Several studies were performed to define predictors of adverse events after percutaneous coronary intervention (PCI). Patients whose post-procedure myocardial fractional flow of reserve (FFR) is <0.90 have an incidence of major cardiac events at 6 months at least 3 times higher than those whose FFR is≥0.90. The aim of this study was to identify clinical, angiographic and procedure-related factors associated with a post-PCI FFR <0.90.
MethodsOne hundred and ninety-three patients (256 lesions) treated between 2004 and 2005 were included, and FFR was measured before and after PCI in all of the treated vessels. Patients were divided into groups with FFR <0.90 and FFR≥0.90. Logistic regression multivariate analysis was used to determine the adjusted odds ratio (OR).
ResultsFFR was measured in all lesions. No difference was observed in clinical parameters between groups. There were differences in angiographic and procedure-related parameters, however, when the logistic regression model was used, the only variable associated to post-PCI FFR <0.90 was the treatment of the left anterior descending artery (OR, 12.1; 95% CI, 6.4-22.9; P<0.01).
ConclusionsThe only predictor of a FFR <0.90 after PCI was the treatment of the left anterior descending artery.
Utilização do Fluxo Fracionado de Reserva do Miocárdio paraIdentificar Variáveis Preditoras de Pior Prognóstico Após Intervenções Coronárias Percutâneas
IntroduçãoVários estudos foram realizados para definir preditores de eventos adversos pós-intervenção coronária percutânea (ICP). Pacientes cujo fluxo fracionado de reserva do miocárdio (FFR) pós-procedimento é <0,90 apresentam índice de eventos cardíacos adversos maiores em 6 meses pelo menos 3 vezes maior do que aqueles cujo FFR é≥0,90. Este estudo teve por objetivo identificar fatores clínicos, angiográficos e do procedimento associados à FFR pós-ICP <0,90.
MétodosForam incluídos 193 pacientes (256 lesões) tratados entre 2004 e 2005, e o FFR foi medido antes e depois da ICP em todos os vasos tratados. Os pacientes foram divididos nos grupos FFR <0,90 e FFR≥0,90. Análise multivariada por regressão logística foi utilizada para determinar as razões de chances (odds ratio−OR) ajustadas.
ResultadosFoi possível obter o FFR em todas as lesões. Não se observou diferença nos parâmetros clínicos entre os dois grupos de pacientes. Houve diferença em alguns parâmetros angiográficos e do procedimento, porém, ao aplicarmos o modelo de regressão logística, a única variável que se associou com FFR pós-ICP <0,90 foi o tratamento da artéria descendente anterior (OR=12,1; IC 95% 6,4-22,9; P <0,01).
ConclusõesA única variável preditora de FFR pós-ICP <0,90 foi o tratamento da artéria descendente anterior.
It is known that the most important prognostic factor in patients with coronary artery disease (CAD) is the presence of myocardial ischemia.1,2 Conversely, the value of the myocardial fractional flow reserve (FFR) in myocardial ischemia detection has been broadly established.3−5 Vessels with FFR≥0.80 can be safely treated conservatively, while FFR <0.80 is a signal of myocardial ischemia and thus, sometimes percutaneous or surgical revascularization is indicated.6−9
A multicenter registry published in 2002 showed that the post-percutaneous coronary intervention (PCI) value of FFR has important prognostic implications.10 Patients who’s post-procedure FFR was <0.90 showed an at least three-fold higher rate of major adverse cardiac events after six months than those with FFR≥0.90.
The aim of this study was to analyze the predictive variables of a post-procedure FFR <0.90 in patients undergoing PCI in an interventional cardiology service, thus identifying factors that could lead to an unfavorable clinical outcome at the long-term follow up.
METHODSThe study sample consisted of patients referred to the Interventional Cardiology Service of Clínica Santa Helena, Cabo Frio, RJ, Brazil, from October 2004 to April 2005, for elective PCI. Patients with chronic vessel occlusion or left main coronary artery lesion≥50% were excluded from the analysis.
Cardiac catheterization and intracoronary blood pressure measuresCatheterization was performed via femoral artery, using 6F or 7F guide catheters with no side holes. Before the angiography, 10,000 IU of intravenous heparin and 0.5mg of nitroglycerin were administered via intracoronary route. Subsequently, intracoronary pressure measurements were performed in all vessels with stenosis≥50% by visual estimation, using a 0.014” guide wire (PressureWire® 4 Sensor; RADI Medical Systems – Uppsala, Sweden) placed on the distal bed of each coronary to be analyzed, one at a time.11 Intravenous adenosine was administered through a sheath placed in the femoral vein, at a dose of 140mg/kg/min, in order to induce maximal hyperemia. FFR was automatically determined as the ratio between the mean distal coronary pressure and mean aortic pressure, measured by the guide catheter during maximal hyperemia.3 All stenoses responsible for ischemia (FFR <0.75 cutoff value used before the FAME study) were treated by PCI, when technically possible. After the procedure, FFR was measured again, and lesions and patients were divided into two groups: FFR≥0.90 (85 patients/107 lesions) and FFR <0.90 (108 patients/149 lesions). In cases where the patient had more of one treated lesion, and one of them showed post-procedure FFR <0.90 and the other(s) FFR≥0.90, the patient was allocated to the FFR < 0.90 group.
Quantitative coronary angiographyQuantitative coronary angiography was performed offline, considering the projection according to which it would be possible to demonstrate more severe lesion, using a software with automatic contour detection algorithm (CAAS II – Pie Medical Imaging; Maastricht, the Netherlands), as previously described.12
Statistical AnalysisAll variables were tested for normality by the Shapiro-Wilks and/or Kolmogorov-Smirnov test. In the descriptive analysis, numerical variables were shown as mean±standard deviation or median and interquartile range [IQ], and categorical variables as numbers (n) and percentages (%). Chi-squared or Fisher’s exact tests were used for categorical variables; the unpaired Student’s t-test and Wilcoxon-Mann–Whitney test were used for continuous variables. All variables with significant difference between the groups (P<0.05) were used for the multivariate logistic regression analysis to determine independent predictors of FFR <0.90 after the procedure. Statistical analysis was performed using the R software, release 3.0.1 (R Core Team – Vienna, Austria). P-values <0.05 were considered statistically significant, and all tests were two-tailed.
RESULTSFor seven consecutive months, 284 patients were admitted to the service to undergo PCI with or without stenting. Of these, nine had myocardial infarction, 25 had chronic occlusion of culprit vessel, and 57 had lesions whose pre-PCI FFR was > 0.75 and thus, were excluded from this analysis. Therefore, 193 patients (256 lesions) were included in this study. Diagnostic coronary angiography had been performed in all patients two days to three weeks before. The clinical characteristics of these patients are shown in Table 1. The mean age was 61.6±10.5years, 34.7 % were females, and 24.4 % were diabetics. Most patients had stable angina (71.5%), multivessel disease (71%), and preserved left ventricular ejection fraction (59±15%). No statistically significant differences were observed between the clinical characteristics of two groups analyzed.
Baseline clinical characteristics
| Clinical characteristics | Groups | P-value | ||
|---|---|---|---|---|
| Patients(n=193) | Post-PCI FFR<0.90(n=108) | Post-PCI FFR>0.90(n=85) | ||
| Age, years | 61.6±10.5 | 60.8±10 | 62.6±11.1 | 0.24 |
| Female gender | 67 (34.7) | 33 (30.6) | 34 (40.0) | 0.22 |
| Symptoms, n (%) | 0.30 | |||
| Stable angina | 138 (71.5) | 82 (75.9) | 56 (65.9) | |
| Silent ischemia | 39 (20.2) | 19 (17.6) | 20 (23.5) | |
| Unstable angina | 16 (8.3) | 7 (6.5) | 9 (10.6) | |
| Risk factors, n (%) | ||||
| Arterial hypertension | 162 (83.9) | 90 (83.3) | 72 (84.7) | 0.85 |
| Dyslipidemia | 90 (46.6) | 52 (48.1) | 38 (44.7) | 0.66 |
| FH of CAD | 74 (38.3) | 47 (43.5) | 27 (31.8) | 0.10 |
| Diabetes | 47 (24.4) | 27 (25) | 20 (23.5) | 0.87 |
| Smoking | 43 (22.3) | 24 (22.2) | 19 (22.4) | > 0.99 |
| Previous AMI, n (%) | 92 (47.7) | 50 (46.3) | 42 (49.4) | 0.77 |
| Previous PCI, n (%) | 31 (16.1) | 19 (17.6) | 12 (14.1) | 0.56 |
| Previous CABG, n (%) | 6 (3.1) | 4 (4.7) | 2 (1.9) | 0.41 |
| LVEF, n (%) | 59±15 | 56.9±15.3 | 60.7±14.6 | 0.08 |
| Multivessel disease, n (%) | 137 (71) | 79 (73.1) | 58 (68.2) | 0.52 |
FFR=fractional flow reserve of the myocardium; PCI=percutaneous coronary intervention; FH-CAD=family history of coronary artery disease; AMI=acute myocardial infarction; CABG=coronary artery bypass graft surgery; LVEF=left ventricular ejection fraction.
FFR measurement was successfully obtained in all analyzed lesions. PCI with stenting was performed in 238 stenoses of 179 patients, with 100% of procedural success. In 18 stenoses (14 patients), PCI was performed with a balloon catheter, also with angiographic and clinical success in all cases.
Angiographic and procedural characteristics of the studied lesionsThe angiographic characteristics of the lesions are shown in Table 2. The left anterior descending (LAD) artery was the most often treated vessel in the group with FFR <0.90. The patients in this group had smaller reference vessel diameters (2.61 [2.29 to 2.93] mm vs. 2.84 [2.49 to 3.11] mm, P<0.01), larger pre minimum luminal diameters (0.89 [0.7 to 1.14] mm vs. 0.80 [0.63 to 1] mm, P=0.02) and the same extent of lesion (14.0 [9.7 to 20.1] mm vs. 15.1 [10.3 to 20.2] mm, P=0.41).
Angiographic and procedure characteristics (analysis by lesion)
| Clinical characteristics | Groups | P-value | ||
|---|---|---|---|---|
| Lesions(n=256) | Post-PCI FFR<0.90(n=149) | Post-PCI FFR>0.90(n=107) | ||
| Coronary artery, n (%) | <0.01 | |||
| Left descending artery | 140 (54.7) | 117 (78.5) | 23 (21.5) | |
| Right coronary artery | 61 (23.8) | 13 (8.7) | 48 (44.9) | |
| Left circumflex artery | 55 (21.5) | 19 (12.8) | 36 (33.6) | |
| ACC/AHA B2 or C, n (%) | 127 (49.6) | 69 (46.3) | 58 (54.2) | 0.25 |
| Calcified lesions, n (%) | 117 (45.7) | 75 (50.3) | 42 (39.3) | 0.10 |
| Number of stents per lesion | 1.02±0.39 | 0.97±0.43 | 1.08±0.34 | 0.02 |
| Mean stent diameter, median [IQ], mm | 3 [2.5-3.5] | 2.75 [2.5-3] | 3.0 [2.75-3.5] | <0.01 |
| Pre-RD, median [IQ], mm | 2.7 [2.35-2.99] | 2.61 [2.29-2.93] | 2.84 [2.49-3.11] | <0.01 |
| Pre-MLD, median [IQ], mm | 0.83 [0.66-1.10] | 0.89 [0.7-1.14] | 0.80 [0.63-1] | 0.02 |
| Pre-stenosis diameter, median [IQ], mm | 68 [60-75] | 66 [58-73] | 70 [64.5-78] | <0.01 |
| Lesion extension, median [IQ], mm | 14.5 [10.1-20.2] | 14.0 [9.7-20.1] | 15.1 [10.3-20.2] | 0.41 |
| Pre-FFR, median [IQ], mm | 0.60 [0.47-0.69] | 0.61 [0.49-0.69] | 0.57 [0.46-0.69] | 0.63 |
| Post-RD, mm | 3.0±0.49 | 2.9±0.50 | 3.14±0.46 | <0.01 |
| Post-MLD, mm | 2.63±0.50 | 2.52±0.52 | 2.78±0.43 | <0.01 |
| Post-stenosis diameter, median [IQ], mm | 12 [9-16] | 12 [8-14.5] | 12 [9-16] | 0.10 |
| Post-PCI FFR, median [IQ], mm | 0.88 [0.82-0.93] | 0.84 [0.80-0.86] | 0.95 [0.92-0.97] | <0.01 |
FFR=fractional flow reserve of the myocardium; PCI=percutaneous coronary intervention; RD=reference diameter; MLD=minimuluminal diameter.
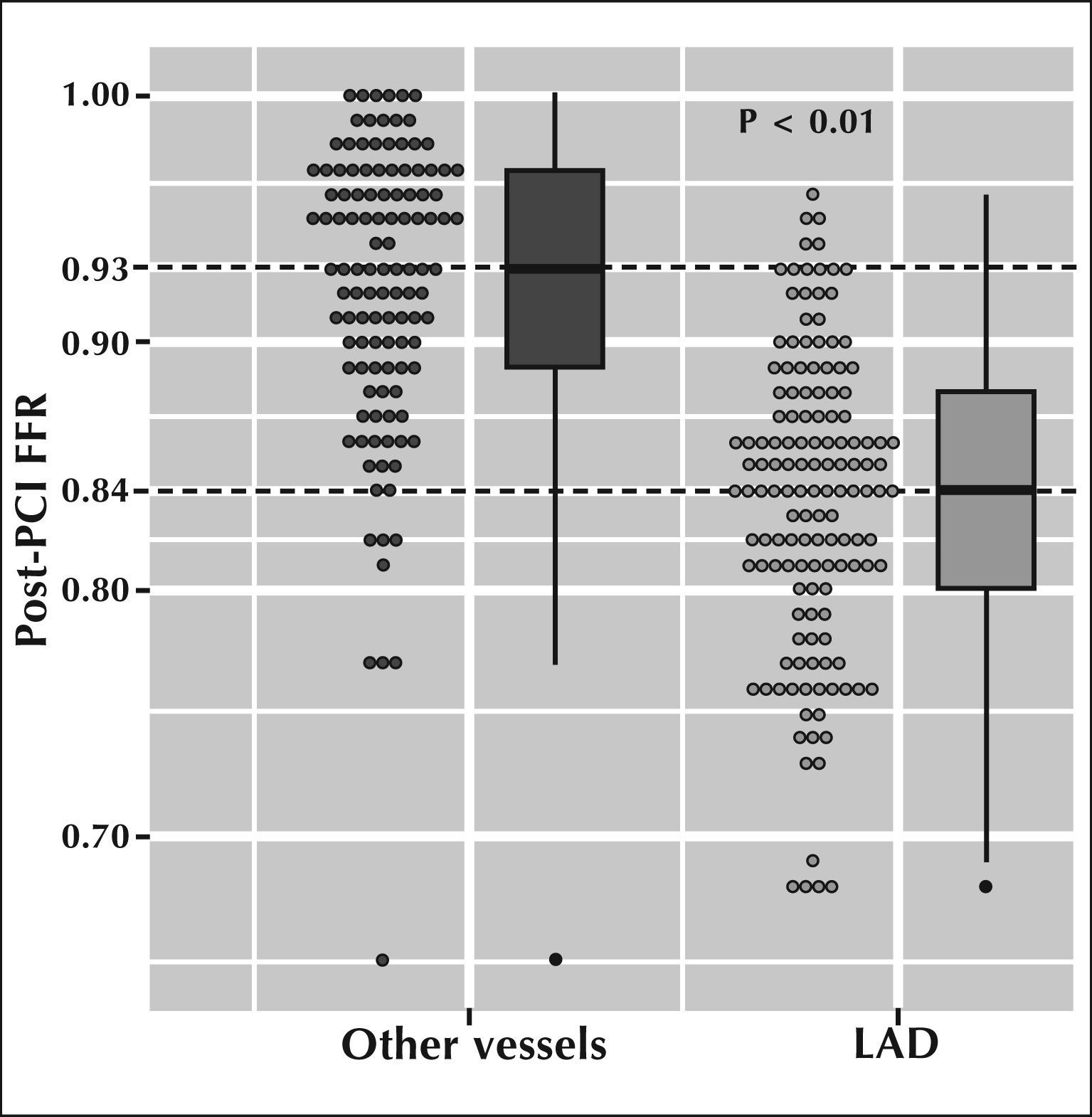
The functional significance of the lesion, measured by pre-PCI FFR, was the same in both groups (0.61 [0.49 to 0.69] vs. 0.57 [0.46 to 0.69], P=0.63). Figure 1 presents the median [IQ] FFR after PCI when the treated vessel was the LAD, as compared with other vessels. It can be clearly observed that the median values of FFR after PCI in the LAD were significantly lower than those measured in other vessels (0.84 vs. 0.93; P<0.01).
– Distribution of fractional flow reserve infarction (FFR) after stent implantation, when treating the left anterior descending artery (LAD) vs. other vessels. It is observed that the median FFR after stent implantation in ADA is significantly lower than in other coronary arteries (0.84 vs. 0.93; P<0.01).
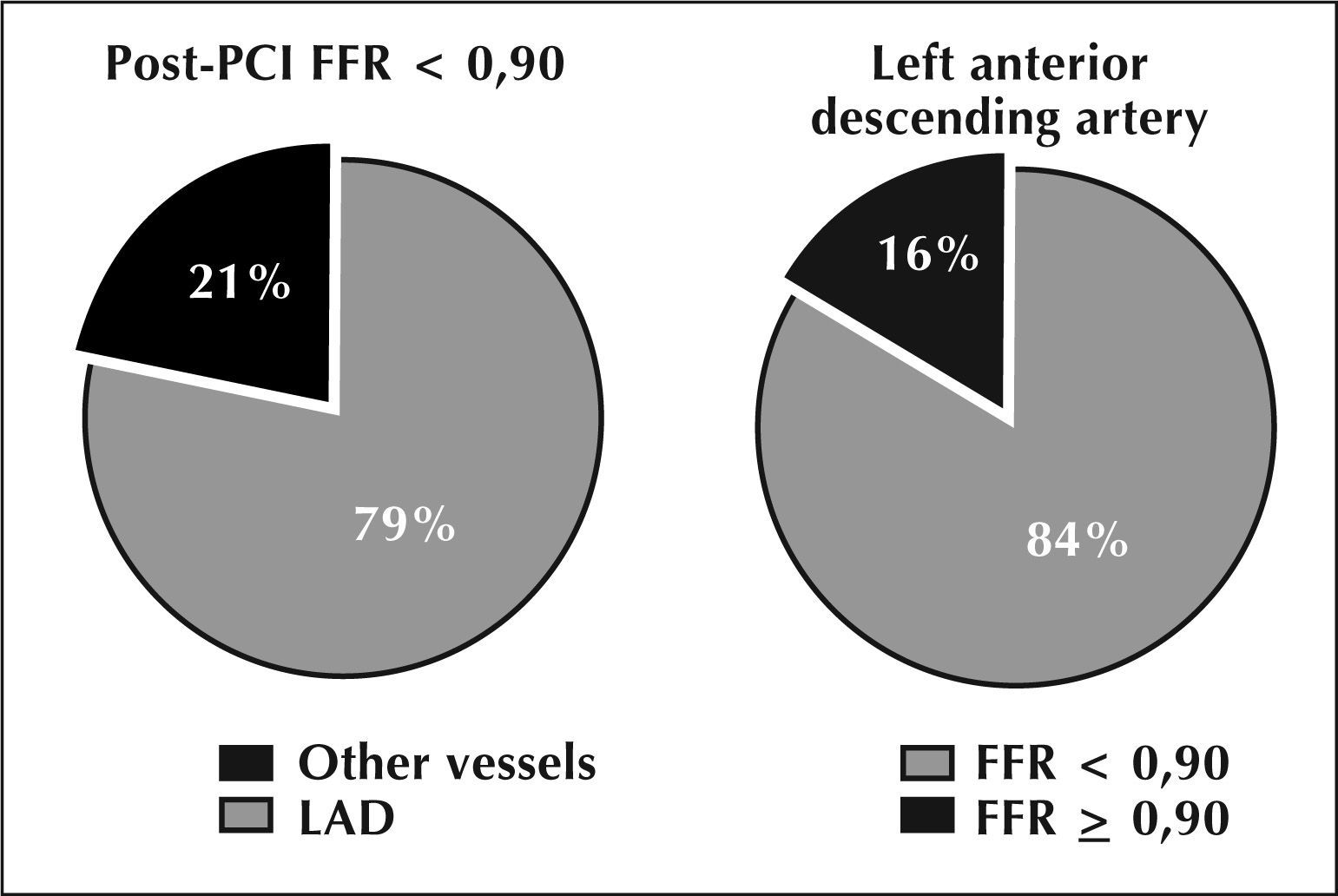
When the variables whose statistical tests showed significant differences were inserted between the two groups in the multivariate logistic regression analysis model, it was observed that only treatment of LAD showed to be an independent predictor of FFR <0.90 after PCI (Table 3 and Figure 2). The treatment of this vessel showed a 12-fold increased risk to obtain post-PCI FFR <0.90 compared to the other coronary arteries (OR=11.9, 95% CI: 6.4 to 23.2, P <0.01). Regarding the vessels which, after treatment, had FFR <0.90, in approximately 79% of all cases the lesion was located in the LAD artery; conversely, of all lesions treated in the LAD, 84% produced post-PCI FFR <0.90 (Figure 3), and in over 75% of cases this FFR was due to the presence of diffuse atherosclerotic disease found along the vessel, according to the results obtained by the guide wire withdrawal curve after PCI. Moreover, in 23% of lesions treated in the LAD (33/140 lesions), the post-PCI FFR was <0.80, thus, this vessel still showed signs of myocardial ischemia despite the fact that the angiographically visible lesions had already been approached by PCI.
Odds ratio (OR) for flow fractional reserve of the myocardium post-percutaneous coronary intervention (PCI) <0.90 with a 95% confidence interval (95% CI)
| OR | 95% CI | P-value | |
|---|---|---|---|
| Left anterior descending artery | 11.9 | 6.4-23.2 | <0.01 |
| Number of stents per lesion | 0.08 | 0.3-2.3 | 0.69 |
| Mean stent diameter | 0,7 | 0.3-1.4 | 0.32 |
| Pre-PCI RD | 0.3 | 0.03-2.9 | 0.33 |
| Pre-PCI MLD | 15.8 | 0.08-329 | 0.30 |
| pre-PCI % stenosis | 57.2 | 0.08-752 | 0.56 |
| Post-PCI RD | 0.7 | 0.07-7.8 | 0.81 |
| Post-PCI MLD | 0.8 | 0.08-8.4 | 0.89 |
RD=reference diameter; MLD=minimum luminal diameter.
– Odds ratio (OR) determined by multivariate logistic regression analysis. Observe that only the treatment of the left anterior descending artery (LAD) is a significant independent predictor of a post-percutaneous coronary intervention (PCI) fractional flow reserve (FFR) <0.90 (OR=12.1, 95% CI: 6.5 to 23.4, P<0.01).
MLD=minimum lumen diameter; RD=reference diameter.
The results of this study confirm that treatment of the LAD is an important predictor of post-percutaneous coronary intervention FFR <0.90, which may have prognostic significance. The other clinical and angiographic variables analyzed were not independent predictors after the multivariate analysis.
A multicenter study coordinated by Pijls et al.,10 involving 750 elective patients treated with PCI with baremetal stent (BMS) and followed for six months showed an overall event major adverse cardiac event (MACE) rate of 10.2%. However, when the patients were divided according with the post-stent FFR, it was observed that those whose FFR was≥0.90 had MACE rates of only 6.2%, whereas in those with post-stent FFR <0.90, the MACE rate was 20.3 % (P<0.001). In 6% of patients, post-stent FFR was <0.80 and, in these patients, the rate of events was as high as 29.5%. In other words, the higher the FFR after stent implantation, the lower the rate of late-onset events and vice-versa. A less than ideal post-stent FFR can be briefly explained in three ways: (1) suboptimal stent implantation not detected by angiography, which could be recognized and corrected with the use of intracoronary ultrasonography; (2) the presence of a persistent gradient along the segment treated by stenting indicates abnormal flow pattern in that place, with low and heterogeneous shear stress, which may favor the process of restenosis despite the good anatomical result;13 and (3) FFR measurement after PCI not only indicates abnormalities in the treated area, but also throughout the remainder of the vessel.14 Although not detectable by angiography or even by ultrasound, diffuse disease may be responsible for the higher rate of events in the FFR <0.90 group.
The present study raises the possibility that a suboptimal FFR after PCI (<0.90) is a consequence of the approach of vessels with diffuse atherosclerotic disease, and indicates LAD as the most common site of occurrence of this abnormal physiological pattern. Unfortunately, the angiographic appearance of the vessel does not always allow for the inference of the presence of this disease, as previously demonstrated.14,15
The advent of drug-eluting stents used in the FAME 1 and 2 studies8,9 decreased the importance of FFR measurement after PCI, as the rate of late-onset adverse cardiac events with the use of these stents is very low. Still, no data are available regarding FFR after drug-eluting stent (DES) implantation and its impact on clinical outcomes of treated patients, comparing those in whom FFR normalized to those who still showed some residual ischemia. Additionally, DES use is not a reality in Brazil, in which the majority of patients submitted to PCI receive bare-metal stents, the only type subsidized by the Brazilian Unified Health System (Sistema Único de Saúde – SUS). Records from the Hospital Information System of SUS (SIH/SUS)16 demonstrate that in 2008, approximately 47,000 hospitalizations occurred through SUS for percutaneous coronary intervention, and that this number has tended to increase.
In this context, the present study is of great importance, as the measurement of FFR after PCI was able to identify patients in whom the physiological post-PCI outcome was not adequate and which should be clinically monitored more closely, with the use of the available therapy arsenal aiming to minimize the risk of late events.
There are some limitations to this study: first, it was a retrospective, nonrandomized study, and patient clinical follow-up was not performed. However, the purpose of the study was to identify variables predictive of myocardial ischemia post-PCI, and not to evaluate the clinical outcome of these patients. Thus, this study is more apt to generate hypotheses to be investigated than to draw clinical conclusions and recommend conduct. Secondly, DES were not used in the treated population, which decreases, as previously mentioned, the importance of physiological assessment after PCI. As 90% of PCI procedures performed in Brazil use BMS, the authors believe that the present study adequately reflects, in this respect, the clinical practice of this institution.
CONCLUSIONSThis study showed that the only clinical or angiographic variable that can adequately predict myocardial FFR after stent implantation of the treated vessel. Treatment of the left anterior descending artery was associated with a post-PCI myocardial FFR <0.90 in most cases, which could result in an unfavorable clinical evolution in this group of patients; this finding should be adequately investigated in future clinical trials.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.