Recent studies have demonstrated the efficacy of the transcatheter valve-in-valve implantation for the treatment of bioprosthesis dysfunction in high-risk surgical patients. This study presents the initial experience with valve-in-valve implantation.
MethodsClinical, echocardiographic, and procedural profiles were characterized, and the mid-term results of patients with surgical bioprosthesis dysfunction submitted to valve-in-valve implantation in the aortic position were reported.
ResultsSeven male patients were included, aged 72.6 ± 10.0 years. The STS score was 9,6 ± 10,5%, and the logistic EuroSCORE was 22.7 ± 14.7%. Three patients had combined aortic bioprosthesis failure; two had isolated regurgitation; and two had isolated stenosis. The transfemoral access was used in six cases, and the transapical access in one case. Implanted devices included Sapien XT (n = 5) and CoreValve (n = 2) prostheses. Procedural success was achieved in six (85.7%) cases. After the procedure, the mean gradient decreased from 38.2 ± 9.6mmHg to 20.9 ± 5.9mmHg, and the valve area increased from 1.2 ± 0.4cm2 to 1.5 ± 0.5cm2. After 1 year, there were no deaths and no other significant adverse outcomes; 80% of patients were in NYHA functional class I/II. The transvalvular gradients and valve area remained unchanged in this period.
ConclusionsThe valve-in-valve procedure was effective in most high-risk surgical patients with bioprosthesis dysfunction. When performed in well-selected patients, it results in satisfactory clinical and hemodynamic outcomes.
Estudos recentes têm demonstrado a eficácia do implante transcateter valve-in-valve para o tratamento de disfunção de biopróteses em pacientes de alto risco cirúrgico. Apresentamos nossa experiência inicial com o implante valve-in-valve.
MétodosCaracterizamos o perfil clínico, ecocardiográfico e do procedimento, e reportamos os resultados de médio prazo de pacientes com disfunção de bioprótese submetidos a implante valve-in-valve em posição aórtica.
ResultadosIncluímos sete pacientes do sexo masculino, com idade de 72,6 ± 10,0 anos. O escore STS foi 9,6 ± 10,5%, e o EuroSCORE logístico foi 22,7 ± 14,7%. Três pacientes apresentavam dupla disfunção; dois tinham insuficiência; e dois exibiam estenose isolada. A via transfemoral foi utilizada em seis casos, e a transapical, em um caso. Os dispositivos implantados incluíram as próteses Sapien XT (n = 5) e CoreValve (n = 2). O sucesso do procedimento foi obtido em seis (85,7%) casos. Após o procedimento, o gradiente médio reduziu-se de 38,2 ± 9,6mmHg para 20,9 ± 5,9mmHg, e a área valvar elevou-se de 1,2 ± 0,4cm2 para 1,5 ± 0,5cm2. Ao final de 1 ano, não ocorreram óbitos e nem outros desfechos adversos significativos; 80% dos pacientes encontravam-se em classe funcional NYHA I/II. Os gradientes transvalvares e a área valvar permaneceram inalterados nesse período.
ConclusõesO procedimento valve-in-valve foi eficaz na maioria dos pacientes de alto risco cirúrgico com disfunção de bioprótese. Quando realizado em pacientes bem selecionados, resulta em desfechos clínicos e hemodinâmicos satisfatórios.
Patients with surgical bioprosthesis valve dysfunction represent a clinical challenge because, although a new surgical replacement is considered the standard treatment, the reoperation is associated with high morbidity and mortality.1,2 These patients are characterized as high surgical risk or inoperable, due to multiple comorbidities, advanced age, clinical frailty, or reduced ventricular ejection fraction.3
Originally developed for the approach of the native valve stenosis, transcatheter aortic prosthesis implantation is the standard treatment for symptomatic patients considered inoperable, in addition to representing an alternative therapeutic strategy to surgical valve replacement in high surgical-risk individuals.4–8 Recent studies demonstrate the clinical efficacy of transcatheter valve-in-valve (VIV) prosthesis implantation for the treatment of aortic surgical bioprosthesis dysfunction. This is a less invasive treatment option, especially because it does not expose the patient to extracorporeal cardiopulmonary circulation and the inherent risks of reoperation. Although the prostheses have not been designed for this purpose, the published results have been encouraging.9–14
This study aimed to characterize the initial experience of a multidisciplinary cardiovascular team in employing the VIV procedures in patients with surgical bioprosthesis dysfunction in the aortic position. Clinical and echocardiographic profiles and the aspects related to the procedure were described, as well as the clinical results of the mid-term follow-up.
MethodsPatient selection and indication for the valve-in-valve procedureThis analysis included patients older than 18 years with symptomatic aortic bioprosthesis dysfunction, consecutively submitted to VIV procedure at two tertiary cardiology centers between January 2009 and June 2015. Patients with previous transcatheter aortic valve procedures or active infective endocarditis were excluded from the sample. The project was approved by the institutional Ethics Committee, and the patients signed an informed consent. Data were prospectively recorded in appropriate forms developed for the study, stored in spreadsheets, and collected from the database of each institution.
Pre-procedure clinical assessmentIn general, patient assessment for the VIV procedure was similar to that performed in patients candidates for transcatheter aortic valve implantation in native position. The treatment indication was based on surgical risk, determined by clinical characteristics or technical reasons. For risk estimation, the Society of Thoracic Surgeons score (STS, available at http://riskcalc.sts.org/de.aspx) and the European System for Cardiac Operative Risk Evaluation score (logistic EuroSCORE, according to http://www.euroscore.org/calcold.html) were used. Risk factors not included in these scores, such as the presence of “porcelain aorta”, frailty, hostile thorax caused by previous chest irradiation, liver diseases, and coagulation disorders, were also considered in this decision. All cases were analyzed and discussed by a multidisciplinary group (the Heart Team), consisting of clinical and interventional cardiologists, cardiovascular surgeons, and cardiac imaging specialists.
Specific characteristics of the surgical prosthesis were assessed to support the indication of VIV procedure. The type, model, size, and position (intra- or supra-annular) of the surgical valve prosthesis were identified. The internal diameter of each bioprosthesis was obtained from the manufacturer's information. Technical aspects of the employed surgery, such as the need for reconstruction of the aortic root and the presence of venous or arterial grafts, were also elucidated.
Complementary pre-procedure examinationsLaboratory tests, electrocardiogram, chest X-rays, transesophageal echocardiography, computed tomography angiography (CT-angiography) of the heart and total aorta, and coronary angiography were performed.
The main parameter considered for the choice of transcatheter aortic prosthesis to be implanted was the internal diameter of the previous surgical bioprosthesis, obtained from the manufacturer or as reported by the VIV Aortic application, developed by Bapat and UBQO Ltd. (London, United Kingdom).15 Echocardiography was used to assess the mechanism and consequences of prosthetic dysfunction, defining the integrity and mobility of the leaflets, left ventricular function, and the presence of pulmonary hypertension and associated valve diseases. In cases of dysfunction due to prosthesis regurgitation, the transesophageal echocardiography excluded the presence of paravalvular reflux. The CT-angiography of the aorta was the method used to determine the best approach. In case of non-availability of previous surgical data, the CT-angiography helped to analyze the surgical prosthesis diameters and to choose the most appropriate transcatheter prosthesis for VIV procedure. Coronary angiography was used for the assessment of associated coronary artery disease and to estimate the risk of coronary occlusion during valve implantation.
Technical aspects of the procedureDual antiplatelet therapy (acetylsalicylic acid, 300mg, and clopidogrel, 300mg) was initiated with a loading dose 24hours before the procedure. The procedures were preferably performed in the hybrid room. The decision regarding use of general anesthesia and transesophageal echocardiography was made at the discretion of the operators.
The femoral vascular access was the first choice for the implantation, and a specific hemostatic device was used for arterial repair mediated by ProGlide® suture (Abbott Vascular®, Santa Clara, USA). In case of the impossibility of using the femoral approach, the transapical access was used. After establishing the vascular access, a bolus of unfractionated heparin was administered (80 to 100 U/kg).
Considering the fact that, in most cases, the surgical bioprosthetic annulus is radiopaque, the identification of the best angiographic projection for the implant was obtained by fluoroscopy: a coplanar angle was sought and used as a reference for the adequate positioning of the transcatheter prosthesis. Aortography with visualization of the coronary ostia was obtained in order to assess the occurrence of coronary obstruction during the procedure.
The self-expanding CoreValve system (Medtronic, Minneapolis, USA) and the balloon-expandable Edwards Sapien XT system (Edwards Lifesciences, Irvine, USA) were used. Both the choice of prosthesis and of the need for pre- or post-dilation were made at the discretion of the operators.
Follow-upClinical, laboratory, and echocardiographic data were collected 30 days and 6 months after hospital discharge. Dual antiplatelet therapy was recommended at maintenance doses (clopidogrel, 75mg daily for 6 months and aspirin, 100mg daily, continuously). In patients with indication for oral anticoagulant use, the drug regimen consisted of the association of clopidogrel and warfarin, according to the International Normalized Ratio (INR) target between 2.0 and 3.0.
DefinitionsThe outcomes were categorized according to the Valve Academic Research Consortium (VARC)-2 criteria,16 and the events were adjudicated by two experienced cardiologists. The primary outcome analyzed was device success, defined as the implantation of a single prosthesis in the planned location, with no prosthesis-patient mismatch, mean aortic transvalvular gradient < 20mmHg or peak velocity < 3 m/s, and absence of aortic regurgitation ≥ moderate, assessed by echocardiography and aortography. The early safety outcome (up to 30 days post-procedure) was defined as the combination of mortality from all causes; stroke; life-threatening bleeding; acute kidney injury stages 2 or 3, according to the Acute Kidney Injury Network (AKIN) score;17 major vascular complications, or valvular dysfunction requiring reintervention. The clinical efficacy criteria (30 days after the procedure) consisted of the combined outcome involving mortality from all causes, stroke, rehospitalization for heart failure, New York Heart Association (NYHA) functional class III or IV, or VIV prosthesis dysfunction (stenosis or regurgitation).16
Other complications were also assessed, such as the need for conversion to conventional surgery, occurrence of coronary obstruction, cardiac tamponade, ventricular septal defect, valve malposition, endocarditis, valve thrombosis, conduction disorders, and need for definitive pacemaker implantation.16
Statistical analysisContinuous variables with normal distribution were described as mean and standard deviation or median and interquartile range for asymmetric variables. Categorical variables were described as absolute numbers and percentages. The comparison between continuous variables was performed by paired Student's t-test. P-values ≤ 0.05 were considered statistically significant. R software version 3.1 was utilized (The R Foundation for Statistical Computing, Vienna, Austria).
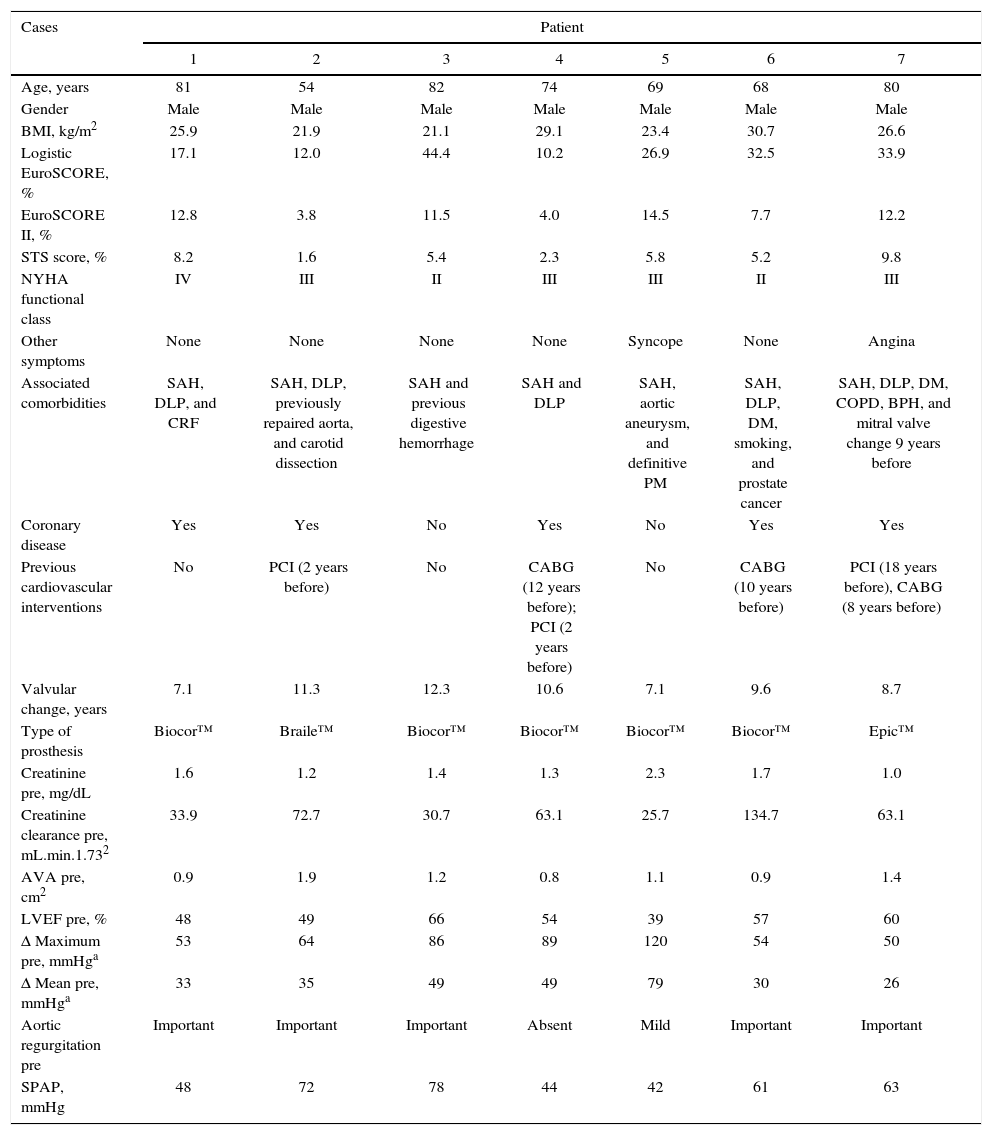
ResultsBaseline clinical characteristicsAll patients were males, mean age of 72.6 ± 10.0 years, and had high surgical risk, with STS score of 9.6 ± 10.5% and logistic EuroSCORE of 22.7 ± 14.7% (Table 1). Most patients (71%) were in NYHA class III or IV. Four of the seven patients had previously undergone coronary artery bypass surgery. The time between surgical bioprosthesis implantation and VIV procedure was 9.5 ± 2.0 years.
Demographic and clinical characteristics.
| Cases | Patient | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Age, years | 81 | 54 | 82 | 74 | 69 | 68 | 80 |
| Gender | Male | Male | Male | Male | Male | Male | Male |
| BMI, kg/m2 | 25.9 | 21.9 | 21.1 | 29.1 | 23.4 | 30.7 | 26.6 |
| Logistic EuroSCORE, % | 17.1 | 12.0 | 44.4 | 10.2 | 26.9 | 32.5 | 33.9 |
| EuroSCORE II, % | 12.8 | 3.8 | 11.5 | 4.0 | 14.5 | 7.7 | 12.2 |
| STS score, % | 8.2 | 1.6 | 5.4 | 2.3 | 5.8 | 5.2 | 9.8 |
| NYHA functional class | IV | III | II | III | III | II | III |
| Other symptoms | None | None | None | None | Syncope | None | Angina |
| Associated comorbidities | SAH, DLP, and CRF | SAH, DLP, previously repaired aorta, and carotid dissection | SAH and previous digestive hemorrhage | SAH and DLP | SAH, aortic aneurysm, and definitive PM | SAH, DLP, DM, smoking, and prostate cancer | SAH, DLP, DM, COPD, BPH, and mitral valve change 9 years before |
| Coronary disease | Yes | Yes | No | Yes | No | Yes | Yes |
| Previous cardiovascular interventions | No | PCI (2 years before) | No | CABG (12 years before); PCI (2 years before) | No | CABG (10 years before) | PCI (18 years before), CABG (8 years before) |
| Valvular change, years | 7.1 | 11.3 | 12.3 | 10.6 | 7.1 | 9.6 | 8.7 |
| Type of prosthesis | Biocor™ | Braile™ | Biocor™ | Biocor™ | Biocor™ | Biocor™ | Epic™ |
| Creatinine pre, mg/dL | 1.6 | 1.2 | 1.4 | 1.3 | 2.3 | 1.7 | 1.0 |
| Creatinine clearance pre, mL.min.1.732 | 33.9 | 72.7 | 30.7 | 63.1 | 25.7 | 134.7 | 63.1 |
| AVA pre, cm2 | 0.9 | 1.9 | 1.2 | 0.8 | 1.1 | 0.9 | 1.4 |
| LVEF pre, % | 48 | 49 | 66 | 54 | 39 | 57 | 60 |
| Δ Maximum pre, mmHga | 53 | 64 | 86 | 89 | 120 | 54 | 50 |
| Δ Mean pre, mmHga | 33 | 35 | 49 | 49 | 79 | 30 | 26 |
| Aortic regurgitation pre | Important | Important | Important | Absent | Mild | Important | Important |
| SPAP, mmHg | 48 | 72 | 78 | 44 | 42 | 61 | 63 |
Values measured by echocardiography.
BMI: body mass index; EuroSCORE: European System for Cardiac Operative Risk Evaluation; STS score: Society of Thoracic Surgeons; NYHA: New York Heart Association; SAH: systemic arterial hypertension; DLP: dyslipidemia; CRF: chronic renal failure; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; BPH: benign prostatic hyperplasia; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; AVA: aortic valve area; LVEF: left ventricular ejection fraction; Δ: gradient; SPAP: systolic pulmonary artery pressure.
Three patients had combined aortic bioprosthesis failure; two had isolated regurgitation; and two patients had isolated stenosis. One patient had been previously submitted to mitral valve replacement. All surgical prostheses were bovine pericardium prosthesis, with SJ Medical Biocor™ (St. Jude Medical Biocor, St. Paul, USA) being used in five patients, SJ Medical Epic™ (St. Jude Medical Biocor, St. Paul, USA) in one patient, and Braile™ prosthesis (Braile Biomédica, São José do Rio Preto, SP, Brazil) in another, with diameters ranging from 21 to 25mm. The mean internal diameters of the bioprosthesis were 24.0 ± 3.9mm, obtained with CT-angiography. The maximum and mean gradients, according to the echocardiography, were 73.7 ± 25.9mmHg and 38.2 ± 9.6mmHg, respectively. The mean areas of the surgical prostheses were 1.2 ± 0.4cm2. Left ventricular ejection fraction ranged from 39 to 66%, with a mean of 53.3 ± 8.9%. Four patients had significant pulmonary hypertension, with mean systolic pulmonary artery pressure (SPAP) of 58.3 ± 14.0mmHg.
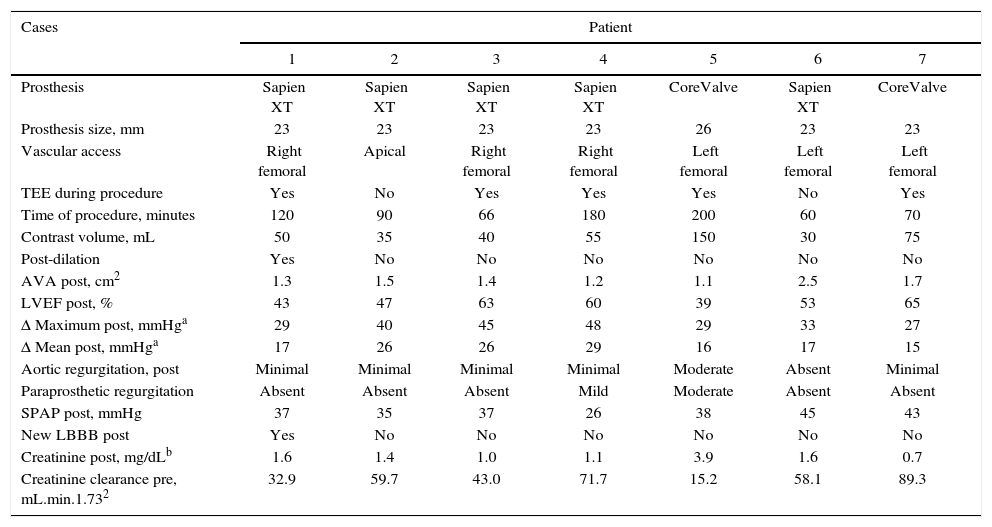
Technical aspects of the interventionMost procedures (six patients) were performed under general anesthesia and monitored by transesophageal echocardiography; in one patient, sedation and local anesthesia were selected (Table 2). The transfemoral access was used in six cases; in these individuals, hemostasis was achieved without vascular complications, using a Perclose™ device. Due to the presence of endoprosthesis in the descending aorta, the transapical access was used in one patient. Pre-dilation was performed in two patients, and post-dilation was required in two patients. The procedure time was 112.3 ± 57.0minutes, and the contrast volume was 63.3 ± 45.4mL.
Characteristicsof the procedure.
| Cases | Patient | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Prosthesis | Sapien XT | Sapien XT | Sapien XT | Sapien XT | CoreValve | Sapien XT | CoreValve |
| Prosthesis size, mm | 23 | 23 | 23 | 23 | 26 | 23 | 23 |
| Vascular access | Right femoral | Apical | Right femoral | Right femoral | Left femoral | Left femoral | Left femoral |
| TEE during procedure | Yes | No | Yes | Yes | Yes | No | Yes |
| Time of procedure, minutes | 120 | 90 | 66 | 180 | 200 | 60 | 70 |
| Contrast volume, mL | 50 | 35 | 40 | 55 | 150 | 30 | 75 |
| Post-dilation | Yes | No | No | No | No | No | No |
| AVA post, cm2 | 1.3 | 1.5 | 1.4 | 1.2 | 1.1 | 2.5 | 1.7 |
| LVEF post, % | 43 | 47 | 63 | 60 | 39 | 53 | 65 |
| Δ Maximum post, mmHga | 29 | 40 | 45 | 48 | 29 | 33 | 27 |
| Δ Mean post, mmHga | 17 | 26 | 26 | 29 | 16 | 17 | 15 |
| Aortic regurgitation, post | Minimal | Minimal | Minimal | Minimal | Moderate | Absent | Minimal |
| Paraprosthetic regurgitation | Absent | Absent | Absent | Mild | Moderate | Absent | Absent |
| SPAP post, mmHg | 37 | 35 | 37 | 26 | 38 | 45 | 43 |
| New LBBB post | Yes | No | No | No | No | No | No |
| Creatinine post, mg/dLb | 1.6 | 1.4 | 1.0 | 1.1 | 3.9 | 1.6 | 0.7 |
| Creatinine clearance pre, mL.min.1.732 | 32.9 | 59.7 | 43.0 | 71.7 | 15.2 | 58.1 | 89.3 |
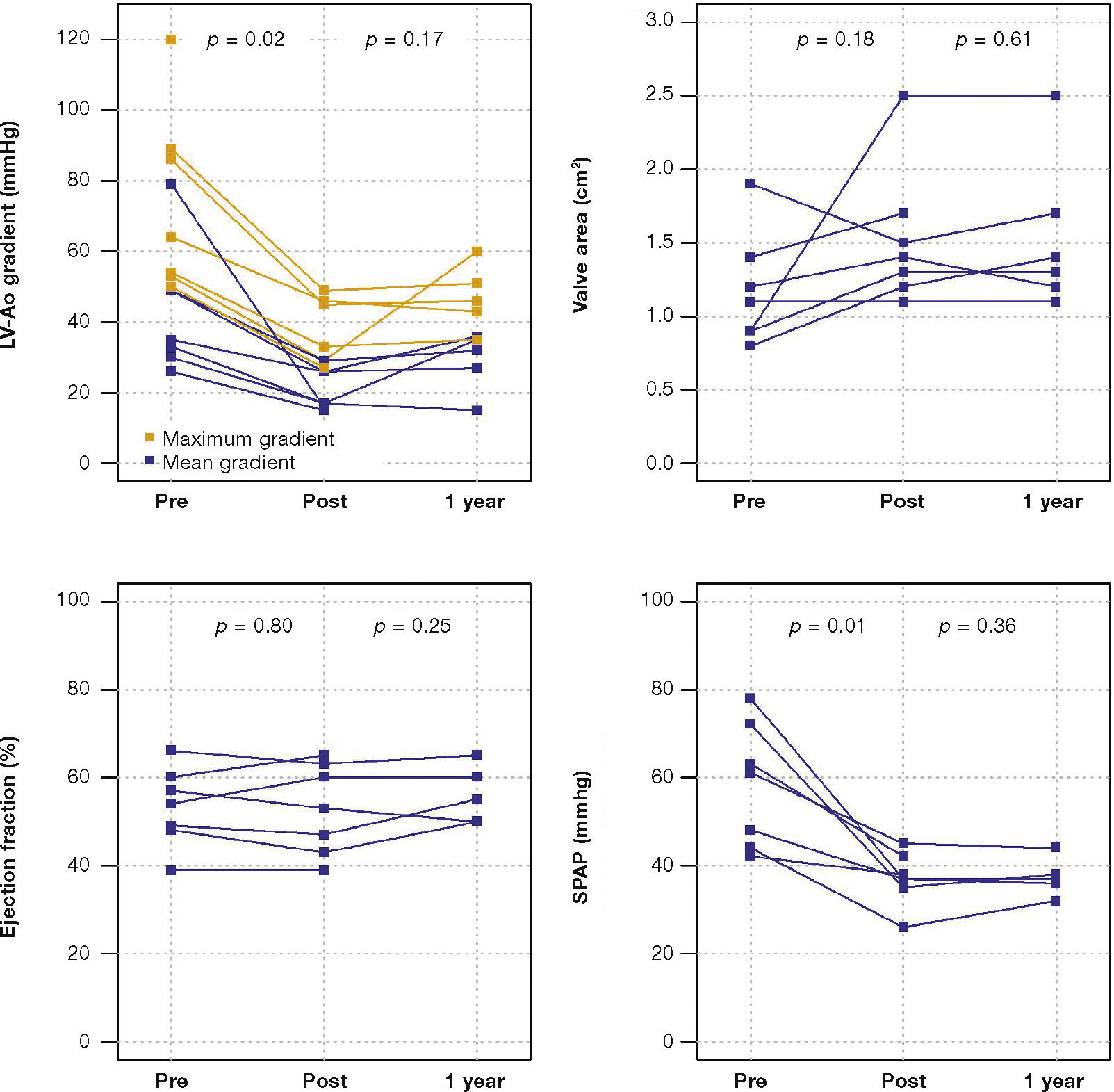
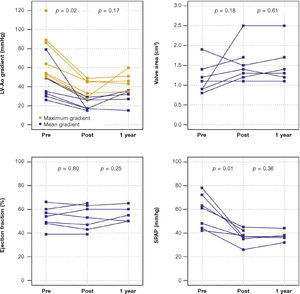
The maximum left ventricular/aortic gradient decreased from 73.7 ± 25.9mmHg to 38.2 ± 9.6mmHg (p = 0.01) and the mean gradient, from 38.2 ± 9.6mmHg to 20.9 ± 5.9mmHg (p = 0.02). There was a significant decrease in SPAP, from 58.3 ± 14.0mmHg to 37.1 ± 6.0mmHg (p = 0.01) and increase in valve area from 1.2cm2 ± 0.4 to 1.5 ± 0 5cm2 (p = 0.18) (Fig. 1).
Device success was achieved in six of the seven patients (85.7%). The first patient in the series had moderate patient-prosthesis mismatch, with the indexed effective orifice area of 0.67cm2/m2, moderate aortic regurgitation, moderate paravalvular regurgitation, and need for a second prosthesis immediately after the procedure. During hospitalization, the patient developed cardiogenic shock, acute kidney injury (AKIN stage 3) requiring hemodialysis, and respiratory failure, and died 48hours after the procedure.
Two patients had left bundle branch block after the procedure, without the need for permanent pacemaker implantation. In-hospital recovery of six patients was uneventful, and all were discharged on the seventh day after the procedure.
Clinical follow-upAt the 30-day follow-up, early safety outcome was achieved in 85.7% of patients.
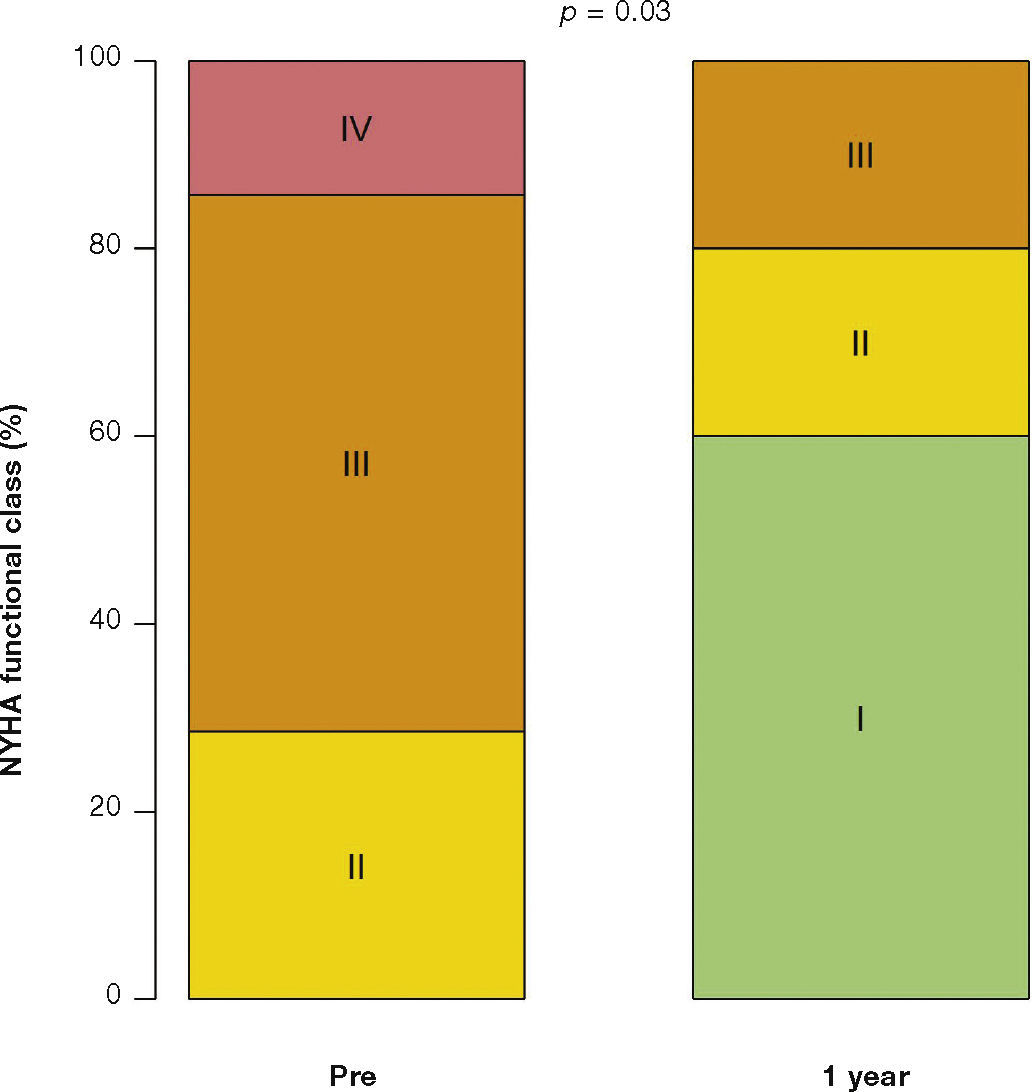
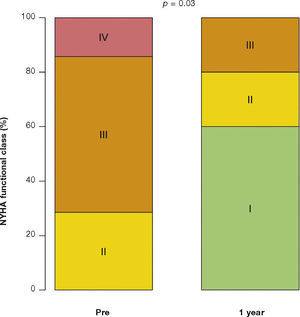
After a mean follow-up of 585 days (288-776 days), only one patient had not yet reached one year post-procedure follow-up. Up to 12 months, there was no need for re-hospitalization, and 80% of patients were in NYHA functional class I or II (p = 0.03), thus reaching the outcome of clinical efficacy. No significant differences were found in transvalvular gradients, in valve area, in left ventricular ejection fraction, or in SPAP, when compared to immediate results: the mean left ventricular-aortic gradient was 29.0 ± 8.6mmHg (p = 0.17); the prosthetic valve area was 1.5 ± 0.5cm2 (p = 0.61); left ventricular ejection fraction was 56.0 ± 6.5% (p = 0.25); and SPAP was 37.4 ± 4.3mmHg (p = 0.36) (Fig. 2). No signs of structural dysfunction or hemodynamic deterioration were identified in any case.
DiscussionThis study described the initial experience of a multidisciplinary group with transcatheter VIV implantation for the treatment of surgical bioprosthesis dysfunction in aortic position in high-risk surgical patients. Data were obtained in a clinical practice setting and confirmed their technical feasibility, providing the treated individuals with clinical and hemodynamic benefits in the first 30 days, which were maintained at the end of 1 year.
Initially described by Wenaweser et al.,18 the VIV implantation has been applied to patients worldwide and its clinical outcomes have been analyzed in several series9,19–22 and multicenter studies.11,14 In these registries, the mean age was 78 years, and surgical risk estimates by logistic EuroSCORE and STS score were 31.3% and 11.3%, respectively. This clinical profile is similar to that of the present cohort, also comprised of patients considered at high surgical risk (logistic EuroSCORE > 20% and STS score > 8%).
In the largest previously published registry, the VIVID (Valve In Valve International Data) study, with 429 patients, the success rate of the procedure was 93.1%. Within 1 month, 7.6% of the patients had died, and 92.6% of surviving patients had NYHA class I/II. The survival rate was 83.2% at 12 months.14 In the present series, the success of the procedure has resulted in significant clinical improvement, with most patients (80%) in NYHA functional class I/II after the first year.
In general, the clinical success of percutaneous transcatheter aortic valve implantation (TAVI) is fundamentally related to the baseline characteristics of the treated patients, to the technique employed, and to the devices used. Accumulated knowledge plays an important role to improve its results. In recent years, in particular, important concepts have been applied to VIV procedures. In this series, the first treated patient developed moderate mismatch and paraprosthetic regurgitation, potentially associated with the size of the transcatheter prosthesis used and its position. This patient had an adverse outcome, with cardiogenic shock after the procedure. At that stage of the learning curve, characteristics currently recognized as fundamental for VIV procedures had not yet been assessed or described.
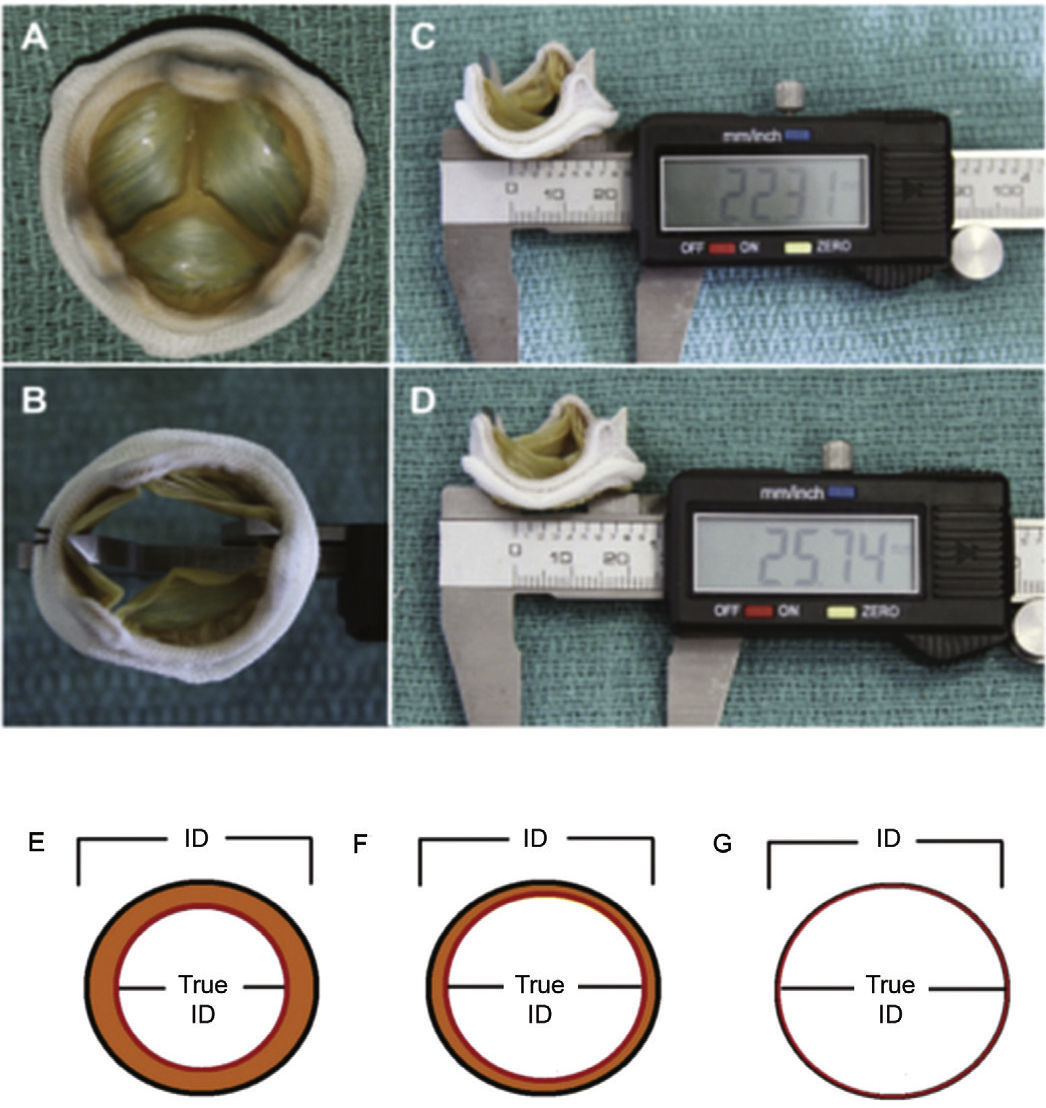
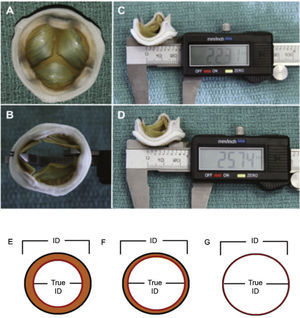
Therefore, important aspects such as model, design, and size of the surgical bioprosthesis with dysfunction should be appraised when choosing the size of the transcatheter prosthesis to be implanted. The assessment of the true internal diameter of the surgical prosthesis is one of the most important factors in this scenario – understanding as true diameter the diameter of the inner annulus of the bioprosthesis, minus the space occupied by leaflets (porcine or bovine pericardium) (Fig. 3).
(A) Surgical bioprosthesis with porcine leaflets. (B) Measurement of the bioprosthesis internal diameter using a caliper. (C) Subtle distortion of the valve caused by the caliper, which can lead to incorrect measurements. First measurement: 22.31mm. (D) Second measurement: 25.74mm, showing the effect caused by the distortion. (E) Porcine valves: true internal diameter (ID) is at least 2mm less than the stent ID. (F) Pericardial valves with leaflets sutured inside the stent frame: true ID is at least 1mm less than the stent ID. (G) Pericardial valves with leaflets sutured outside the stent frame: true ID is the same as the stent ID.
Adapted from Bapat et al.23
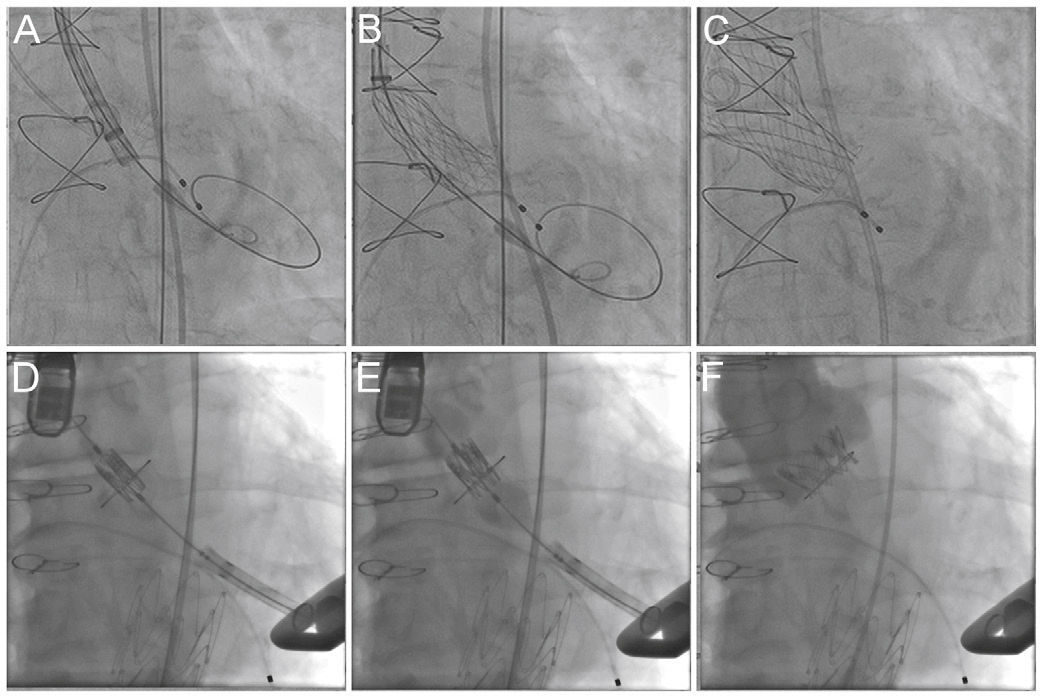
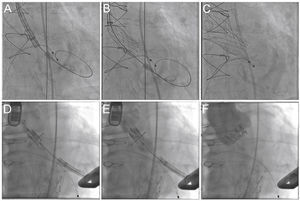
The knowledge of the fluoroscopic aspect of a particular surgical prosthesis defines the optimum position for the transcatheter bioprosthesis implantation (usually 15% below the suture annulus for the Sapien XT and from zero to 4mm for the Core Valve prosthesis)24 (Fig. 4). Currently, the smartphone application developed in 2013 by Bapat15 allows access to the necessary structural information of several surgical prostheses, assisting in the most appropriate choice of size and positioning of the VIV prosthesis, thus reducing the possibility of mismatch and aortic regurgitation.
Valve-in-valve procedure using CoreValve (A-C) and Sapien XT (D-F) prostheses. (A) The bioprosthesis radiopaque suture annulus is viewed through fluoroscopy and the adequate angulation is verified before the procedure and the landing zone. (B) Release of the self-expanding CoreValve prosthesis, slowly and gradually, and control by fluoroscopy. (C) Final prosthesis position. (D) Positioning of the balloon-expandable Sapien XT prosthesis. (E) Release under fluoroscopy and result after the implantation (F).
In this series, the mean transvalvular gradient after the VIV procedure was 20.9 ± 5.9mmHg, and 42% of patients had a mean gradient ≥ 20mmHg. These values are higher than those commonly observed after TAVI in native valve stenosis (≤ 10mmHg). Studies show that the occurrence of severe mismatch (< 0.65cm2/m2) after aortic VIV is 32.1%. Higher transvalvular gradients are, therefore, somewhat expected in these procedures: the VIV prosthesis is implanted in a non-distensible structure (in this case, the surgical prosthesis annulus), resulting in a smaller effective flow area.9,25 Some patients also have mismatch and high mean baseline gradient after surgical valve replacement (especially with bioprosthesis < 23mm). This fact also contributes to the observation of higher gradients after VIV.14,26 In the current series, however, even patients with a mean gradient > 20mmHg after VIV had significant improvement in symptoms.
Due to the small sample size, there was no statistically significant difference between the mean gradient obtained after VIV with self-expandable vs. balloon-expandable prosthesis. In the series by Dvir et al., both the stenosis mode of bioprosthesis failure and the balloon-expandable devices (in comparison with self-expandable devices) were associated with higher post-procedure transvalvular gradients.14
In this study, no complications such as stroke or the need for permanent pacemaker were observed. In fact, evidence shows that the VIV procedure does not increase the risk of conduction disorders, of need for pacemaker, and of stroke, when compared to the treatment of native valve stenosis.27 The paravalvular regurgitation rates are substantially lower when compared to TAVI.9
There were no cases of aortic failure, progressive increase in valvular gradient after implantation, or evidence of leaflet degeneration on the echocardiographic serial evaluations.
ConclusionsIn this initial study, the valve-in-valve procedure was clinically effective, resulting in immediate favorable results in patients with aortic bioprosthesis dysfunction and high surgical risk. The clinical and hemodynamic benefits were maintained in the midterm follow-up. Studies with more patients and longer-term follow-up are still required to consolidate its indication, especially in individuals with lower surgical risk.
Funding sourceNone declared.
Conflicts of interestDr. Dimytri A. Siqueira is proctor for Edwards, Medtronic, and Symetis. Dr. Alexandre Abizaid is a proctor for Edwards, Medtronic, and Symetis. Dr. Magaly Arrais is a proctor for Edwards and Medtronic. The other authors declare no conflicts of interest related to this manuscript.
AknowledgmentsTo the Coronary Angioplasty Section team of Centro de Pesquisa Clínica: Renata Viana, Patricia Stein and Mayra Mika. Mr. Reginaldo Barreto, for the availability, organization, and assistance in collecting database information from the Invasive Cardiology Service of Instituto Dante Pazzanese de Cardiologia database.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.