The manipulation of the pressure guide-wire presents no difficulties, since the diagnostic catheter (JR 5F, AL1 5 MP, or MP 5F) is located near the origin of the collateral vessel. Moreover, its maneuverability is excellent, approaching that which is usually observed during intracoronary procedures.
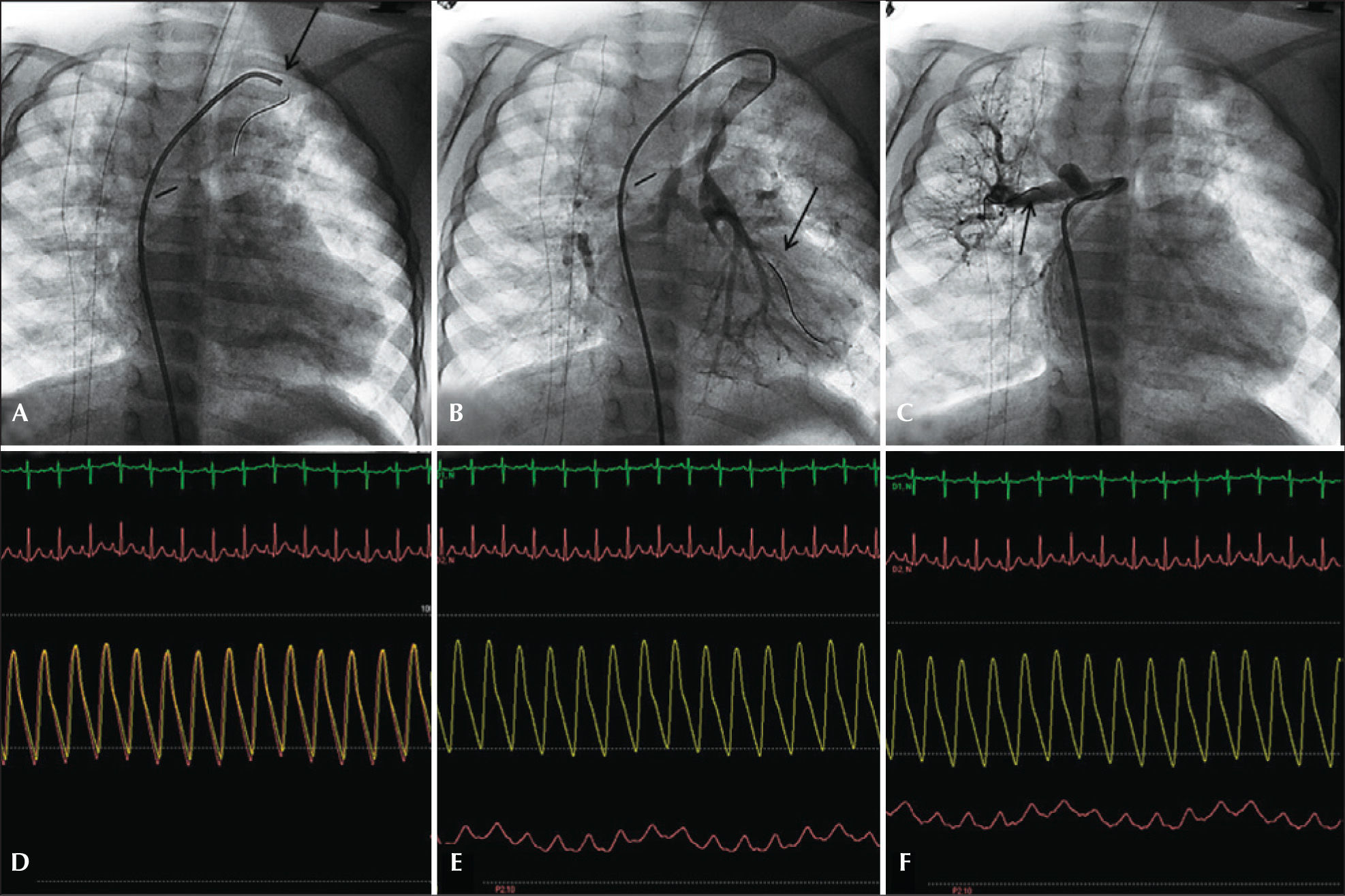
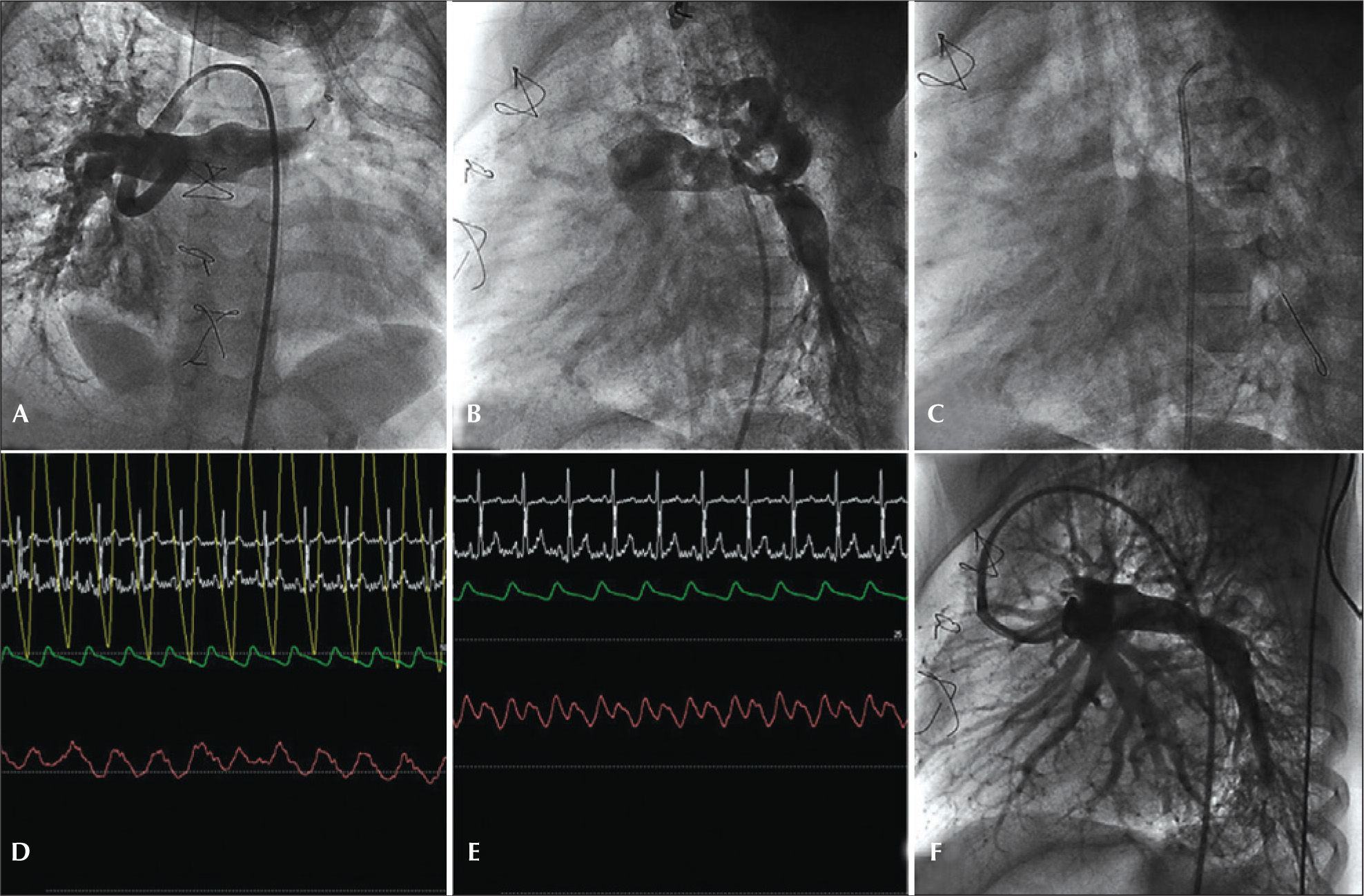
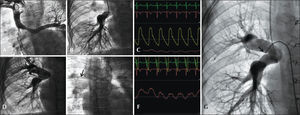
The use of this technique has an additional favorable aspect, i.e., the fact that the only source of lung blood flow in some patients is not compromised. Figure 2 illustrates a case of type-C PAVSD according to the classification of Barbero-Martial,6 wherein all collateral vessels were catheterized, and the corresponding intravascular pressures were documented.
– Pulmonary atresia with ventricular septal defect type C (Barbero-Marcial). (A) Selective catheterization of bifurcated collateral with origin in the left subclavian, with pressure-wire in position of pressure equalization. (B) Pressure-wire in the left pulmonary artery branch through the most stenotic sub-branch of the collateral vessel. (C) Collateral toward the right lung, upper lobe. (D) Pressure equalization. (E) Intravascular pressure in the left pulmonary artery branch, inferior lobe, with a mean of 18mmHg. (F) Intravascular pressure in a branch to the upper lobe of the right lung, with a mean of 21mmHg.
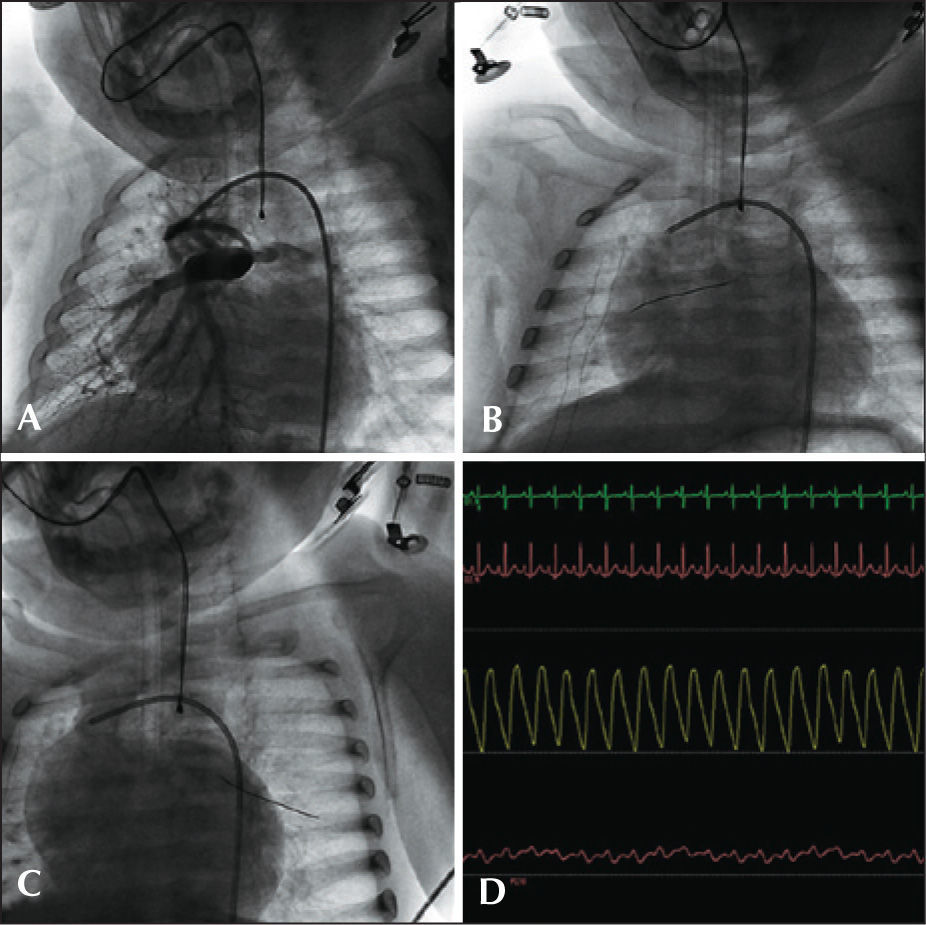
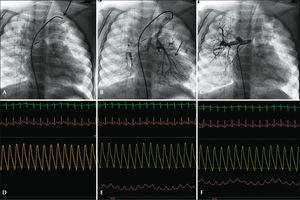
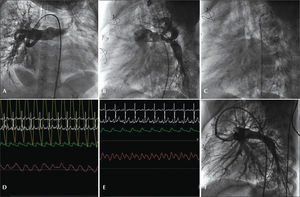
After the original description of the Glenn surgery, its clinical application, in order to perform a terminolateral anastomosis of the superior vena cava to the pulmonary artery, was pioneered by Azzolina et al.7 in 1972. During the preoperatory evaluation of the staged cavopulmonary shunt, the documentation of pulmonary intravascular pressures<15mmHg is critical. Often, in a scenario of pulmonary branch hypoplasia, the patient is initially treated with a modified Blalock-Taussig systemic-pulmonary shunt; sometimes, the selective catheterization of these grafts is laborious and envolves risks. Even when possible, technical difficulties prohibit an accurate assessment of pressures by conventional catheters. In this scenario, the pressure-wire method allows the safe obtaining of curves to provide adequate pressure values, without compromising the flow through these shunts. Another significant aspect is that, in the presence of anomalies in the pulmonary arborization due to the constructed shunt, the presence of the guide-wire does not interfere with the morphology of the pressure curve, and is maneuverable over long distances, contrary to the diagnostic catheter that, in general, has no appropriate dirigibility to reach the obstructed sites. In Figure 3, a case of pre-Glenn catheterization with a modified Blalock–Taussig shunt is documented in an 8-month-old patient with a diagnosis of PAVSD, who was programmed for a univentricular repair.
– Patient with pulmonary atresia with ventricular septal defect undergoing a modified Blalock-Taussig shunt. (A) Pulmonary arteriography with injection through Blalock-Taussig shunt. (B) Pressure-wire positioned through the GORE-TEX® tube in the right pulmonary artery branch. (C) Pressure-wire into the left pulmonary artery branch. (D) Simultaneous aortic and pulmonary pressure curves, with mean pulmonary intravascular pressure of 11mmHg in both branches.
Intraoperatively, in cases of transvalvular pulmonary flow, the flow is usually maintained, unless, after the surgical anastomosis, the pulmonary pressure remains elevated. In the intraoperative phase, the use of pressurewire measures to decide whether or not the pulmonary valve is patent is possible, although in the present service this procedure has not yet been performed.
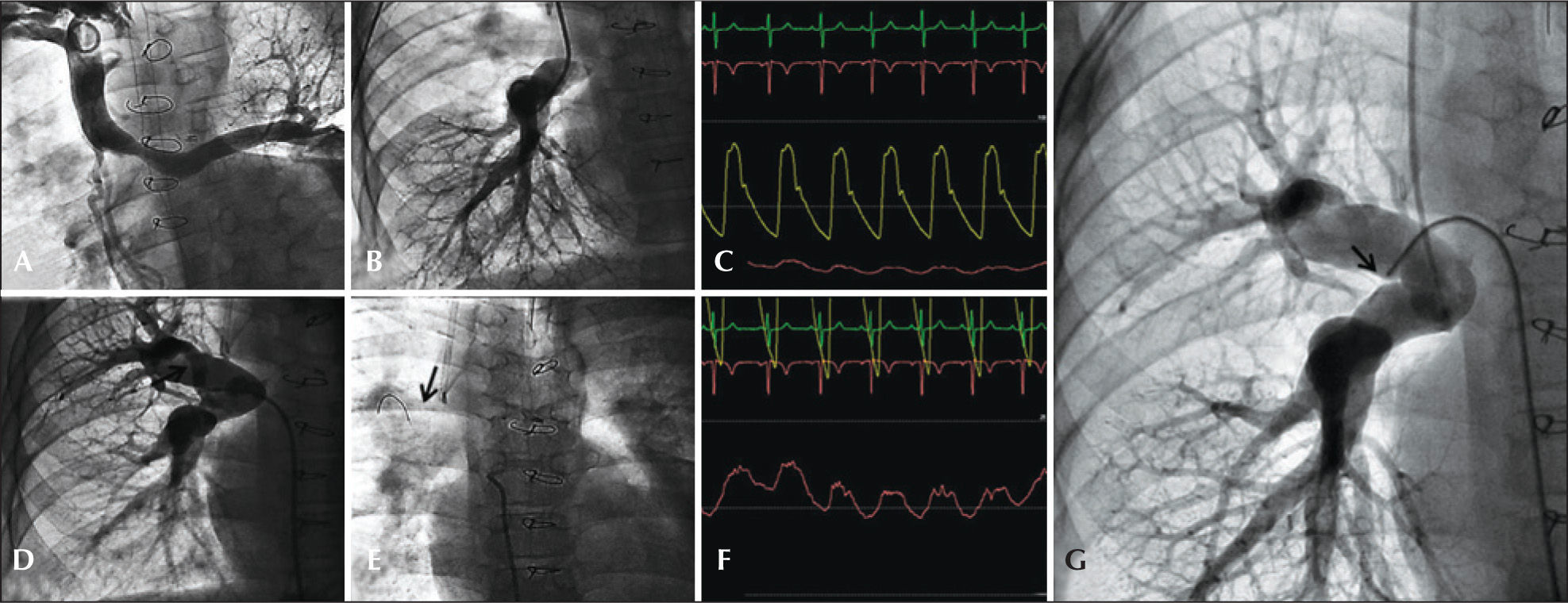
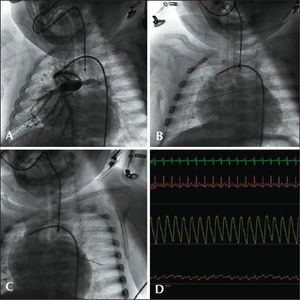
Cardiac catheterization pre-Fontan surgeryThe implementation of this total cavopulmonary shunt must meet prerequisites considered essential for long-term success. Among them, the documentation of mean pulmonary pressure<15mmHg and a pulmonary arteriolar resistance<4 UW/m2 are fundamental. Patients with cardiac disease requiring Fontan surgerytype treatment might not tolerate even small degrees of pulmonary stenosis.8 Usually, pulmonary catheterization by a Glenn shunt presents no difficulties; but more accurate systems, free of artifacts that interfere with the pressure curves, enable the documentation of small anatomical gradients, which may go unnoticed in a scenario of little-elevated pressure regimes. Thus, the pressure-wire method is justified in such circumstances. Figure 4 shows a patient with a scheduled procedure for total cavopulmonary shunt, diagnosed with pulmonary atresia with systemic-to-pulmonary collateral to the right lung, with the aim to evaluate intra-parenchymal interlobar connections and lobar artery continuity with the main pulmonary artery. The presence of arterialization in the pulmonary pressure curve, with a pressure gradient of 4mmHg between the left branch (preferably perfused by the Glenn shunt) and the right branch of the pulmonary artery (perfused by the systemic-pulmonary collateral) angiographically suggests a segmental perfusion deficit in the right lung.
– Pre-Fontan catheterization. (A) Bidirectional Glenn anastomosis with preferential flow to the left lung. (B) Selective angiography of the right pulmonary artery branch through the conduit in the Glenn procedure. (C) Aortic and left pulmonary artery branch pressures, on a scale of 100 (left branch of the pulmonary artery, 10mmHg). (D) Systemic-pulmonary collateral to the right upper lobe. (E) Pressure-wire into the collateral vessel. (F) Intravascular pressure into the right pulmonary artery branch obtained with the pressure-wire (mean of 14mmHg) on scale of 25mmHg. (G) Angiography with Berman catheter occluding the collateral vessel, showing the whole irrigation of the right lung via pulmonary artery.
Anomalies of the pulmonary arborization are frequent with conotruncal malformations, especially in variants of tetralogy of Fallot and, in particular, pulmonary atresia. Such stenoses may be congenital or subsequent to surgical procedures of the Blalock-Taussig type, or to branch unifocalization (Rastelli), due to fibrosis or twisting at the anastomosed site. Sometimes, these stenoses, in the absence of significant pulmonary hypertension, are difficult to evaluate by conducting only the conventional manometric procedure with catheter pullback. In this scenario, the simultaneous measurements of the pressure distally to the stenosis with the pressure-wire and in the pulmonary trunk with the catheter allow the identification of small gradients, and also the study of vessels difficult to approach with the conventional method.
The clinical case of Figure 5 depicts a 4-year-old patient with a diagnosis of pulmonary atresia with unbalanced atrioventricular (AV) septal defect, treated with a modified conventional Blalock-Taussig surgery in his first year of life and with another modified BlalockTaussig shunt at the age of 3, after a suspected stenosis of the first shunt by noninvasive evaluation, without confirmation by angiography. A left branch stenosis was documented by the pressure-wire method, treated with a stent and central shunt occlusion.
– Patient with two central shunts with increased pulmonary blood flow and stenosis of the left branch. (A) A modified Blalock-Taussig shunt. (B) Conventional Blalock-Taussig shunt. (C) Pressure-wire into the left pulmonary artery branch. (D) Manometry of right pulmonary artery branch with an MP 5F catheter (mean pressure of 25mmHg on a scale of 50mmHg). (E) Manometry of distal branch of the left pulmonary artery (mean pressure of 17mmHg on a scale of 25mmHg). (F) Stent implantation into the left pulmonary artery branch with occlusion of the Blalock-Taussig shunt.
The reconstruction of the RV outflow tract is usually performed by the interposition of valved or valveless right ventricle-pulmonary stem (RVPS) tubes that often calcify in the late postoperative period, evolving with acquired stenoses. Generally, the determination of RV systolic pressure suffices for the diagnosis; however, when branch stenoses are present, the documentation of the level of obstruction and of the significance of each of these stenoses is largely facilitated by the pressure-wire. Another relevant aspect is that the location of the gradients in the proximal or distal anastomoses or at the valve topography is sometimes hampered by sudden movements of the catheter, as a result of flow turbulence in these areas. The pressure-wire also makes it easier and more reliable to perform the procedure under these conditions.
DISCUSSIONEssentially, the manometric system, which uses the pressure-wire for functional assessment of coronary stenoses, was standardized to determine the fractional flow reserve. In this sense, the method has gained gradual acceptance for its great practicality in quite varied situations, such as lesions in the left coronary trunk, serial focal stenoses, bifurcations, and even in the presence of diffuse coronary artery tapering.9
The present work broadens the concept of practicality of the manometric method based on the use of the pressure-wire technique for several applications in patients with congenital and acquired heart disease. Thus, the practical applications resulting from this concept are expanded from the use in this laboratory of hemodynamics and interventional cardiology to the specific context of pulmonary atresia, as recently described by Haddad et al.,10 with the demonstration of the technique’s feasibility and safety in ten pediatric patients.
The pressure curves obtained with diagnostic catheters often exhibit artifacts causing reverberation or attenuation, for instance, ringing, overshooting, and damping, limiting the acquisition of accurate pressure values. In such circumstances, the diagnosis of significant venous gradients becomes impracticable, with the aggravating circumstance that, in this area of the circulatory system, even small gradients can be of great clinical relevance. Considering that the manometric system based on the use of pressure-wire is a device that measures pressures without the need of diaphragms and of interposed liquid columns, this allows a more accurate register of pressure values without artifacts, in the diverse contexts discussed in this study. In this hemodynamics and interventional cardiology laboratory, the pressure-wire manometric system has allowed the authors to obtain pulmonary intravascular pressures and pressures in native vascular conduits or in conduits resulting from surgery in a safe manner and with less risk compared to the technique usually used, as exemplified in the cases described.
Another potentially very important aspect will be the use of the pressure-wire technology as a tool for calculation of flow through a conduit. Newer versions of the PressureWire® Certus and of the RADI Analyzer® Xpress console allow for flow measurements and output estimations by a method based on a thermodilution system. In patients with heart diseases concurrent with increased pulmonary blood flow, frequently it is not possible to apply the Fick principle for proper quantification. Moreover, cases with pulmonary atresia and systemic-pulmonary collaterals and/or patent ductus arteriosus are eventually superficially labeled as having a pulmonary hypoflow. However, in theory, this can translate into the contrary, by means of a selective catheterization of all collateral vessels and the calculation of the sum of flows through these collaterals, since the result may actually represent a true pulmonary blood hyperflow. This strategy would also allow for the estimation of individual pulmonary resistances of all pulmonary segments thus perfused. It is worth emphasizing the speculative nature of this condition, which requires data not yet available in the literature for such documentation.
CONCLUSIONSIn the authors’ recent practice of diagnostic cardiac catheterization of various congenital and acquired diseases, the miniaturized manometric method using the pressure-wire has become virtually indispensable in many contexts. This is an important complementary diagnostic method, not only in cases where there is difficulty in using the usual means to acquire intravascular pressures, but also in routine cases, since the pressurewire permits obtaining tracings with less artifacts. The simultaneous recording of pressure curves, without the need for two vascular punctures or of larger diameter catheters, allows for very satisfactory graduating of stenoses with significant hemodynamic repercussion in different clinical and postsurgical contexts. The authors also foresee the future expansion of its use with other complementary diagnostic purposes.
CONFLICT OF INTERESTSThe authors declare no conflicts of interest.
SOURCE OF FINANCINGNone.