Obstructive sleep apnea is an independent cardiovascular risk factor which can be diagnosed by means of a portable device (WatchPAT®) capable to perform automatic analysis of the peripheral artery tonometry, its amplitude and variability, with a high grade of correlation to polysomnography.
ObjectiveTo describe the use of WatchPAT® for diagnosing obstructive sleep apnea in patients with cardiovascular disease.
MethodsA case series study of patients evaluated in cardiovascular consultation who underwent to home sleep monitoring using the WatchPAT®200U system between February 1, 2017 to February 1, 2018, due to suspected obstructive sleep apnea.
Results37 patients were included, with an average age of 57.6 years, most of whom were male. Cardiovascular diseases were: uncontrolled or predominantly nocturnal hypertension (37.8%), recurrent palpitations and/or chronic fatigue (21.6%); sleep disturbances were informed only in 27% of the patients. Obstructive sleep apnea was identified in 97.3% of the patients (29.7% moderate and 56.8% severe), 37.8% had high blood pressure, 49.5% had established heart disease, and 18.9% were diabetic. Only, fifty percent of the patients with severe obstructive sleep apnea and 45% with moderate obstructive sleep apnea snored.
ConclusionCardiovascular disease specialists should participate more actively in the search and diagnosis of obstructive sleep apnea; it is highly prevalent in patients with nocturnal or resistant high blood pressure, heart rhythm disorders or myocardial structural damage. Obstructive sleep apnea can be diagnosed using portable devices such as WatchPAT®.
La apnea obstructiva del sueño es un factor independiente de riesgo cardiovascular. Para su diagnóstico existen dispositivos portátiles (WatchPAT®) que utilizan el análisis automático de la amplitud y variabilidad del tono arterial periférico logrando una alta correlación con la polisomnografía.
ObjetivoDescribir el uso del WatchPAT® para el diagnóstico de apnea obstructiva del sueño en pacientes con patología cardiovascular.
MétodosSerie de casos que incluyó pacientes valorados en consulta cardiovascular a quienes se les realizó monitorización del sueño en casa mediante WatchPAT®200U por sospecha de apnea obstructiva del sueño entre el 1° de febrero de 2017 y el 1° de febrero de 2018.
ResultadosSe incluyeron 37 pacientes, edad promedio 57,6 años, la mayoría hombres. Las enfermedades cardiovasculares principales fueron: hipertensión arterial no controlada o de predominio nocturno (37,8%), palpitaciones recurrentes y/o fatiga crónica (21,6%); se informaron alteraciones del sueño solo en 27% de los pacientes. Se identificó apnea obstructiva del sueño en 97,3% de los pacientes (29,7% moderado y 56,8% severo); 3,8% eran hipertensos, 45,9% tenían enfermedad cardíaca establecida y 18,9% eran diabéticos. Solo 50% de los pacientes con apnea obstructiva del sueño severa y 45% moderada roncaban.
ConclusiónLos especialistas en patología cardiovascular deben tener una participación más activa en la búsqueda y diagnóstico de apnea obstructiva del sueño, altamente prevalente en pacientes con hipertensión arterial nocturna o resistente, en trastornos del ritmo cardiaco o en aquellos con daño estructural del miocardio. Su diagnóstico puede realizarse con dispositivos portátiles como WatchPAT®.
Obstructive sleep apnea (OSA), characterized by partial (hypopnea) or complete (apnea) obstruction of the upper airways during sleep, is an independent cardiovascular risk factor which is highly prevalent in human beings.1–4 Approximately 24% of men and 9% of women experience OSA,5 with even higher estimated figures (14% to 55%) considering the impact of current pandemic obesity, increased longevity and improved technology used for its detection.6–10
Long-lasting apneic episodes induce homeostatic and cardiovascular disturbances with sympathetic discharge, hypoxemia, tachycardia and peripheral vasoconstriction. These phenomena, due to their repetitive and continual occurrence, can cause or aggravate conditions such as high blood pressure, heart failure, atrial fibrillation, coronary artery disease or type 2 diabetes mellitus.10
Polysomnography (PSG) is the gold standard for diagnosing sleep disorders, among which OSA is the most frequent.11 However, this test requires multiple cables and electrodes which gather information regarding respiratory and muscular movements, electroencephalographic and electrocardiographic signals, as well as a nasal cannula and pulse oximeter. As a consequence of this complexity, patient's movement is limited involuntarily forcing adoption of a supine position which is associated with a higher number of apneas. In addition, conditions under which the test is performed are quite different from the usual environment patient sleeps affecting his or her ability to get enough and good quality of sleep.12
Polysomnography requires a complex infrastructure; it can only be performed at few specialized centers by trained personnel, using sophisticated and costly devices. This situation together with high demand of the test led to diagnostic delay and health consequences for those affected.11
Within the new OSA diagnostic armamentarium there are portable devices based on automatic measurement of peripheral arterial tonometry (PAT) amplitude and variability analysis, which besides quantify heart rate and oxygen saturation changes. One of these is the WatchPAT®, which has an almost 90% correlation to PSG in respect to respiratory indices and sleep stages, as well as high sensitivity and specificity for the diagnosis of OSA.12–14 In addition, it is currently recommended and accepted by the American Academy of Sleep Medicine as an outpatient diagnostic test for OSA.11
This study intends to describe the first case series in Colombia of home sleep testing with the WatchPAT® device for diagnosing OSA in patients with cardiovascular diseases.
MethodsThis is a case series study which reviewed clinical charts of patients who underwent to home sleep testing using the WatchPAT® 200U system at a cardiovascular health center between February 1, 2017 and February 1, 2018. All subjects were seen in outpatient clinic due to cardiovascular conditions such as uncontrolled or predominantly nocturnal arterial hypertension, recurrent palpitations with or without documented cardiac arrhythmias (mainly atrial fibrillation), or having undergone radiofrequency ablation of atrial flutter and/or atrial fibrillation with recurrence or paroxysms.
Considering the close relationship between cardiovascular diseases and OSA, an interview was performed focusing on the signs and symptoms of this condition as described in the International Classification of Sleep Disorders.10 As part of OSA risk stratification, the STOP-BANG 15 and Epworth Sleepiness Scale 16 were systematically applied.
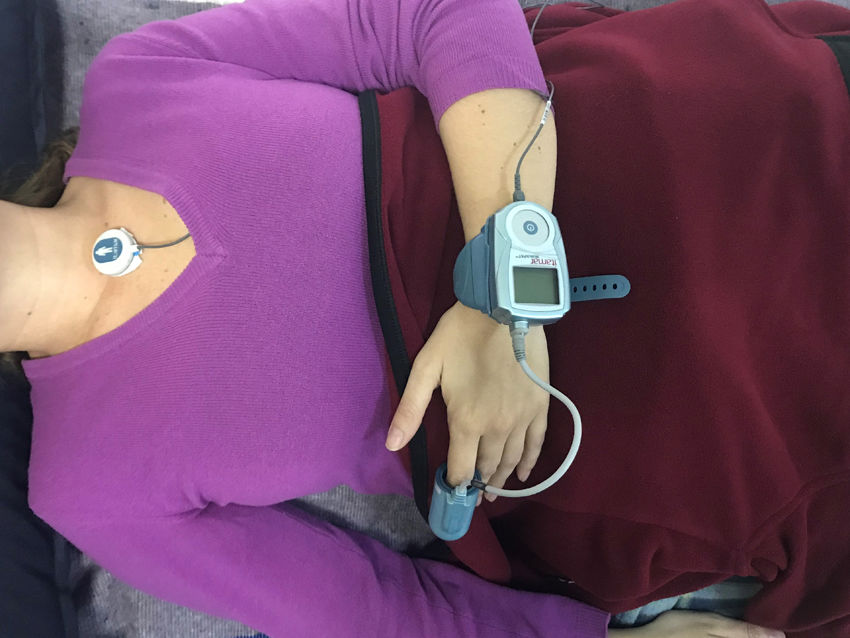
All of the patients had clinical characteristics suggestive of concomitant OSA, and therefore, following the clinical assessment, outpatient sleep monitoring was performed using the WatchPAT® system. This device, which is like a large wristwatch, is connected to a pneumo-optical probe placed on the index finger of the non-dominant hand, with an oxygen saturation (SatO2) sensor in its distal portion. WatchPAT® has a specific algorithm for evaluating dynamic changes in arterial volume. In addition, has a small electronic patch which has to be attached at the suprasternal notch to detect the level of snoring and the patients’ position during sleep. It also includes an actigraph (an activity and movement sensor) which, together with the PAT, discriminates between the various sleep and arousal stages, supplying the real sleep times (fig. 1).
The OSA diagnostic triad using the WatchPAT® system consists of: a) attenuation of the PAT signal, b) increased pulse frequency in the digital arteries, and c) oxygen desaturation. Together, these signs indicate activation of the sympathetic autonomic nervous system.
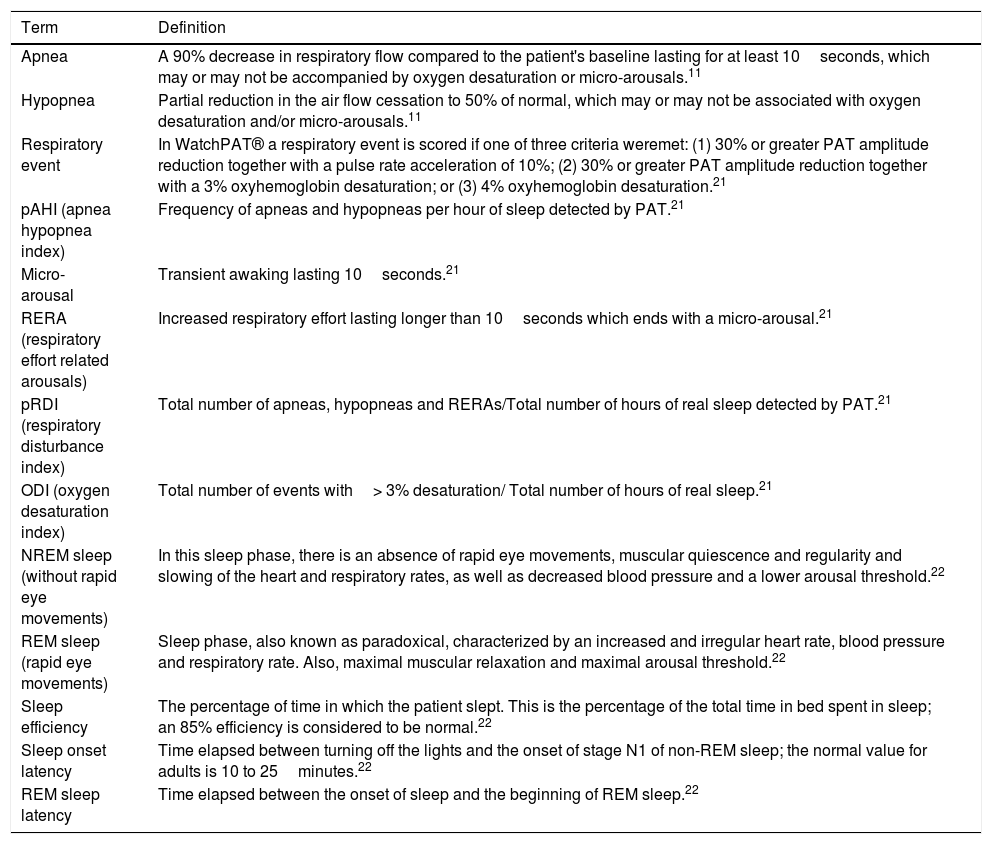
After sleep monitoring using WatchPAT®, according to sleep respiratory indices (see Table 1) obtained, it was determined whether the patients suffered from OSA and its degree of severity, based on the pAHI (Apnea Hypopnea Index), as follows: mild (from 5 to 15), moderate (from 15 to 30), and severe (greater than 30).
Definitions and concepts.
| Term | Definition |
|---|---|
| Apnea | A 90% decrease in respiratory flow compared to the patient's baseline lasting for at least 10seconds, which may or may not be accompanied by oxygen desaturation or micro-arousals.11 |
| Hypopnea | Partial reduction in the air flow cessation to 50% of normal, which may or may not be associated with oxygen desaturation and/or micro-arousals.11 |
| Respiratory event | In WatchPAT® a respiratory event is scored if one of three criteria weremet: (1) 30% or greater PAT amplitude reduction together with a pulse rate acceleration of 10%; (2) 30% or greater PAT amplitude reduction together with a 3% oxyhemoglobin desaturation; or (3) 4% oxyhemoglobin desaturation.21 |
| pAHI (apnea hypopnea index) | Frequency of apneas and hypopneas per hour of sleep detected by PAT.21 |
| Micro-arousal | Transient awaking lasting 10seconds.21 |
| RERA (respiratory effort related arousals) | Increased respiratory effort lasting longer than 10seconds which ends with a micro-arousal.21 |
| pRDI (respiratory disturbance index) | Total number of apneas, hypopneas and RERAs/Total number of hours of real sleep detected by PAT.21 |
| ODI (oxygen desaturation index) | Total number of events with> 3% desaturation/ Total number of hours of real sleep.21 |
| NREM sleep (without rapid eye movements) | In this sleep phase, there is an absence of rapid eye movements, muscular quiescence and regularity and slowing of the heart and respiratory rates, as well as decreased blood pressure and a lower arousal threshold.22 |
| REM sleep (rapid eye movements) | Sleep phase, also known as paradoxical, characterized by an increased and irregular heart rate, blood pressure and respiratory rate. Also, maximal muscular relaxation and maximal arousal threshold.22 |
| Sleep efficiency | The percentage of time in which the patient slept. This is the percentage of the total time in bed spent in sleep; an 85% efficiency is considered to be normal.22 |
| Sleep onset latency | Time elapsed between turning off the lights and the onset of stage N1 of non-REM sleep; the normal value for adults is 10 to 25minutes.22 |
| REM sleep latency | Time elapsed between the onset of sleep and the beginning of REM sleep.22 |
Descriptive statistics were provided as frequencies and proportions for categorical variables and as measures of central tendency and standard deviation or measures of position for numerical variables, according with their distribution. The statistical analysis was performed using SPSS Statistics 24.
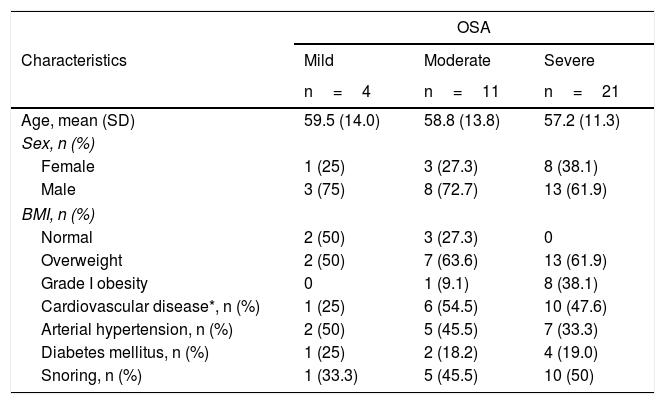
ResultsThirty-seven patients were included mostly males (64.9%). The average age was 57.6 (SD 12.1) years, with the youngest 27 and the oldest 80 years. In 37.8% (14 subjects), indications for home sleep monitoring with WatchPAT® besides suspicion of OSA based on symptoms and/or results of sleep questionnaires, were: uncontrolled or predominantly nocturnal arterial hypertension, and recurrent palpitations with or without documented cardiac arrhythmias. The study was ordered in 21.6% (8 subjects) due to chronic fatigue, and in 27% (10 subjects) due to sleep disturbances such as sleep onset insomnia and drowsiness; in only 8.1% (3 subjects) was it ordered due to snoring. When a focused interview was carried out regarding specific sleep apnea symptoms, 48.6% of the patients self-reported snoring. Approximately a quarter (24.3%) of the patients had grade I obesity and 59.5% were overweight, 37.8% were hypertensive, 45.9% had some type of established heart disease (arrhythmias, ischemic cardiomyopathy, dilated cardiomyopathy and coronary artery disease), being arrhythmia the most common and 18.9% were diabetics. There were no patients with chronic obstructive pulmonary disease. OSA was identified in 36 patients, corresponding to 97.3%, being moderate in 29.7% and severe in 56.8%. 72.7% of patients with moderate OSA and 100% of patients with severe OSA were overweight or had grade I obesity. A greater proportion of patients with a history of established cardiovascular disease (arrhythmias, cardiomyopathy and coronary artery disease) was also seen in these two groups. When questioned specifically about snoring, only half of the patients with severe OSA and 45% with moderate OSA reported having this symptom (Table 2).
Demographic and clinical characteristics.
| OSA | |||
|---|---|---|---|
| Characteristics | Mild | Moderate | Severe |
| n=4 | n=11 | n=21 | |
| Age, mean (SD) | 59.5 (14.0) | 58.8 (13.8) | 57.2 (11.3) |
| Sex, n (%) | |||
| Female | 1 (25) | 3 (27.3) | 8 (38.1) |
| Male | 3 (75) | 8 (72.7) | 13 (61.9) |
| BMI, n (%) | |||
| Normal | 2 (50) | 3 (27.3) | 0 |
| Overweight | 2 (50) | 7 (63.6) | 13 (61.9) |
| Grade I obesity | 0 | 1 (9.1) | 8 (38.1) |
| Cardiovascular disease*, n (%) | 1 (25) | 6 (54.5) | 10 (47.6) |
| Arterial hypertension, n (%) | 2 (50) | 5 (45.5) | 7 (33.3) |
| Diabetes mellitus, n (%) | 1 (25) | 2 (18.2) | 4 (19.0) |
| Snoring, n (%) | 1 (33.3) | 5 (45.5) | 10 (50) |
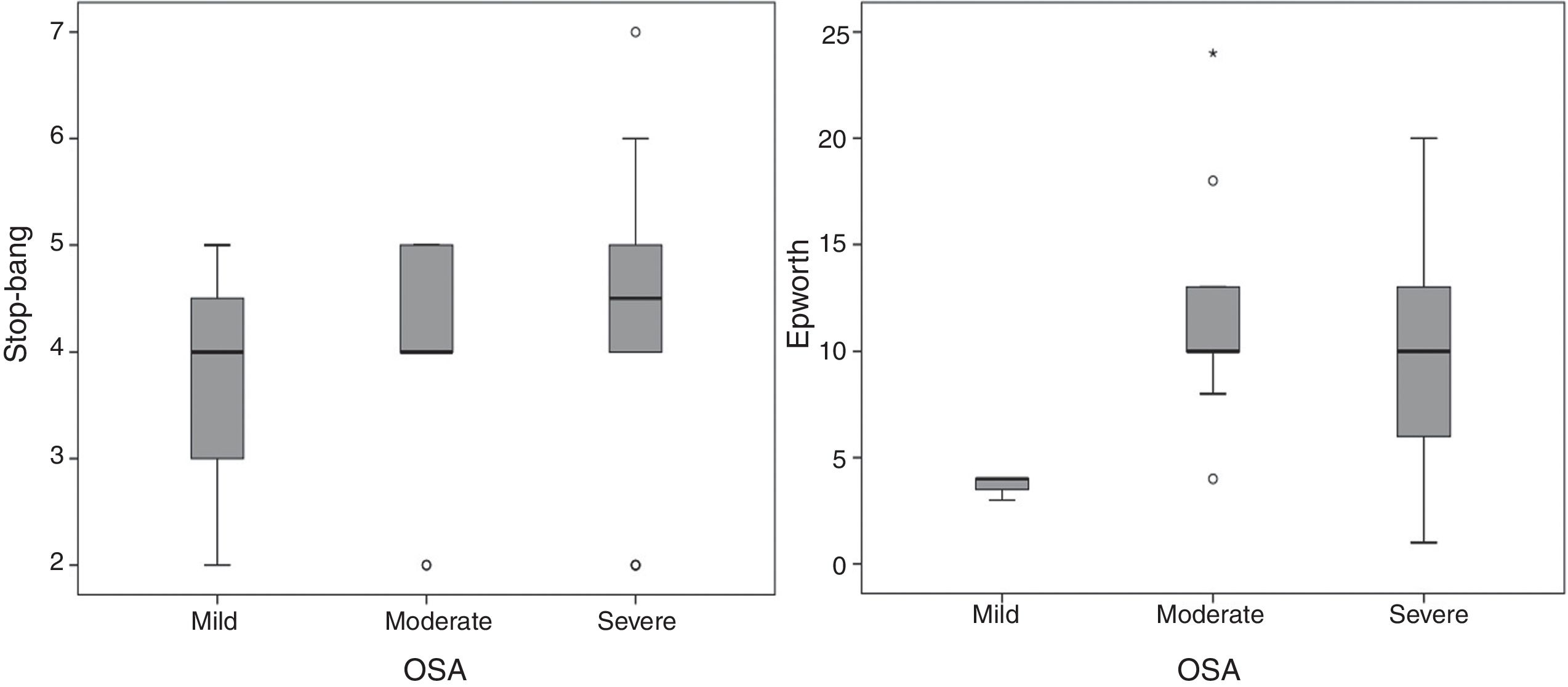
The application of Epworth sleepiness scale 16 revealed that 51.4% of the patients had mild daytime drowsiness and 20% had moderate or severe drowsiness. With regard to the risk of OSA, measured by the STOP-BANG questionnaire,15 50% of the patients were found to have a high risk of OSA and 29.4% a moderate risk. With regard to the applied questionnaires, the Epworth sleepiness scale 16 showed that patients with moderate and severe OSA had median scores between 9 and 10, compared with a score of 4 for mild OSA. On the STOP-BANG questionnaire 15 no differences were seen in the median, but there was a trend towards higher scores in a proportion of patients with moderate to severe OSA (fig. 2).
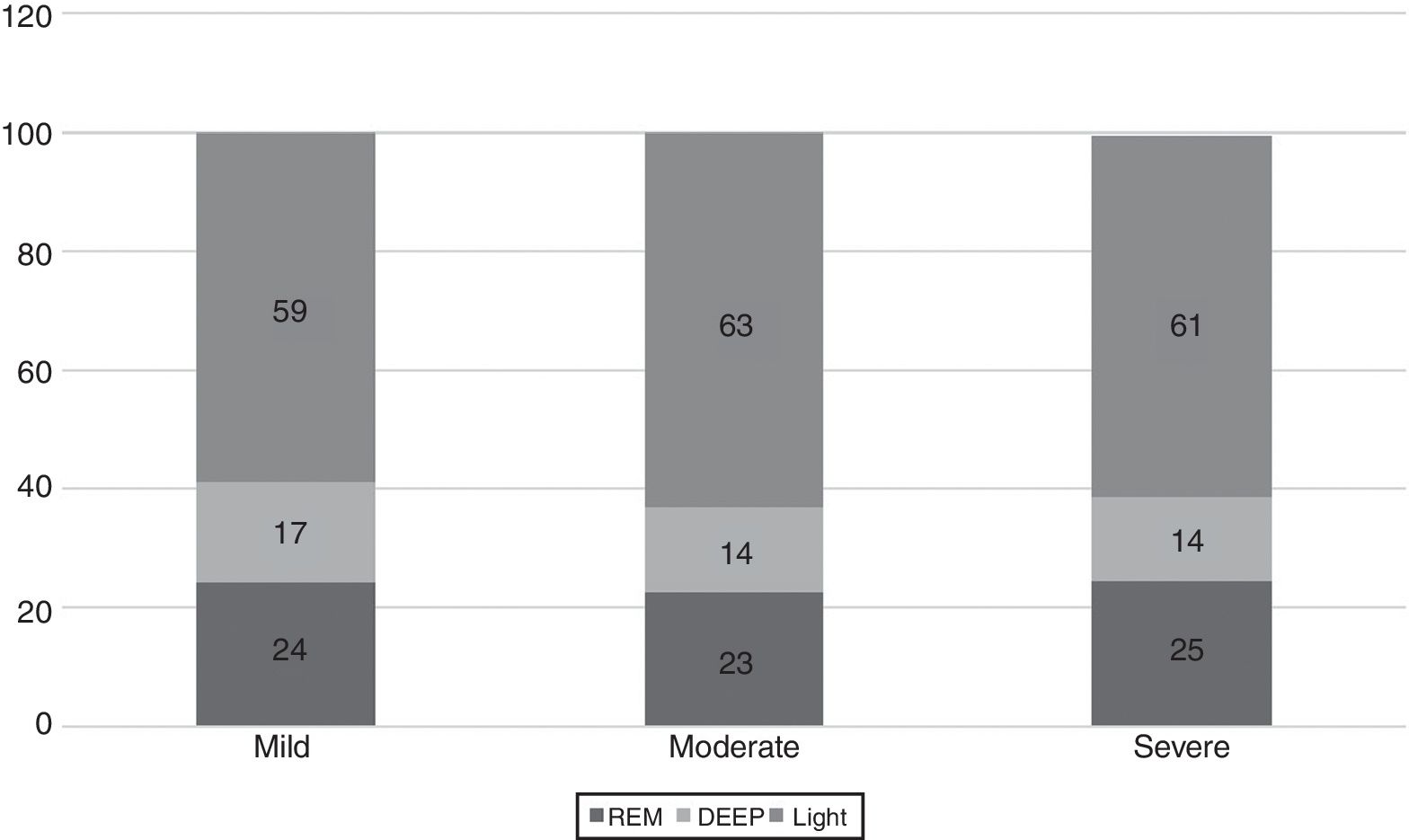
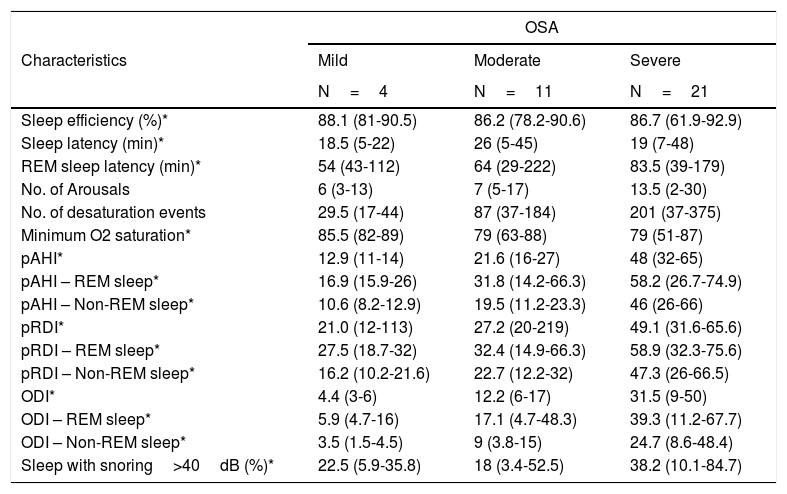
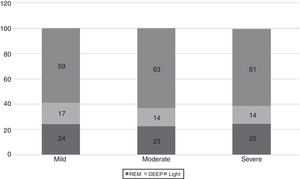
The WatchPAT® monitoring found that sleep efficiency was approximately 87% regardless of the severity of OSA. With regard to sleep architecture, patients with mild OSA were found to have a greater proportion of deep and rapid eye movement (REM) sleep compared to those with moderate and severe OSA (fig. 3).
Sleep latency was greater in patients with moderate OSA (26minutes) than in those with mild and severe OSA (19minutes). REM sleep latency went from 53minutes in mild OSA to 83.5minutes in severe OSA. The minimum desaturation was 85.5%, 79% and 79% for mild, moderate and severe OSA, respectively. The average pAHI was 21.7/hour and 48.3/hour for moderate and severe OSA, respectively. The oxygen desaturation index (ODI) was, on average, 11.4/hour for moderate and 32.1/hour for severe OSA, and the respiratory disturbance index (pRDI) hovered around 50 in both groups. Table 3 discriminates these indices according to sleep stage. Patients with mild and moderate OSA snored at an intensity greater than 40 decibels approximately 20% of the total sleep time, while patients with severe OSA did so 45% of the time (Table 3).
WatchPAT® results according to OSA severity degree.
| OSA | |||
|---|---|---|---|
| Characteristics | Mild | Moderate | Severe |
| N=4 | N=11 | N=21 | |
| Sleep efficiency (%)* | 88.1 (81-90.5) | 86.2 (78.2-90.6) | 86.7 (61.9-92.9) |
| Sleep latency (min)* | 18.5 (5-22) | 26 (5-45) | 19 (7-48) |
| REM sleep latency (min)* | 54 (43-112) | 64 (29-222) | 83.5 (39-179) |
| No. of Arousals | 6 (3-13) | 7 (5-17) | 13.5 (2-30) |
| No. of desaturation events | 29.5 (17-44) | 87 (37-184) | 201 (37-375) |
| Minimum O2 saturation* | 85.5 (82-89) | 79 (63-88) | 79 (51-87) |
| pAHI* | 12.9 (11-14) | 21.6 (16-27) | 48 (32-65) |
| pAHI – REM sleep* | 16.9 (15.9-26) | 31.8 (14.2-66.3) | 58.2 (26.7-74.9) |
| pAHI – Non-REM sleep* | 10.6 (8.2-12.9) | 19.5 (11.2-23.3) | 46 (26-66) |
| pRDI* | 21.0 (12-113) | 27.2 (20-219) | 49.1 (31.6-65.6) |
| pRDI – REM sleep* | 27.5 (18.7-32) | 32.4 (14.9-66.3) | 58.9 (32.3-75.6) |
| pRDI – Non-REM sleep* | 16.2 (10.2-21.6) | 22.7 (12.2-32) | 47.3 (26-66.5) |
| ODI* | 4.4 (3-6) | 12.2 (6-17) | 31.5 (9-50) |
| ODI – REM sleep* | 5.9 (4.7-16) | 17.1 (4.7-48.3) | 39.3 (11.2-67.7) |
| ODI – Non-REM sleep* | 3.5 (1.5-4.5) | 9 (3.8-15) | 24.7 (8.6-48.4) |
| Sleep with snoring>40dB (%)* | 22.5 (5.9-35.8) | 18 (3.4-52.5) | 38.2 (10.1-84.7) |
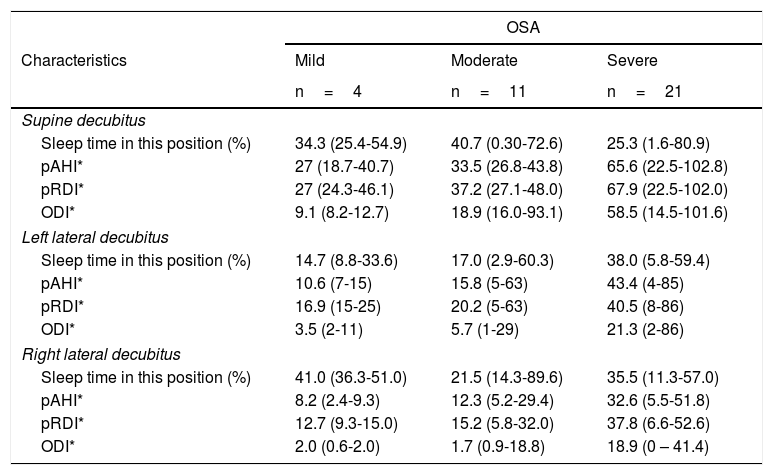
The predominant sleep position was supine in 37.8% of the cases, followed by right lateral decubitus in 35.1%, left lateral decubitus in 24.3% and prone in 2.7%. WatchPAT® also provides information regarding respiratory indices for each of the positions in which the patient sleeps. In general, respiratory indices are seen to be worse in the supine position in all the OSA levels of severity. These are described in depth in Table 4.
Sleep respiratory indices by position.
| OSA | |||
|---|---|---|---|
| Characteristics | Mild | Moderate | Severe |
| n=4 | n=11 | n=21 | |
| Supine decubitus | |||
| Sleep time in this position (%) | 34.3 (25.4-54.9) | 40.7 (0.30-72.6) | 25.3 (1.6-80.9) |
| pAHI* | 27 (18.7-40.7) | 33.5 (26.8-43.8) | 65.6 (22.5-102.8) |
| pRDI* | 27 (24.3-46.1) | 37.2 (27.1-48.0) | 67.9 (22.5-102.0) |
| ODI* | 9.1 (8.2-12.7) | 18.9 (16.0-93.1) | 58.5 (14.5-101.6) |
| Left lateral decubitus | |||
| Sleep time in this position (%) | 14.7 (8.8-33.6) | 17.0 (2.9-60.3) | 38.0 (5.8-59.4) |
| pAHI* | 10.6 (7-15) | 15.8 (5-63) | 43.4 (4-85) |
| pRDI* | 16.9 (15-25) | 20.2 (5-63) | 40.5 (8-86) |
| ODI* | 3.5 (2-11) | 5.7 (1-29) | 21.3 (2-86) |
| Right lateral decubitus | |||
| Sleep time in this position (%) | 41.0 (36.3-51.0) | 21.5 (14.3-89.6) | 35.5 (11.3-57.0) |
| pAHI* | 8.2 (2.4-9.3) | 12.3 (5.2-29.4) | 32.6 (5.5-51.8) |
| pRDI* | 12.7 (9.3-15.0) | 15.2 (5.8-32.0) | 37.8 (6.6-52.6) |
| ODI* | 2.0 (0.6-2.0) | 1.7 (0.9-18.8) | 18.9 (0 – 41.4) |
The use of the WatchPAT® system in patients with cardiovascular diseases and a clinical presentation highly suggestive of OSA posits important considerations which may lead to a change in its diagnostic, treatment and follow up paradigm.
In this case series, 86.5% of the patients were shown to have moderate to severe OSA, especially those with established cardiovascular disease (arrhythmias, heart diseases and coronary artery disease). This finding deserves special attention, since in spite of having a sufficient body of evidence demonstrating a causal association between sleep apnea and the incidence and morbidity related to, arterial hypertension, coronary artery disease, cardiac arrhythmias, heart failure and cerebrovascular disease,17 OSA is not suspected, searched and studied in patients with cardiovascular diseases as frequently as it should be.
Several scales have been used to evaluate the risk of OSA. In this series, the STOP-BANG and Epworth sleepiness scales were applied, and there were no score differences according to OSA severity. The Epworth median for moderate and severe OSA corresponds to mild daytime drowsiness, and the STOP-BANG median for all degrees of severity corresponds to an intermediate risk of OSA. These findings coincide with the American Academy of Sleep Medicine (AASM) guidelines, which recommend that these questionnaires or predictive algorithms not be used to diagnose OSA in adults, in the absence of polysomnography or home sleep apnea testing (strength of recommendation: strong).11
The association of obesity and overweight with sleep apnea is widely recognized, and therefore this condition, which per se increases the cardiovascular risk, should be suspected in any patient with these characteristics. However, OSA may also present in patients whose body mass index is within normal limits,18 as was seen in a third of the patients with moderate OSA in this series.
One symptom which has traditionally been linked with OSA is snoring. However, only 8% of the patients included in the study looked for medical consultation specifically due to this symptom, and half (50%) reported it on the directed questionnaire. Therefore, it is important to take into account other signs and symptoms when it comes to suspecting this sleep disorder.
In the third edition of the International Classification of Sleep Disorders (ICSD-3), cardiovascular diseases (arterial hypertension, coronary artery disease, heart failure, and atrial fibrillation) are included as part of the diagnostic criteria for OSA, together with the following symptoms: drowsiness, fatigue or nonrestorative sleep; and signs: apnea, gasping or nocturnal asphyxia; along with observation of these by a third party.10 In this context, the cardiologist should consider OSA to be a promoting factor of the mentioned cardiovascular diseases, through an active search for symptomatology suggestive of OSA, together with the clinical picture, and, in cases where it is required, sleep monitoring.
This study used the WatchPAT® device which allows the ambulatory diagnosis of this sleep disorder with less logistical complexity compared to polysomnography, obtaining a quick and accurate diagnosis which permits prompt treatment.
The American Academy of Sleep Medicine establishes that this type of home sleep test is useful for diagnosing OSA in patients with a moderate to high probability of having this condition, as long as they do not have any major neurological disorder (principally neuropathies), take narcotic, sedative or neuroleptic medications, or have severe lung diseases (strength of recommendation: strong).11
The WatchPAT® system has been compared to PSG for the diagnosis of mild, moderate and severe OSA, finding a high sensitivity and specificity for the three groups of patients, as follows: mild OSA: 92% sensitivity – 78% specificity; moderate OSA: 96% sensitivity, 75 to 100% specificity; severe OSA: 96% sensitivity – 88% specificity.19,20 Previous meta-analyses carried out by the University of Chicago group led by Yalamanchali et al.,12 found a high correlation between WatchPAT® and PSG for the various respiratory indices, mainly pAHI and ODI, with correlation coefficients of 0.889 and 0.942, respectively. Pinto et al., 13 in Brazil, and Korkuyu et al.,14 in Turkey also found a high correlation with regard to these respiratory indices, confirming and extending the previous findings to different population groups.
Although no data inference can be made based on this study, since it is a case series, it is important to highlight its purpose, which is to call attention to the importance of suspecting OSA in patients with cardiovascular disease, even if they do not report specific sleep disorder symptoms. Also, to present a new sleep study method (WatchPAT®) which has proven to have an adequate performance in diagnosing OSA, and may decrease the waiting time for this test, since it is a portable device which may be used at home without the assistance of technical personnel.
ConclusionFrom an epidemiological and healthcare cost point of view, it is imperative for physicians specialized in cardiovascular diseases to take a more active role in the approach to and diagnosis of OSA, given the high prevalence of the problem in the general population, and especially in patients with resistant arterial hypertension, heart rhythm disorders or myocardial structural damage. Obstructive sleep apnea hypopnea syndrome may be diagnosed with portable devices such as WatchPAT®, which has high specificity and sensitivity in the diagnosis of obstructive sleep apnea, with less complexity and on an outpatient basis, resulting in lower healthcare costs and greater efficiency.
FinancingFundarritmia – Fundación Cardiovascular.
Conflicts of interestNone.