Traumatic brain injury (TBI) is one of the most common neurological disorders at the present time. The consequences are so devastating that up to 39% of the patients die from trauma and 60% of the survivors will have cognitive and/or motor deficits.
ObjectiveTo analyse the current evidence on the management of severe traumatic brain injury and the clinical outcome achieved with the use of hypertonic sodium lactate.
MethodologyA search of the scientific literature was conducted in the EMBASE, PubMed/Medline, OVID and Science Direct databases with the aim of preparing a reflection article, using the words “traumatic brain injury”, “hypertonic sodium lactate”, “metabolism in brain injury”, “management of traumatic brain injury”, focusing on the potential benefits of hypertonic sodium lactate, regardless of the date of publication.
ResultsThe use of hypertonic sodium lactate has been shown to have a successful impact on the dismal prognosis of TBI, modulating intracranial hypertension and cerebral oxidative metabolic dysfunction. This has been proven in vitro, in animal models, and in humans.
ConclusionEfforts to find better clinical outcomes in patients with TBI have confirmed the need for new management alternatives supported by the understanding of the pathophysiology. Given its multiple modulating endocrine-metabolic effects on secondary injury, lactate has been found to be a promising therapy in the management of TBI.
El trauma cráneo-encefálico (TEC) es uno de los desórdenes neurológicos más comunes actualmente, presenta consecuencias tan devastadoras, que el 39% mueren a causa del trauma. De los sobrevivientes, el 60% tendrán déficit en las competencias cognitivas y/o motoras.
ObjetivoAnalizar a través de la evidencia actual, el manejo del trauma cráneo-encefálico severo y el desenlace clínico logrado con el uso del lactato sódico hipertónico.
MetodologíaSe realizó una búsqueda en bases de datos de literatura científica como EMBASE, Pubmed/Medline, OVID, Science Direct para elaborar un artículo de reflexión, usando las palabras “traumatic brain injury” “hypertonic sodium lactate” “metabolism in brain injury” “management of traumatic brain injury” haciendo hincapié en los beneficios potenciales del lactato sódico hipertónico, sin tener en cuenta la fecha de publicación.
ResultadosEl lactato sódico hipertónico ha demostrado impactar de forma exitosa el pronóstico sombrío del TEC, modulando la hipertensión endocraneana y disfunción metabólica oxidativa cerebral, lo cual se ha demostrado en modelos in vitro, animales y humanos.
ConclusiónLos esfuerzos por mejorar los desenlaces clínicos han llevado a buscar nuevas alternativas del manejo del TEC, derivadas del entendimiento de la fisiopatología, de allí surge el lactato como terapia prometedora en el manejo del TEC, dado sus múltiples efectos endocrino-metabólicos moduladores de la injuria secundaria.
Traumatic brain injury (TBI) is a heterogenous condition in terms of aetiology, severity and outcomes, and it is one of the most common neurological disorders in this day and age.1 According to WHO forecasts, TBI and motor vehicle accidents will be the third cause of morbidity in the world by 2020.2 This review of the literature focuses on severe TBI, defined as the “action produced by external mechanical forces leading to gross tissue damage,”3 with a score of 3–8 on the Glasgow coma scale.4
With an incidence ranging between 108 and 332 new hospital admissions for every 100,000 inhabitants per year, severe TBI is a global problem.3
Despite medical and pharmacological treatment, average mortality in TBI is 39%5 and “approximately 60% of those who survive end up with cognitive and/or motor impairment.”6 In Colombia there are no updated data coming from multiple centres focusing on this problem.
Because of the impact on morbidity and mortality, new management options have been explored, including the use of hypertonic sodium lactate as a means to improve short and long-term outcomes.7–9 The aim of this article is to describe the benefits of hypertonic sodium lactate as a therapy in head injury, and to explore the feasibility of it becoming the best management option.
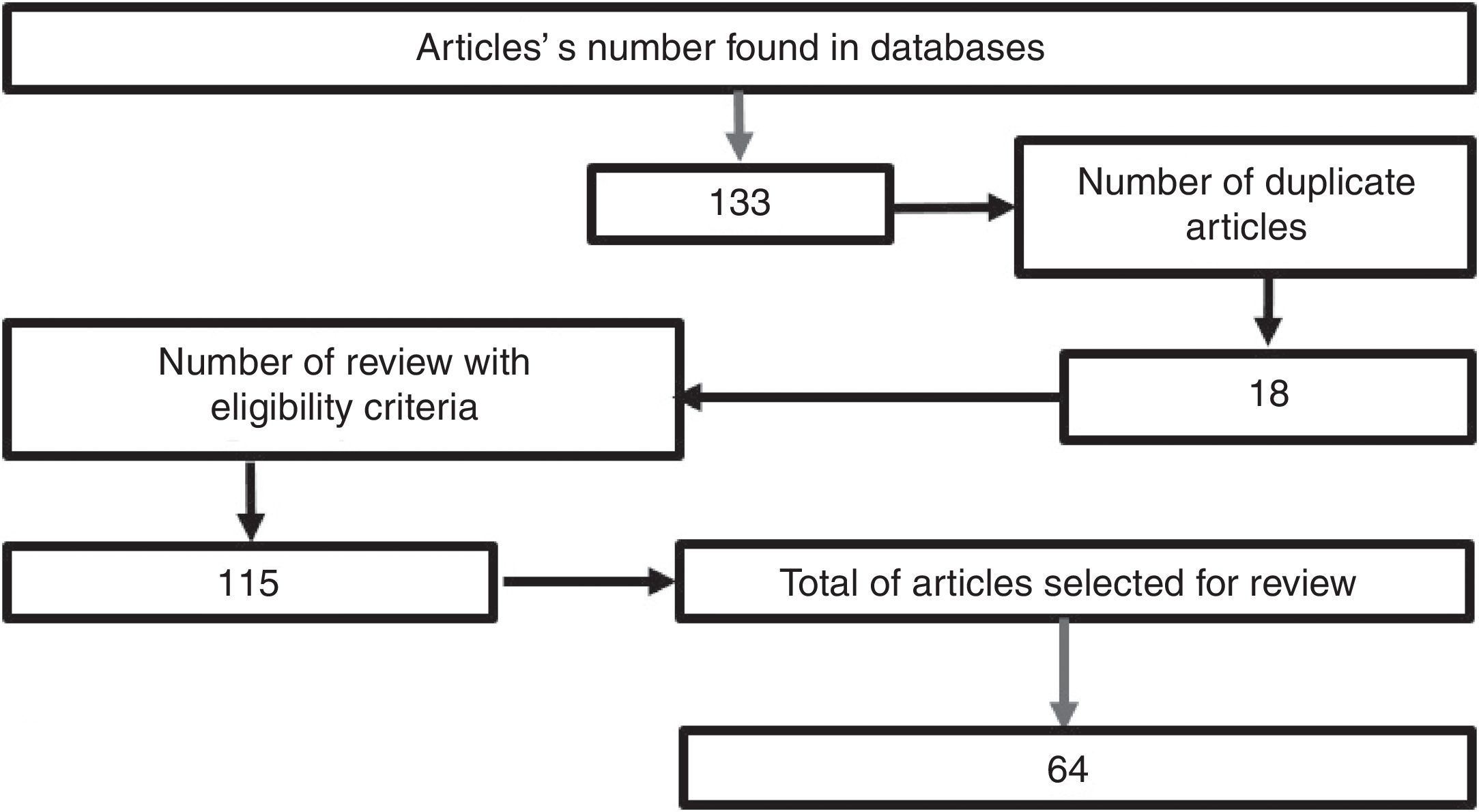
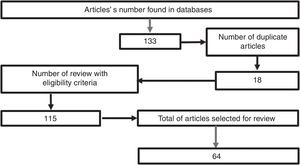
MethodologyA search was conducted in the scientific literature, including articles published in Pubmed/Medline, Science Direct, EMBASE, and OVID, using the words “hypertonic sodium lactate”, “traumatic brain injury”, “management of traumatic brain injury”, “metabolism in brain injury”, using hypertonic sodium lactate as the reference term for all the searches. No date limit for publication was considered. The selected articles were published in the English language, except those related to the local epidemiology, which are in Spanish. A total of 133 papers were found in the reference databases and, of these, 18 were duplicated. An assessment was then made of 115 articles, leading to the selection of 65 relevant documents, including systematic reviews, meta-analyses, clinical trials, and reviews of the literature with the aim of elucidating the pathophysiology following head injury, clarifying the goal of pharmacological therapy in TBI, and determining the outcomes obtained with the use of hypertonic sodium lactate in patients with head injury (Fig. 1).
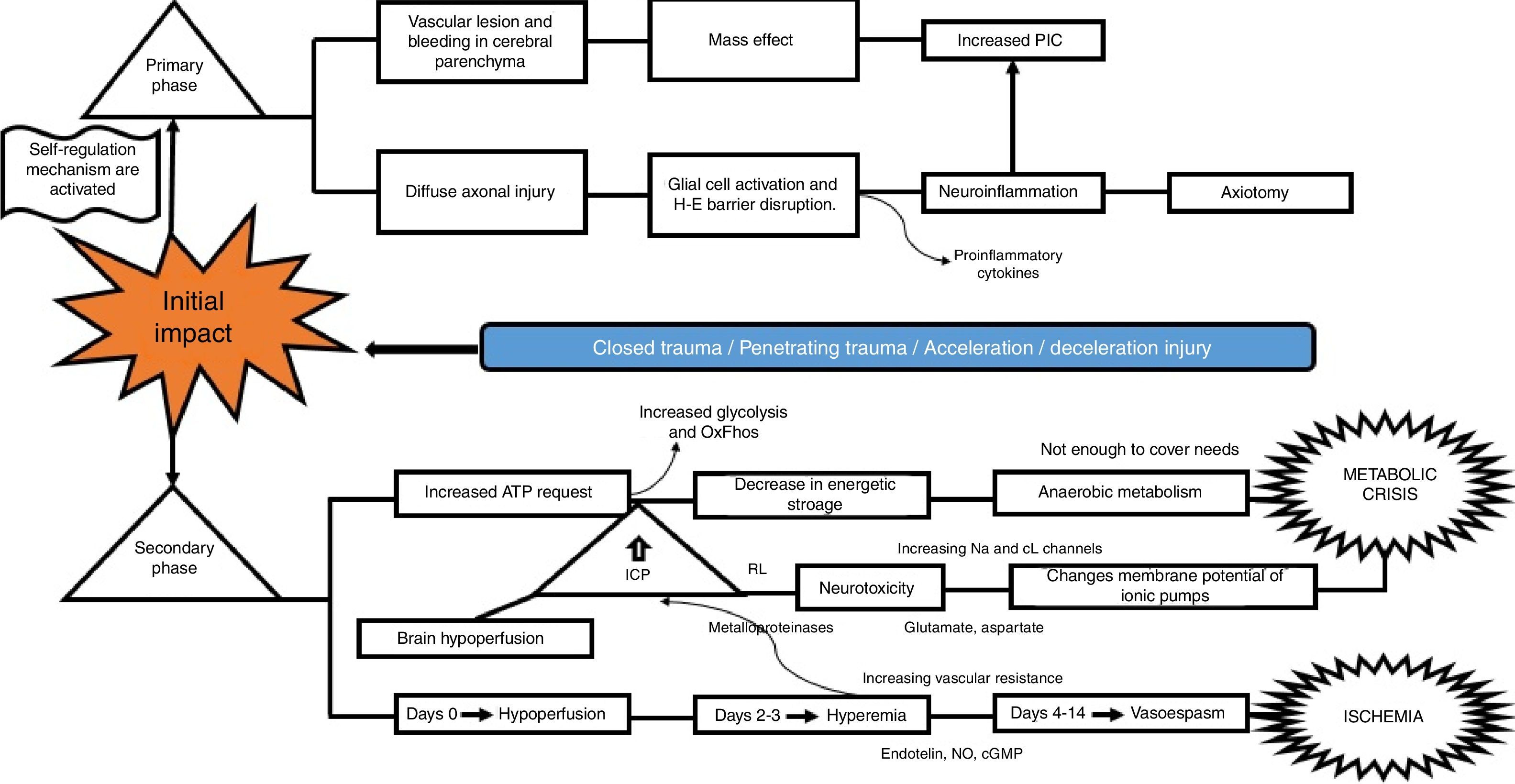
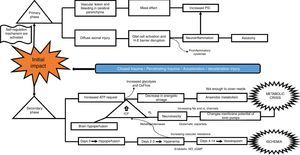
What is the pathophysiological mechanism of damage in head injury?Insults from TBI are divided into two main phases, depending on the time course following trauma. The primary phase is the immediate insult associated with the mechanical impact, consisting of vascular trauma and cerebral parenchymal bleeding with subsequent neuronal damage due to mass effect, and increase in intracranial pressure (ICP). Neuronal injury leads to axonal degeneration due to cytoskeleton disruption. This results in diffuse axonal damage and disconnection, activation of quiescent glial cells, and massive neuroinflammation, both axonal as well as of the brain parenchyma. This process causes glial activation and migration of pro-inflammatory cytokines as a result of blood-brain barrier disruption, and results in an imbalance of neurotransmitter homeostasis, axonal degeneration and cell death10–12 (Fig. 2).
The secondary phase is characterised by disrupted homeostasis of various intrinsic mechanisms of cerebral autoregulation.13 Energy failure is one of the main abnormalities that ensues following TBI as a result of the high requirement of energy to meet the demands created by the trauma: at first, there is increase oxidative phosphorilation and glycolysis to compensate for the energy deficit until ATP reserves are depleted, at which point anaerobic metabolism takes over until it also becomes insufficient, leading to the depletion of energy reserves and resulting in metabolic stress and cell death14,15 (Fig. 3).
This serious metabolic failure leads to ion pump dysfunction as a result of increased permeability and membrane potential alteration. This causes ion and neurotransmitter redistribution, with massive pre-synaptic glutamate release and post-synaptic membrane ionic balance disruption,16 leading to greater neurotoxicity, development of cerebral oedema, increased intracranial pressure and cerebral hypoperfusion.17–20
Regarding blood homeostasis, 3 stages of changes are recognised: the first stage of “cerebral hypoperfusion” starts on the day of injury due to progressive accumulation of cellular oedema, leading to mild-to-moderate increase in intracranial pressure. This is followed by a “hyperemic” stage on days 2 and 3 that triggers major cellular oedema. During this stage, there is a sharp increase of intracranial pressure in 40% of the patients, and initiation of a stage of “vasospasm” that develops between 96h and 14 days after TBI, eventually leading to increased distal vascular resistance and narrowing of the microcirculation and subsequent ischaemia and worsening of the degree of injury.21
Given these considerations, what should be the focus of medical pharmacological management?According to the management guidelines for head injury, the cornerstone of management is the reduction of significant clinical repercussions of the secondary injury, including intracranial hypertension and cerebral hypoperfusion.22–24 Prognosis is dismal if these two medical conditions are not managed appropriately.25
One of the most important studies on the subject was the meta-analysis conducted by Stein et al.,26 which showed that patients in whom intracranial pressure was monitored had a 12% reduction in mortality and a 6% improvement in neurological prognosis compared to those who were not monitored. Farahvar et al. showed that therapy focused on ICP monitoring and reduction of intracranial hypertension episodes (defined as ICP increases over 25mmHg) helps reduce the risk of dying within the first 2 weeks after the event by 64%, compared to patients in whom ICP was not monitored, but who received treatment for lowering intracranial pressure.27
ICP greater than 20mmHg has been found to be the most important independent prognostic factor.28–30 As shown in a study with 846 cases that assessed Glasgow score, age, hypoxia, pupillary response, hyperthermia and increase in ICP, the clinical condition with the most consistent data regarding improved prognosis, less severe deficit and lower mortality was an ICP lower than 20mmHg.31 This confirms that the most important factor to address in order to achieve lower morbidity and mortality in patients with TBI is prevention of ICP elevation.32,33
What is the origin of the use of hypertonic lactate as a chemical mediator in the management of TBI?Multiple reviews have been conducted over the past few years with the aim of exploring other management options in head injury, finding a dichotomy between the use of crystalloids vs. colloids. When assessing overall mortality in the management with intravenous fluids of trauma and burned patients or in post-operative states, colloids have similar mortality to that of crystalloids.34 Moreover, it has also been found that the administration of synthetic colloids (gelatines, dextrans, hydroxyethyl starch) gives rise to coagulation homeostasis alteration, reduction of factors VII, VIII and Von Willebrand, and fibrinogen. This leads to altered erythrocyte and platelet aggregation, aside from different degrees of hypersensitivity reactions and renal function decline, greater in magnitude in people with pre-existing renal disease.35,36
Unlike hypertonic saline solution (HSS), hypo-osmolar and iso-osmolar solutions do not guarantee an osmotic effect.37 However, when comparing the use of HSS with other solutions (including mannitol, 0.9% saline solution, Ringer's lactate, hypertonic sodium and hypo-osmolar solutions) for the management of TBI, there were no differences in mortality rate or ICP control according to the meta-analysis performed by Pelletier et al.38 To date, there are no studies comparing HSS with hypertonic sodium lactate.
A study in humans showed that cerebral atrophy develops after head injury, mainly in the frontotemporal lobes. This finding was associated with attention deficit, and altered operational and psychomotor skills 12 months after TBI. Cortical atrophy is triggered by the energy-metabolic imbalance unleashed by head injury; those findings suggest that neuropsychological outcomes may improve in the short and medium term with the use of pharmacological therapy aimed at modulating damage.39,40
Based on the above, not only is there a need for the osmotic effect in order to avoid secondary damage caused by TBI, but also of a solution that can modulate damage.10,16,41,42 In this search for new management options for TBI, Pellerin and Magistretti introduced the concept of lactate shuttles in 1994,43 in what marks a milestone in the knowledge of lactate as a critical chemical compound for metabolism coordination in different tissues.44 This hypothesis prompted multiple authors to study the subject and assess the veracity of this postulate.
One of the findings that led to the study of this organic compound as a metabolic pathway was the understanding of TBI pathophysiology, because lactate diminishes the effects on metabolic-oxidative imbalance45–47 and attenuates the onset of the damage cascade.41 One of the great contributions of lactate in TBI is that it becomes a dynamic energy source as it acts as the main alternative to glucose during cellular metabolism stress in the brain.48
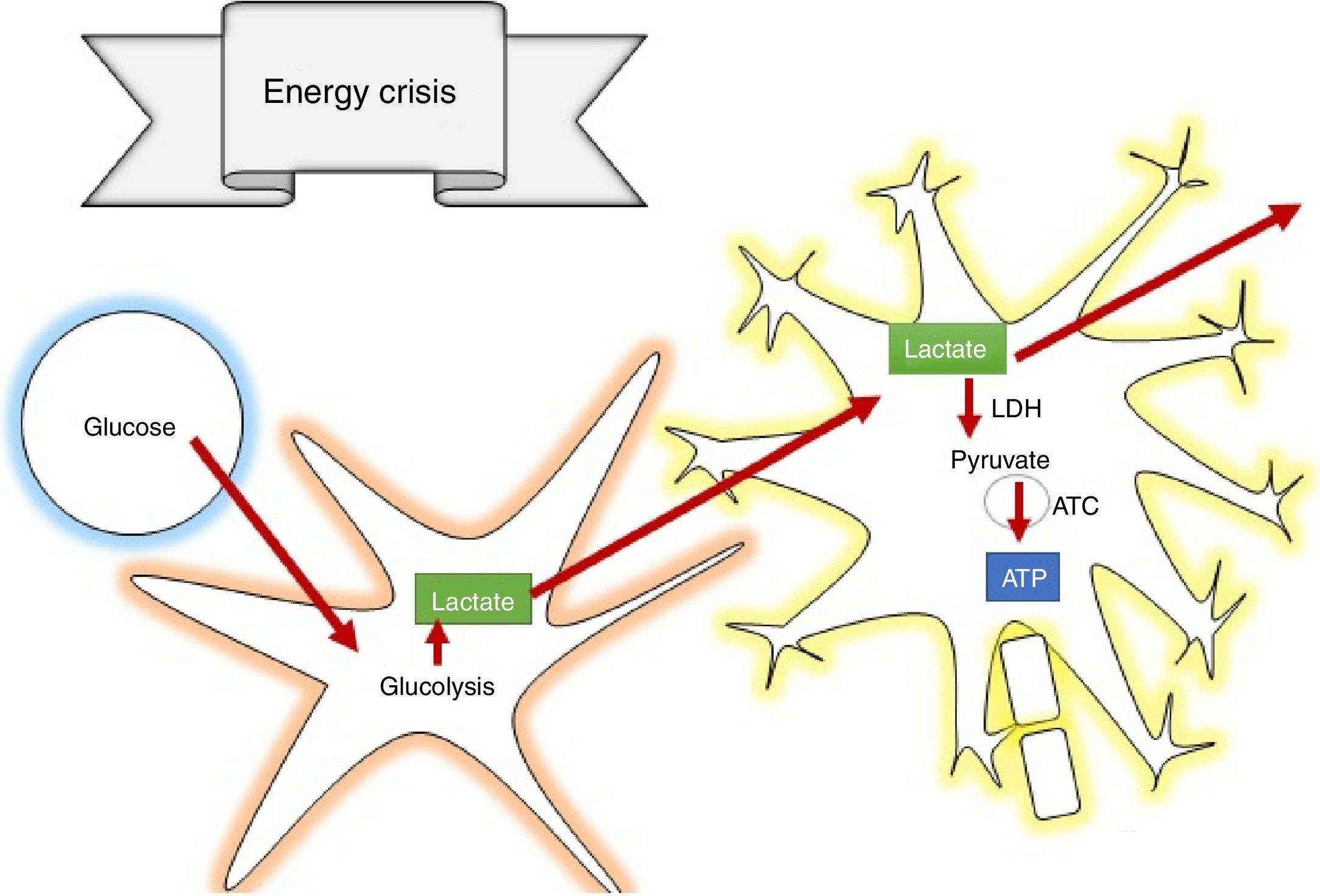
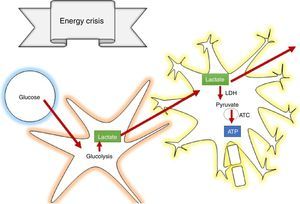
How does lactate act to prevent secondary brain injury?According to the astrocyte-neuron lactate shuttle (ANLSH) hypothesis, astrocytes use glycolysis to produce lactate which enters the neurons through monocarboxylate transporters where it is then transformed into pyruvate by the lactate dehydrogenase enzyme and enters into oxidative metabolism, producing energy.42 Lactate is, therefore, an essential precursor of anaerobic as well as aerobic metabolism.
One of the first attempts to assess the efficacy of lactate for the management of TBI was a trial in animal models that showed less cell death, less hemispheric atrophy, as well as smaller brain injury size and improved neurological outcomes in the animals treated with lactate.49–51
In the human brain, higher levels of lactate (within physiological range) resulted in a substantial reduction of catecholamine, growth hormone and cortisol concentrations. There was also a diminished symptomatic response to hypoglycaemia and a lower glucose level for the onset of hypoglycaemia52 when lactate was used as an alternative source of energy.
Lactate is oxidised in the brain, contributing 8% of the energy requirements53; in supraphysiologic conditions, it can contribute more that 60%,54 avoiding cerebral damage caused by low glucose levels and the increase in the size of cerebral penumbra.55 It was also determined that lactate acts by inhibiting glutamate-dependent excitatory pathways, expanding the neuroprotective effect in the damage cascade proposed in TBI pathophysiology.56
“Lactate transport from the astrocyte to the neuron is essential for synaptic plasticity and long-term memory, as well as for the attenuation of underlying molecular and synaptic changes.”57,58 For example, during periods of stress, the fall in O2, the increase in extracellular lactate and adenosine promote astrocyte-mediated vasodilation and improve long-term results.59,60 These benefits are not just hypothetical because it was shown in animal models treated with hypertonic lactate following TBI that there was significant improvement in cognitive skills and neuronal plasticity compared to the models that were managed with saline solution.61,62
Hypertonic sodium lactate also has a therapeutic potential as it “diminishes cerebral inflammation and restores the blood-brain barrier because it reduces endothelial activation and is equivalent to a metabolisable anion (causing chlorine outflow from the endothelial cells towards the vasculature). As a result of its hypertonic action, it produces neutral fluid balance, reducing the need for blood products and, consequently, leading to a lower risk of induced coagulopathy.”45
What has been the benefit of hypertonic sodium lactate in human studies?A multi-centre study involving 60 patients found that those treated with hypertonic sodium lactate for 48h had a lower number of intracranial pressure elevation episodes, compared to patients treated with 0.9% saline solution, with a rate of episodes of ICP elevation of 36% vs. 66%, respectively. It was also found that urinary output and fluid balance were better in the patients treated with hypertonic sodium lactate. This study did not find neurologic improvement six months after TBI,63 but it points to a promising effect of lactate in controlling the number of ICP rises following TBI, which in itself in an independent marker of dismal prognosis.
Another study involving 17 patients treated with hypertonic sodium lactate and 17 patients treated with mannitol staged ICP reduction using invasive techniques, and showed a larger, more prolonged reduction of −5.9±1 in intracranial pressure 4h after the initiation of intravenous fluids in patients treated with hypertonic sodium lactate, versus −3.2±0.9mmHg in the patients treated with mannitol.64 Another advantage of the use of hypertonic sodium lactate is that perfusion pressure is higher than in patients treated with mannitol.
An important finding regarding hypertonic sodium lactate is the dose-dependent therapeutic effect. This was evident in animal models in which the use of an excessively high dose created cell damage due to inflammation, while the use of lower doses led to cell damage modulation. However, further human studies are required in order to confirm the potential benefits identified so far in vitro and in animal models,65 considering that the only clinical trials in humans are those mentioned in this article.
ConclusionTraumatic brain injury being a significant public health issue, efforts at achieving better clinical outcomes have led to the search of new management alternatives based on the understanding of the pathophysiology. Hypertonic sodium lactate has emerged as a promising therapy in the management of TBI, as it has been shown to have a beneficial potential in terms of endocrine and metabolic modulation effects on secondary brain injury. Likewise, it has been shown to be effective for modulating cerebral vascular tone and acting as vasodilator, membrane stabiliser, and regulator of active transport, fluid balance and energy functional reserve, becoming an alternative carbon source. It has also been found to have beneficial effects on cognitive repercussions following TBI, indicating that it could be an effective therapy to improve short and long-term outcomes and impact morbidity and mortality. Larger scale human studies on the use of hypertonic sodium lactate are needed in order to confirm the promising results found in animals. Also needed are clinical trials comparing mortality and long-term neurological results with the use of hypertonic saline solution, considering that there are not studies comparing these two treatment models.
FundingThe authors did not receive any sponsorship for their work on this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Betancur-Calderón JM, Veronesi-Zuluaga LA, Castaño-Tobón HF. Terapia con lactato sódico hipertónico en trauma cráneo-encefálico ¿Se convertirá en la mejor alternativa de manejo? Rev Colomb Anestesiol. 2017;45:51–57.