Diabetic foot is one of the most fatal outcomes for patients with diabetes; the importance of control in a disease that progresses until presenting important macroscopic changes in the lower limb is absolutely relevant. Along diabetes progression, the disease can lead to increased morbidity and invasive and limiting interventions for the patient, hence the importance of early and timely detection and intervention of the pathology by the medical team. These recommendations are addressed to general practitioners and specialized faculty in various medical branches, emphasizing how a comprehensive approach to the patient with diabetic foot should be carried out. Covering prevention, initial diagnosis, evaluation of the progression of the pathology, stratification with the proposed classifications, and finally the treatment according to the stage in which the patients are, is actually well described herein in order to minimize unsatisfactory outcomes, interventions and complications derived from the progression of diabetic foot. We are talking about recommendations and not guidelines due to the absence in a large number of opportunities of properly structured scientific evidence (I and II). Perhaps, the most important thing to emphasize in all these recommendations is to remind the reader that in cases of treating a diabetic foot, it should always be kept in mind that the contralateral foot is not healthy because it has also been subjected to the same disease, for the same period of time and stressed equally as well. Therefore, even if the contralateral foot does not have symptoms, it should be considered equally ill and should be examined and treated likewise.
Evidence level: IV.
El ataque de pie diabético es uno de los desenlaces más fatídicos para el paciente con diabetes, lo que demuestra la importancia del control en una enfermedad que avanza hasta presentar cambios macroscópicos importantes en el miembro inferior. Durante la progresión de la Diabetes, la enfermedad puede derivar en un aumento de la morbilidad e intervenciones invasivas y limitantes para el paciente, de ahí la importancia de la detección e intervención temprana y oportuna de la patología por parte del equipo médico. Estas recomendaciones van dirigida a médicos generales y especialistas en diversas ramas médicas, con el objetivo de enfatizar el cómo se debe realizar el abordaje integral del paciente con pie diabético. Abarcando la prevención, diagnóstico inicial, evaluación de la progresión de la patología, estratificación con las clasificaciones propuestas, y por último el tratamiento según el estadio en el que se encuentre el paciente. Esto con el fin de minimizar desenlaces, intervenciones y complicaciones derivadas de la progresión del pie diabetico. Hablamos de recomendaciones y no de guías debido a la ausencia en un gran número de oportunidades de evidencia científica debidamente estructurada (I y II). Tal vez lo más importante por recalcar en todas estas recomendaciones es recordarle al lector que en los casos de afectación de un pie diabético, siempre se debe tener en cuenta que el pie contralateral también ha estado sometido a la misma enfermedad durante el mismo tiempo y por lo tanto aunque no tenga síntomas se debe considerar igualmente enfermo y se debe examinar también.
Nivel de evidencia: IV.
Diabetes mellitus (DM) is a public health concern. According to the International Diabetes Federation, 425 million cases were recorded by 2017.1,2 This disease is more frequent in developing countries where maintaining a healthy lifestyle is difficult and risk factors for DM are not always identified.3,4
Inadequate monitoring of DM results in multiple complications, both at the micro and macrovascular levels.5 Moreover, it is one of the main causes of nontraumatic lower extremity amputation, and it has been reported that 50% of patients who have undergone their first amputation will require further amputations over the next 3 years.6,7 Thus, identifying risk factors is essential to prevent these complications.8–10
General and specialized physicians often forget the importance of examining patients comprehensively for early diagnosis of the microvascular complications of diabetes [retinopathy, nephropathy, diabetic foot (DF) at risk, etc.].11,12 Patients must be monitored constantly by medical staff in order to detect complications associated with diabetes as well as its risk factors. This will enable us to address the problem specifically and a direct intervention can be proposed for inclusion in the primary health care model, regardless the type of facility at which the interventions are conducted, i.e., at an inpatient or outpatient center.13,14
One of the main aspects of preventing the occurrence of complications in patients with DM is raising awareness among patients regarding changes in their lifestyles, with special focus on unhealthy nutritional habits and any degree of malnutrition that they may have.15,16 Despite its impact on patients’ health, limited studies on DM have been conducted in Colombia and there is a lack of in-depth studies on the implementation of preventive strategies for type 2 DM (DM2) and DF.17,18
The general objective of this paper is to propose recommendations for the management of patients with DF receiving outpatient services or those admitted to the hospital or emergency care units from the perspective of orthopedics and foot surgery. These recommendations would be useful in different health institutions. In order to develop these recommendations, prior evidence-based publications were considered. Expert consensus was obtained whenever a systematic review or meta-analysis could not be performed due to limited evidence.
The most important aspect of these recommendations may be that they focus on the comprehensive care of patients not only in an interdisciplinary but also in a transdisciplinary manner. As much as the orthopedist should know about DM, the internist should also know about the foot, their knowledge being comparable to all other specialists, general practitioners, and therapists.
EpidemiologyDM is one of the most frequent diseases and has 6% prevalence in Colombian community .19 On the other hand, the worldwide prevalence of DF among patients with DM is 8%–13%, mainly affecting patients aged between 45 and 65. Amputation risk is up to 15 times higher in patients with diabetes than in patients without diabetes. Amputation incidence in patients with diabetes is around 2.5–6/1000 patients/year.
By 2010, it was estimated that more than 152 million people were diagnosed with diabetes worldwide, with a high incidence in Africa and Asia.20 The International Diabetes Federation estimated that by 2011–2012, 336 million people would be diagnosed with DM and 280 million people would be at risk of developing DM.21
In Colombia, DM2 is one of the main reasons for consulting professionals from different healthcare services, and it shows a high mortality rate in the population aged >45.22 In Bogotá, DM2 has a prevalence of 5.16% in men and 3.8% in women.23 In 2010, according to Colombia's basic health indicators, diabetes ranked fourth in the mortality rate ranking for women aged> 45.24,25
In Colombia, DM is one of the 10 main reasons for hospitalization, seeking outpatient care, and mortality among the population aged> 45. DF accounts for 20% of hospitalizations associated with DM,26,27 and up to 10% of patients admitted due to DF injuries require extended hospital stay or amputation of the affected limb.
Amputation incidence is 5.97 for every 100,000 patient with diabetes/year, and it increases to up to 9.15 every 100,000 patient with diabetes/year for patients aged> 45.28
Definition of DFThe DF syndrome is one of the most serious complications of diabetes and includes infection, ulceration, and destruction of depth tissues associated with diabetic neuropathy (DN) and peripheral arterial disease as well as articular, dermatological, and soft tissue damage.29,30
DF can also be defined in a general and inaccurate way as a clinical alteration of a typically neuropathic origin induced by an increase in blood glucose sustained over time in addition to secondary ischemic events that, with repetitive traumatic triggering factors, result in foot injury and/or ulceration and/or articular destruction of the ankle and foot.31
The Latin American Saint Elian model provides an extensive definition of the DF Attack:
“It is a syndrome that occurs as an acute or chronic attack on the foot characterized by one or more wounds whose etiology, complexity, and severity varies in terms of the extent and depth of tissue destruction, anatomical areas, and aspects that may be worsened by ischemia, infection, edema, and neuropathy, with risk for amputation and/or death in patients with diabetes.” This definition clearly differentiates DF injuries from soft tissue infections and arterial or venous ulcers occurring in the lower extremities of patients with diabetes. Moreover, it states that ulcers are one type of DF injury and is not the one that defines it, avoiding its overuse as an umbrella term to include all the injuries of the syndrome.32
Ulcers tend to result in loss of functionality, amputation, and deterioration (both psychical and economic), becoming the most frequent disability observed in patients with diabetes.33 For this reason, early identification of patients at risk is the first step, followed by provision of education to patients and their families to promote self-care activities.34 In underdeveloped and developing countries (such as ours), there is a lack of prompt care and efficient promotion programs aimed at preventing these injuries.35
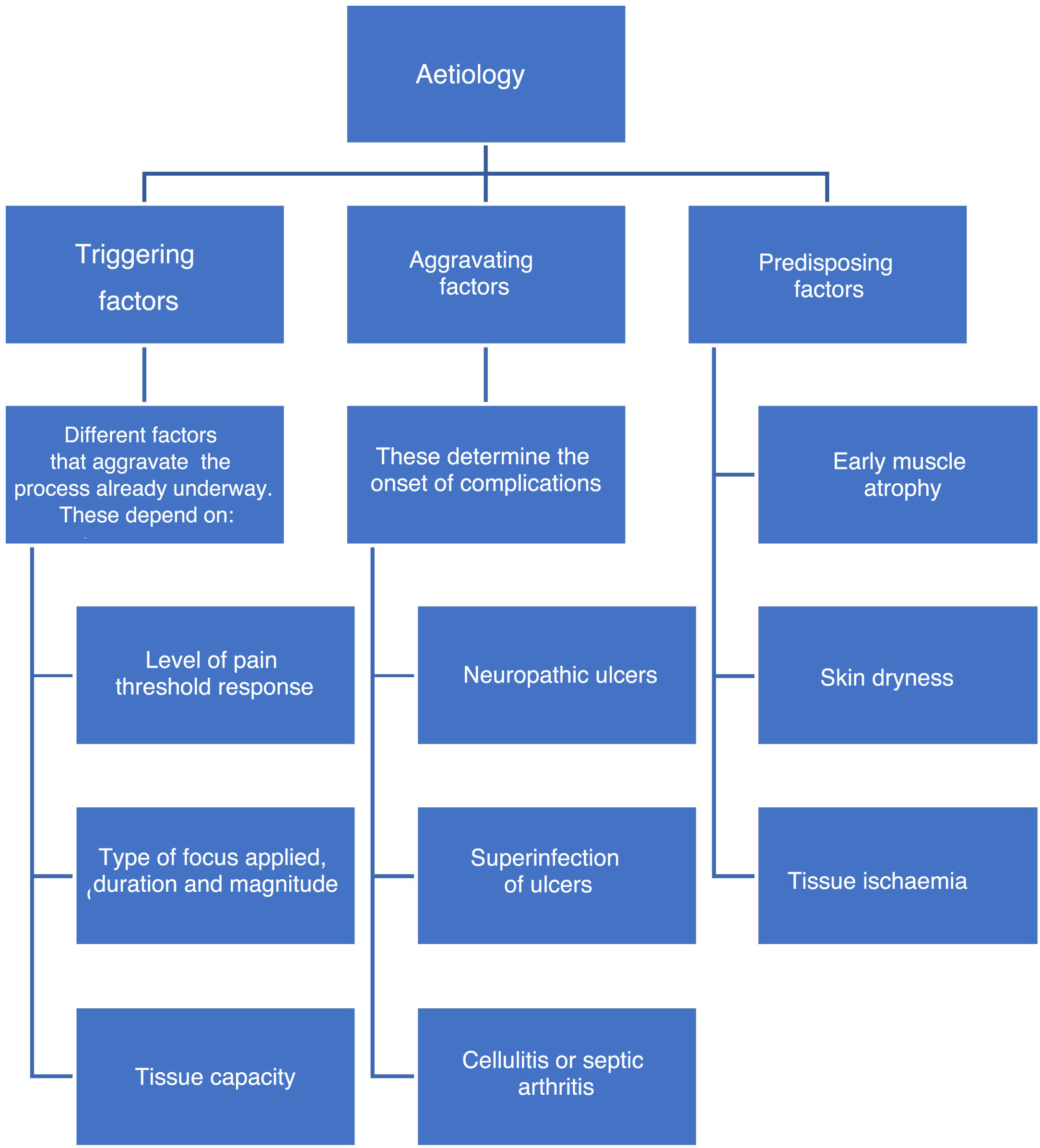
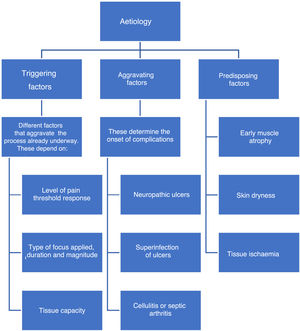
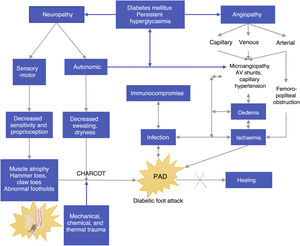
EtiologyFactors associated with the onset of DF include triggering, worsening, or predisposing factors, which are collectively called intrinsic factors. (See Figure 1).
Besides the factors mentioned above, extrinsic factors also exist. Some extrinsic factors include mechanical, thermal, and chemical factors, which may be associated to the use of inadequate shoes and account for 50% of new DF cases. The thermal factor is associated with the loss of heat sensitivity caused by neuropathy and can lead to injuries through prolonged exposure to high temperatures (such as hot water) in the absence of an efficient “withdrawal” reflex, which is triggered by the feeling of heat and pain.
Intrinsic factors include prior foot deformities (hammer toes, claw toe, hallux valgus, Charcot neuroarthropathy, etc.),36 which increase plantar pressure and cause progressive injuries due to repetitive trauma in areas that should be healthy. As sensitivity decreases, these injuries affect deeper tissues and become severe injuries. This becomes a vicious circle in which muscle atrophy caused by denervation or Charcot neuroarthropathy results in deformities which in turn alter foot pressure areas and promote ulcer formation.37
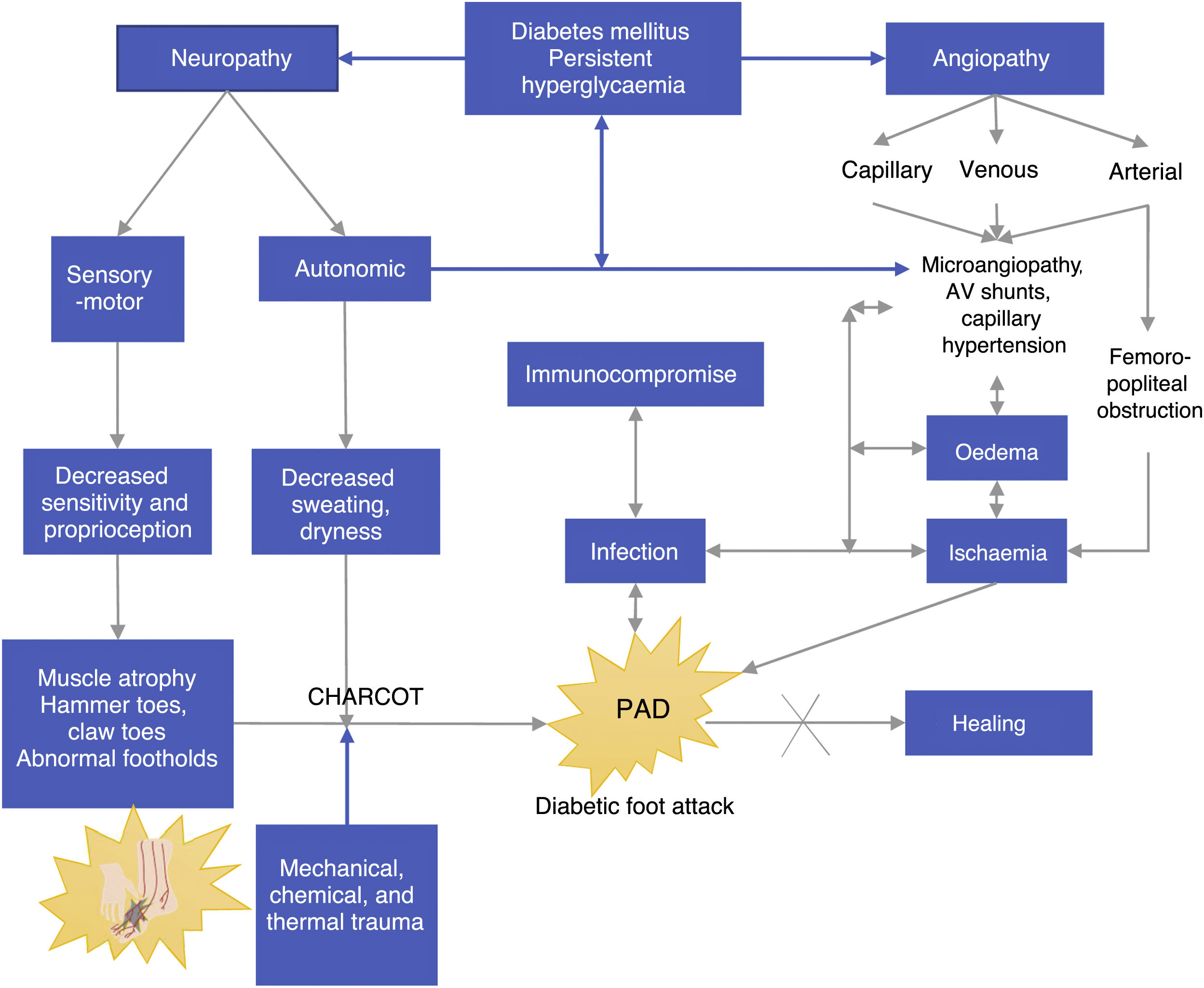
Pathophysiology of DFPersistent hyperglycemia, a chronic complication of DM, is the biological substrate for vascular and neuropathic endothelial damage as well as for arterial layer and immunological activity damage (see Figure 2). The four aggravating factors as per the Saint Elian model are neuropathy, ischemia, infection, and edema. Neuropathy can either be motor sensory or autonomic, the former being the cause of insensitivity and reduced proprioception, which results in loss of pain, an essential protection mechanism. All the aggravating intrinsic factors result in adverse conditions that enhance the onset of ulcers.38,39
Loss of muscle tone due to motor neuropathy may cause muscle contracture in some areas and weakness in others (alteration of the intrinsic muscles of the foot). Loss of lower extremity proprioception enhances articular cartilage damage and stiffness or deformity. Moreover, the synchronic muscle activation and contraction movements that allow for foot load transfer (neuromuscular control in the kinetic chain of gait) gradually decrease until different types of deformities occur, in which the normal plantar morphology of the foot changes and the normal support patterns necessary to walk are reduced.40,41
Abnormal support points are located in metatarsal bone prominences, mainly in the hallux if occurring concomitantly with hallux valgus. Feet with neuropathic muscle atrophy have a higher tendency for deformation if the patient uses inadequate shoes because such shoes cause the toes to press against each other and may lead to the development of “kissing ulcers.” This is more evident in patients with ischemia.
Autonomic neuropathy leads to dry foot because foot sweating, which is usually regulated by the autonomic nervous system, does not occur. Infectious agents such as fungi aggravate the development of foot cracks and small and medium wounds, creating a biological environment ideal for the development of infections. If both (motor neuropathy and autonomic neuropathy) disorders are present, the foot of a patient with diabetes becomes highly susceptible to ulcerations; therefore, even the smallest chemical, physical, mechanical, or thermal trauma will result in an inevitable injury, which generally becomes an ulcer and gets infected. Peripheral neuropathy affecting arterioles results in an increase in arteriovenous shunts and replacement by surrounding angiosomes, a physiological compensation which only occurs in the foot.
Based on Figure 2, we can observe another component of DF (most probably a different disease state), i.e., angiopathy, which is associated with the involvement of big blood vessels such the arterial enviroment, and capillary environment. Regardless of whether it is a macro or microvascular involvement, its direct result is foot ischemia. Venous insufficiency and capillary involvement due to infection result in edema, and these angiopathic vascular injuries have even been reported as a triggering factor of Charcot neuroarthropathy caused by bone hyperemia.
A positive correlation has been found between arteriovenous shunt and disease duration (r=0.62, p<0.001). Arteriovenous shunts are directly correlated with the degree of autonomic neuropathy, mainly in advanced motor sensory neuropathy cases.
Autonomic neuropathy caused by arteriovenous shunting (AV) may be associated with the pathophysiology of DF, and it causes gangrene or chronic neuropathic ulcers. Even when there is no ischemia secondary to femoral popliteal obstruction, this is the reason why gangrene or chronic ulcers persist even when neuropathy is predominant: their pathophysiological component is actually mixed. Ischemia promotes sepsis persistence or increase since it hinders adequate tissue oxygenation and adequate flow of formed defense elements in the injury site; when the edema is corrected, healing improves. If these factors persist and are not reverted, the healing and treatment response will be insufficient and consequences will be fatal.42
Within DF, there are three factors that play a significant role in this complication—peripheral neuropathy, occlusive arterial disease, and aggregated infection.
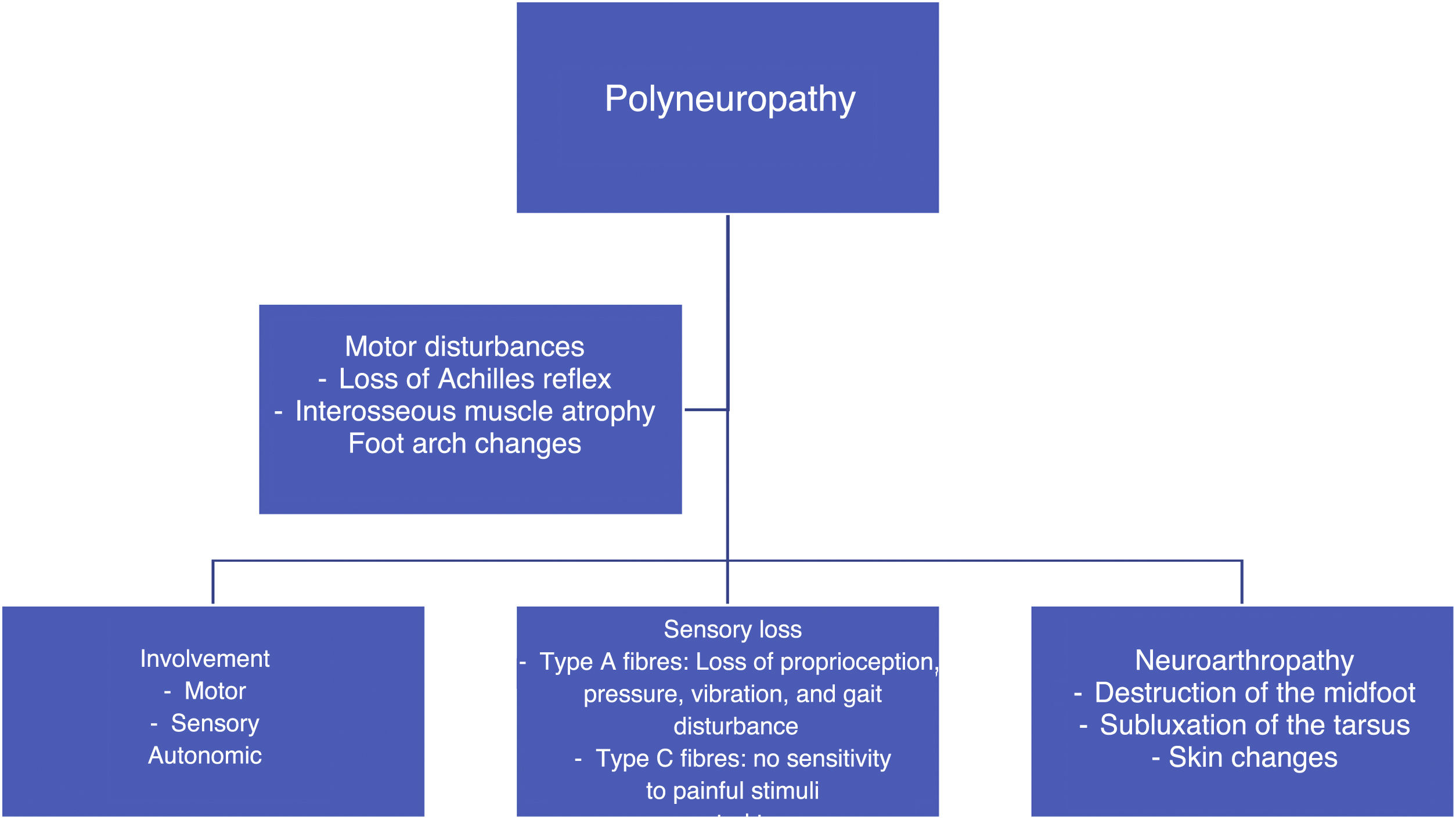
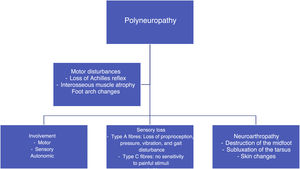
In patients with poorly controlled diabetes, polyneuropathy affects the motor and sensory parts. There is a progressive destruction of motor fibers, and the first consequence of this is the loss of the Achilles reflex, followed by the atrophy of the interosseous muscles,43,44 which changes the anatomy of the foot and leads to deformities, depression of the metatarsal heads, toe contracture, and clubfoot. With respect to the sensory parts, type A and C fibers are affected, which results in the loss of different types of sensitivity (proprioception, pressure, vibration, pain, etc.). As a result, the patient is prone to suffer repeated trauma, develop blisters or fractures, and lose protective pain reflexes. In addition, their skin is dry because of inadequate regulation by the autonomous system and shows the presence of fissures through which pathogenic microorganisms may enter.45,46 (See Figure 3).
Risk factors for DFThe main risk factors for the development of DF and the series of injuries that lead to gangrene and amputation are:
- a.
Peripheral neuropathy
- b.
Inadequate hygiene
- c.
Deformities
- d.
Old age
- e.
High plantar pressure
- f.
Inadequate metabolic control
- g.
Hyperkeratosis
- h.
Smoking
- i.
Prior amputation
- j.
Onychomycosis with toe nail deformity
- k.
Inadequate shoes
- l.
Proprioceptive loss
Patients with two or more risk factors should be monitored every 6 months, and those with four or more risk factors should be monitored every 3 months.
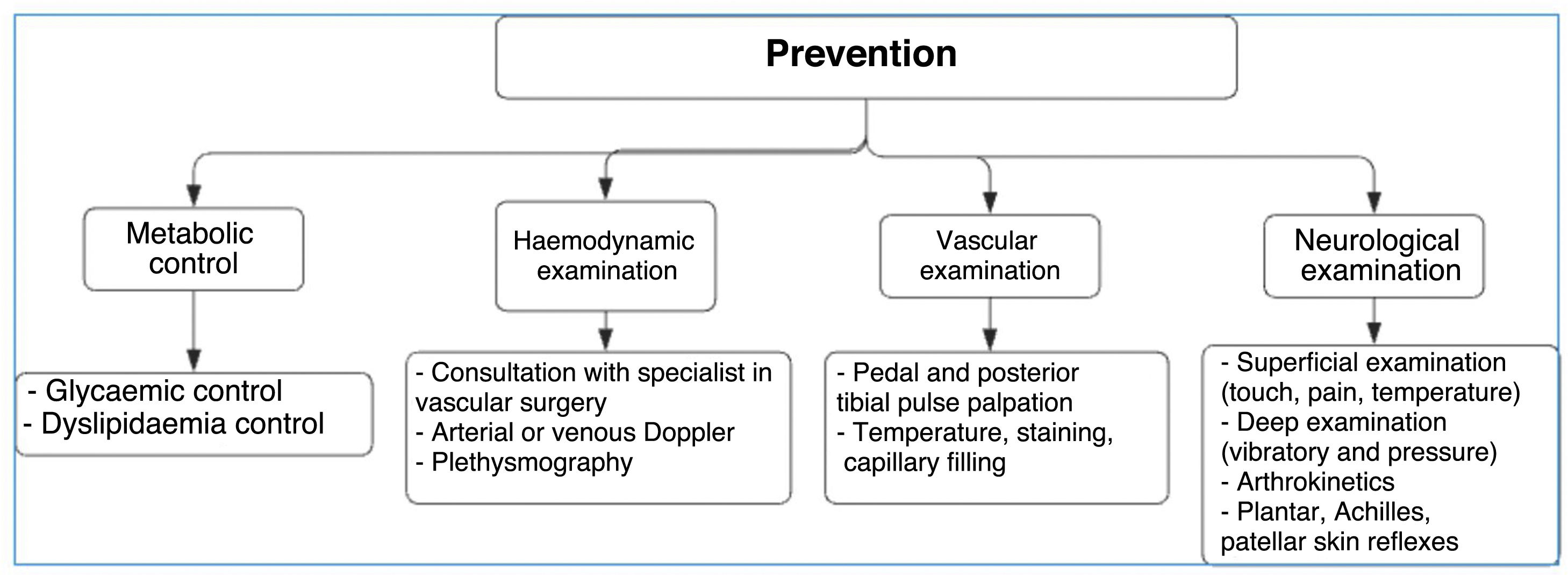
Clinical manifestations and diagnosisAs previously reported in scientific literature, promotion and prevention are the most important actions that can be taken to avoid the development of complications in patients with diabetes. This begins with early diagnosis to prevent the occurrence of infections and potential amputation of the limb.47
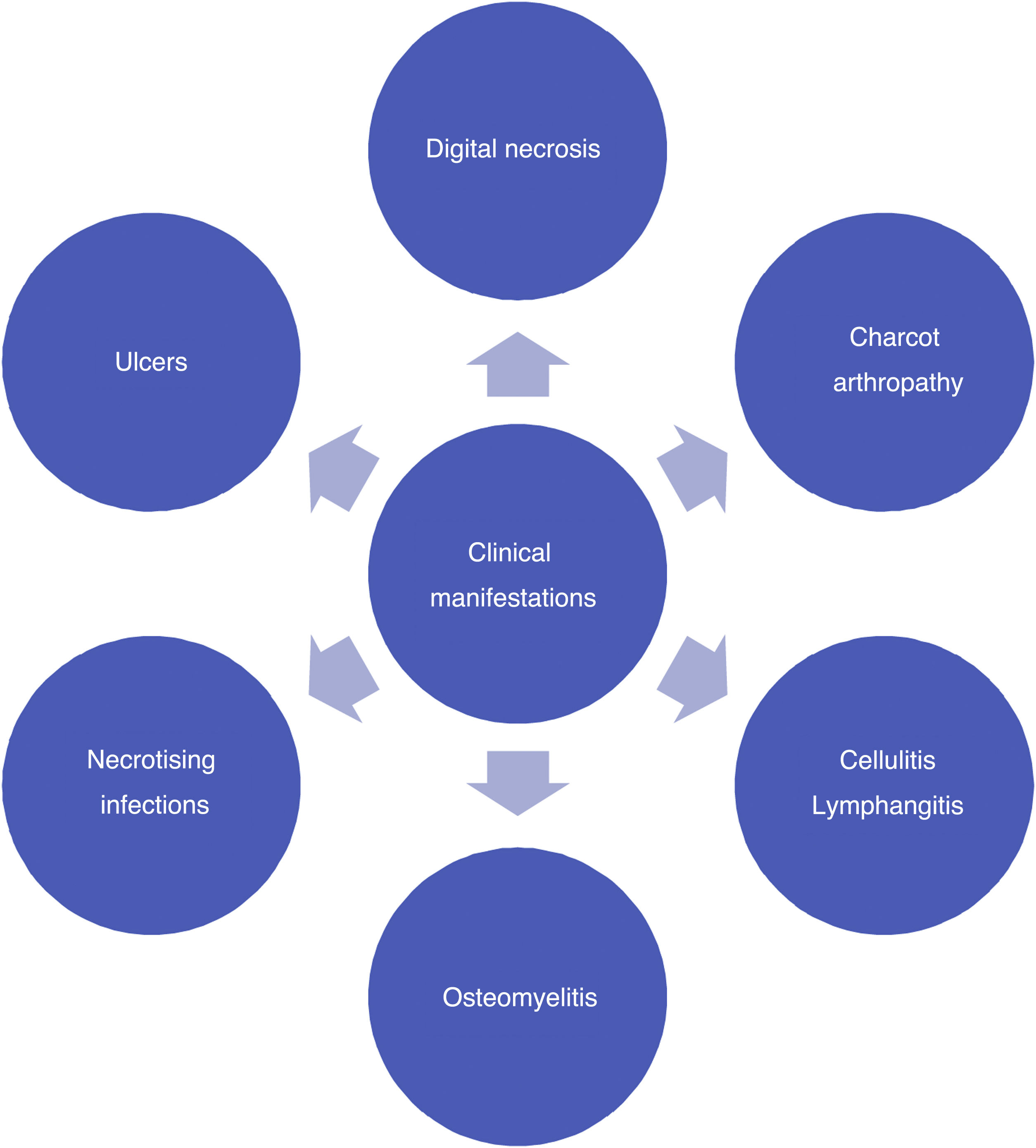
Different classifications have been developed over time (see Figure 4). Some of them are still used despite their obsolete nature and apparent lack of utility in hospital centers with high amputation and death rates associated with DF. Several others are used because they are preferred by healthcare professionals based on the severity of the DF attack or from the perspective of their medical specialty. Texas and Wagner classifications have become popular but do not include Charcot neuropathy and are focused on infection and ischemia, minimizing the focus on other important variables. As a result, their use has gradually decreased.48,49 The Latin American Saint Elian System includes diabetic Charcot neuro-osteoarthropathy as a DF pathology and has a considerable global impact on publications. It is also part of the clinical practice recommendations proposed by the International Diabetes Federation.50,51
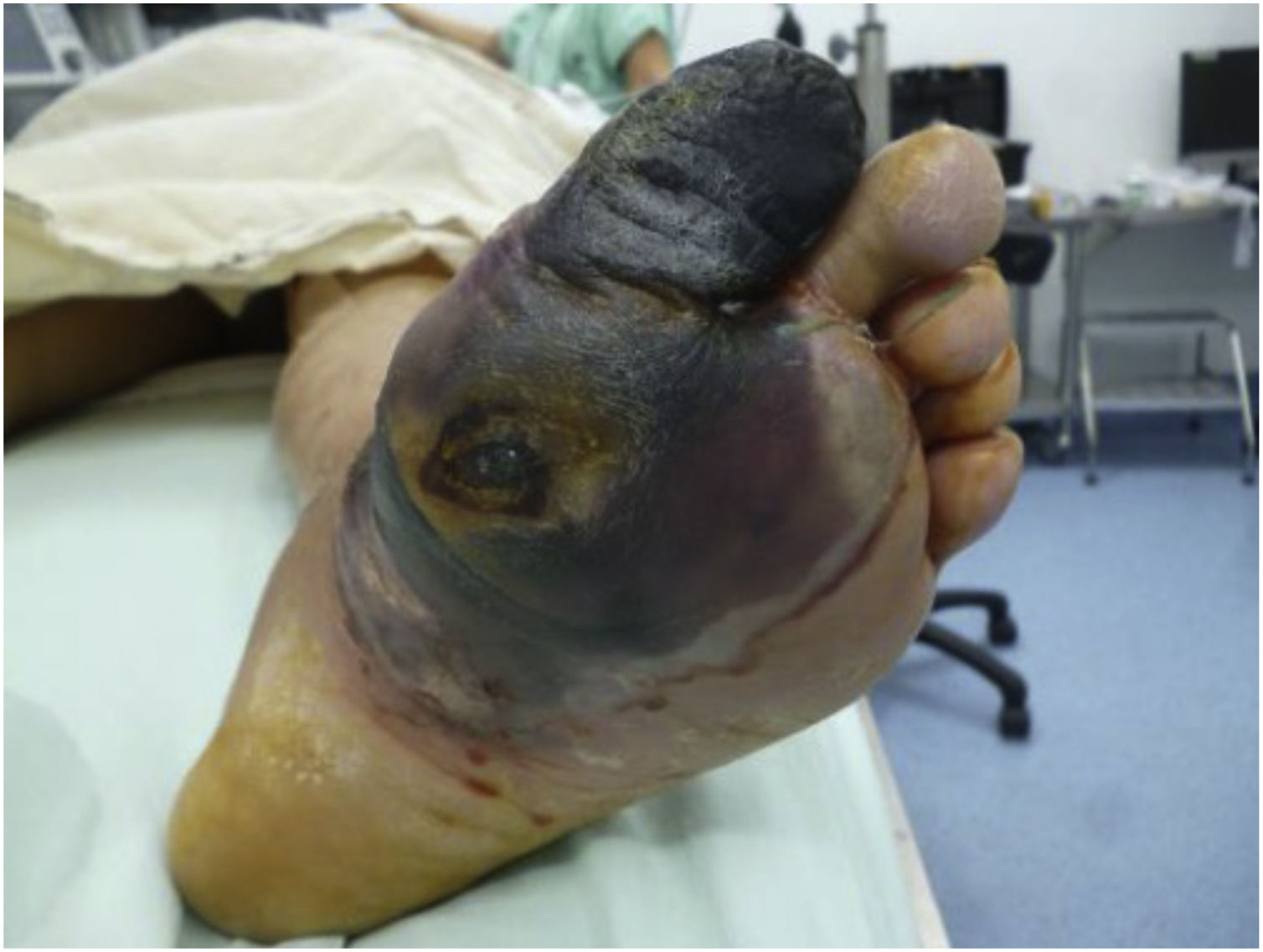
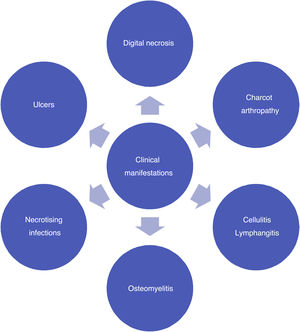
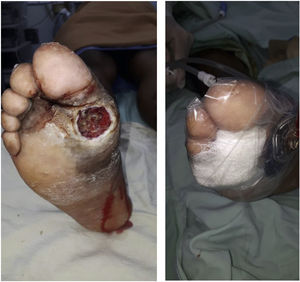
Recently, Vas et al. 52 have defined the term “diabetic foot attack” to describe the DF syndrome. They describe three types of DFA, which include ischemia, infection, and severe neuropathy (Charcot). Based on the Saint Elian definition of DFA, there are five types of attack when including nephropathy-associated severe bilateral edema and mixed attacks. DFA is characterized by a devastating outcome in one foot, with acute inflammation, progressive skin destruction, and tissue necrosis that is sometimes associated with a remarkable systemic response. It can evolve negatively, in a matter of hours, from a simple wound to a wound that risks limb viability. It can occur abruptly, with no prior affection, or result from the exacerbation of a chronic process associated with ischemia, neuropathy, or both. Therefore, late diagnosis or intervention initiation significantly increases the risk of major limb amputation.
Charcot foot, also known as osteopathic foot, is a neuroarthropathy having a multifactorial etiology, and its occurrence and history of association with severe foot deformities has been widely studied. Moreover, multiple theories regarding its pathophysiology have been suggested. Initially, erythema, heat, and edema are evidenced. Radiographically, tarsometatarsal luxation and plantar tarsal subluxation are observed.53,54
DF infections may vary in severity (ranging from cellulitis to necrotizing fasciitis). Inadequate control of metabolic pathology causes immunosuppression, which makes the patient prone to infections, that is further aggravated by the skin-related pathological changes mentioned above.55,56 The most prevailing pathogens include Staphylococcus, Streptococcus, Enterococcus, Escherichia coli, and other gram-negative bacteria, especially Staphylococcus aureus, which has shown methicillin resistance in 30%–40% of cases.57,58
As a result of superinfected ulcers, microorganisms may spread to the lymphatic system, causing lymphangitis with predominant erythematous lines reaching the dorsal foot and the leg. Lymphangitis and cellulitis leads to infections caused by gram-positive bacteria, which may evolve and cause sepsis.59
Necrotizing soft tissue infections occur when an infection reaches beyond the subcutaneous tissue and affects tendons and muscle tissues. They tend to be polymicrobial and involve anaerobic germs.
Neurological foot assessment should include a Semmes Wenstein 5.07 monofilament test in which 10-g pressure is applied when bending the microfilament, which is considered as a determinant value for defining the presence of DN. Alternatively, the Ipswich Touch Test (Ipswich, a city located in Suffolk, England) can be performed. In this test, slight pressure is applied over the second and fifth toe and the dorsal surface of the hallux. The same system used to register monofilaments can also be used, but the test should be performed using a tuning fork (see Figure 5). A monofilament self-exam may be indicated in patients with good support and high educational level and who know which type of monofilament should be used. If the patient does not have this support network, manipulating a limb with devices not adequate as a monofilament may result in skin injuries and ulcer risk due to improper material use.
Recently, the modified Romberg test (normal values: 20–40 s) has been used for examining the proprioception of the ankle and foot. Values lower than 11 s suggest total loss of proprioception and, consequently, that of neuromuscular control, substantially affecting the foot load transfer system and increasing the risk of falls and injuries caused by articular mechanical overload. The modified Romberg test should be performed on a routine basis if DN is suspected, i.e., for those scoring below 11 s. Likewise, the purpose of proprioceptive rehabilitation for patients with diabetes should be the achievement of a minimum of 11-s joint protection value in order to prevent the loss of joint proprioception that may trigger the early development of Charcot arthropathy.60
Differential diagnosis between cellulitis and Charcot arthropathy: Clinically, Charcot arthropathy does not involve a wound. By performing an elevation test of the affected foot edema, erythema, and pain typically decreases after the patient is told to lift their foot for several minutes; however, infected foot does not show a decrease in edema, erythema, and pain when this so called elevation diagnostic test is performed. In case of ulcers, the probe to bone test should be performed. When performing the test, if the bone can directly be felt from the outside, the presence of bone infection is almost certain; however, bone infection should not be ruled out if the bone cannot be felt.61,62
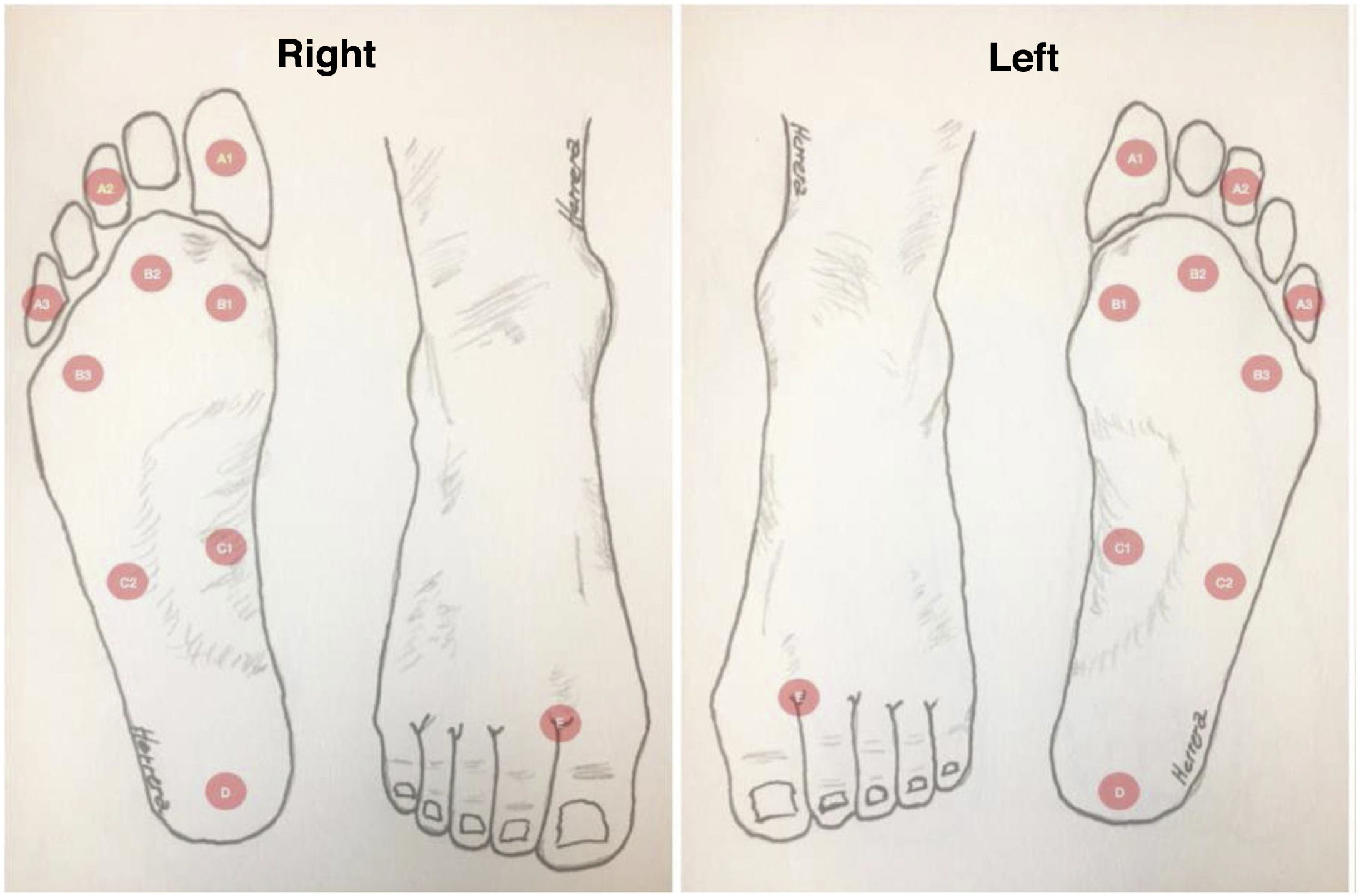
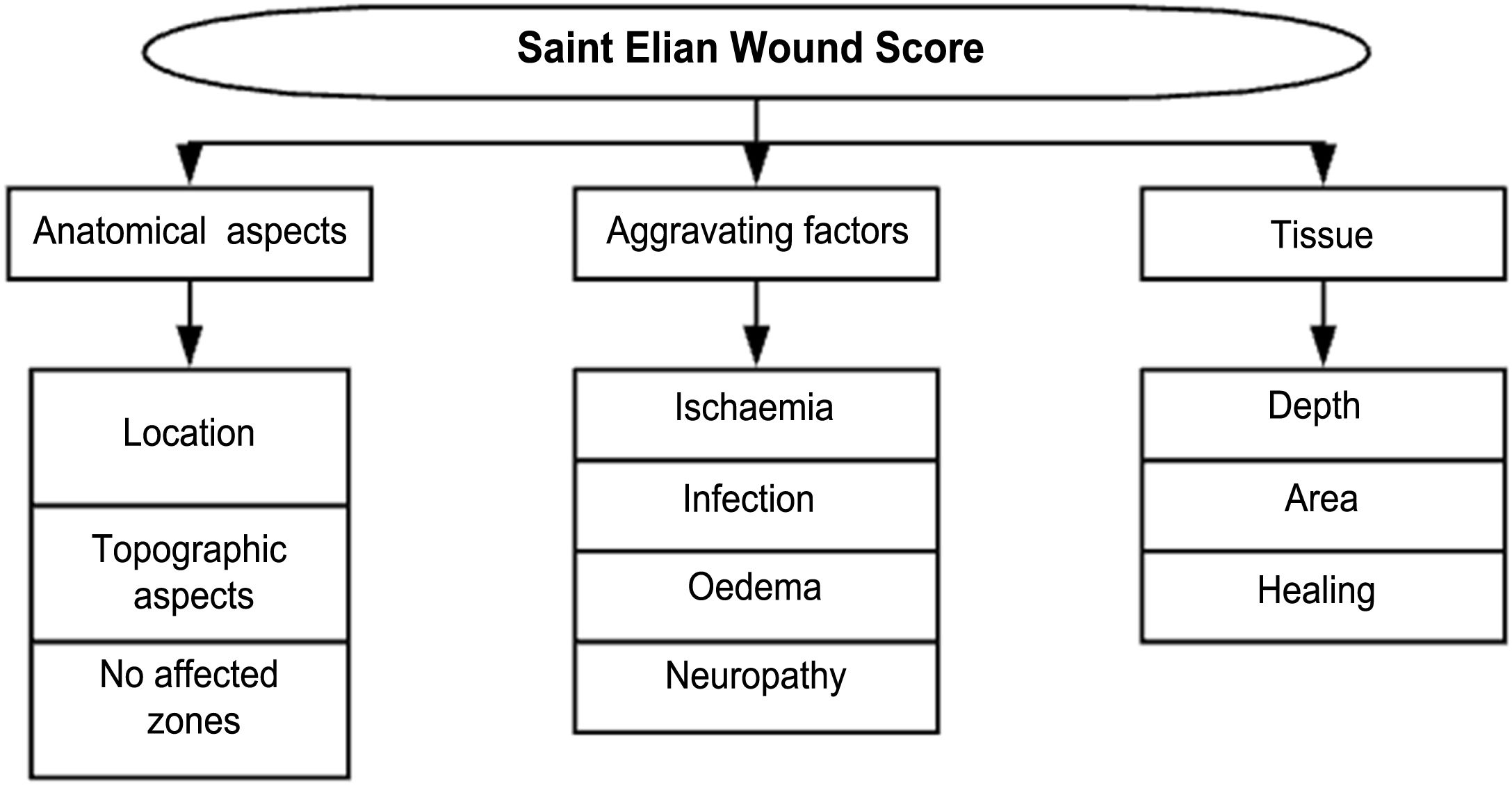
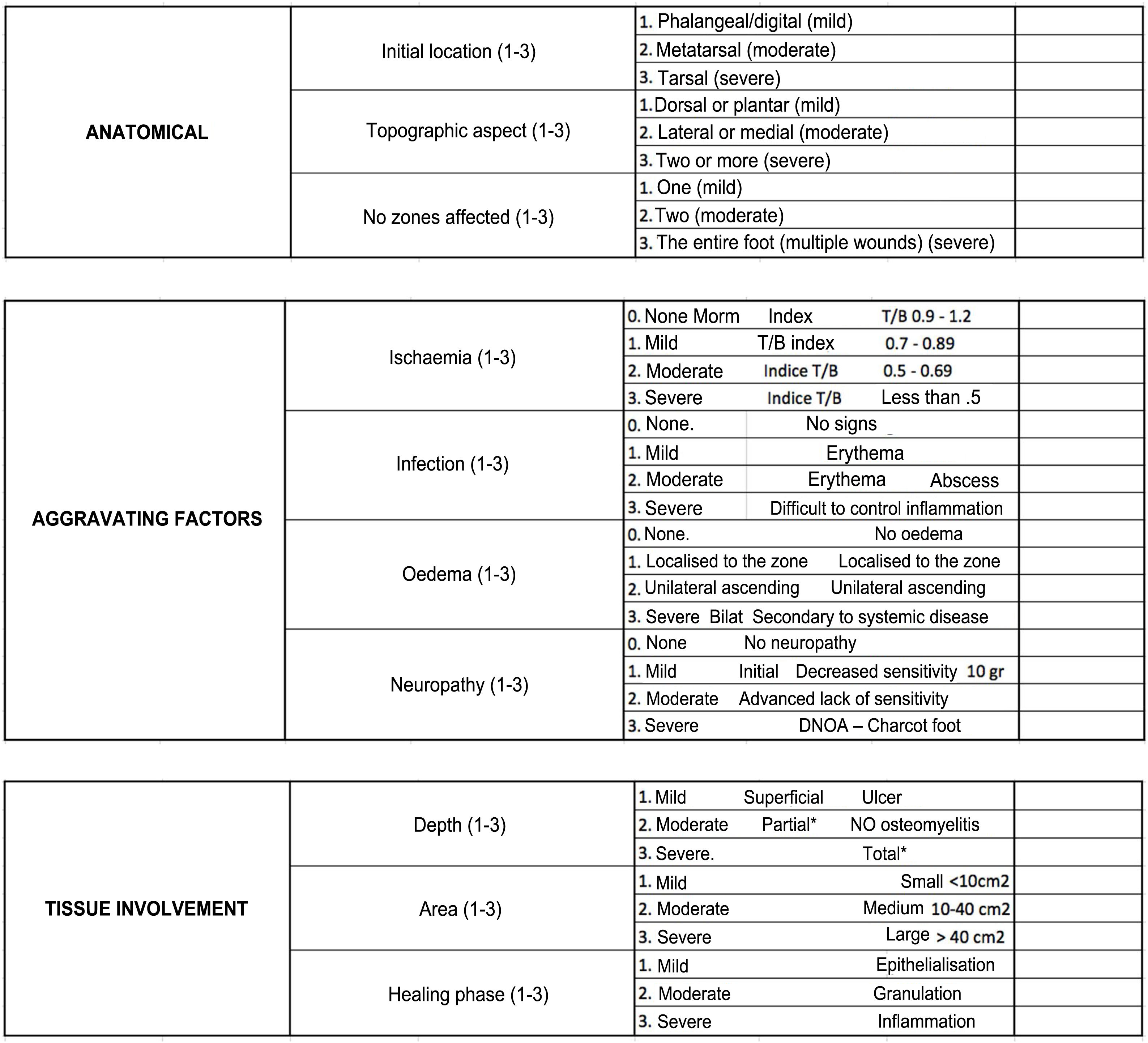
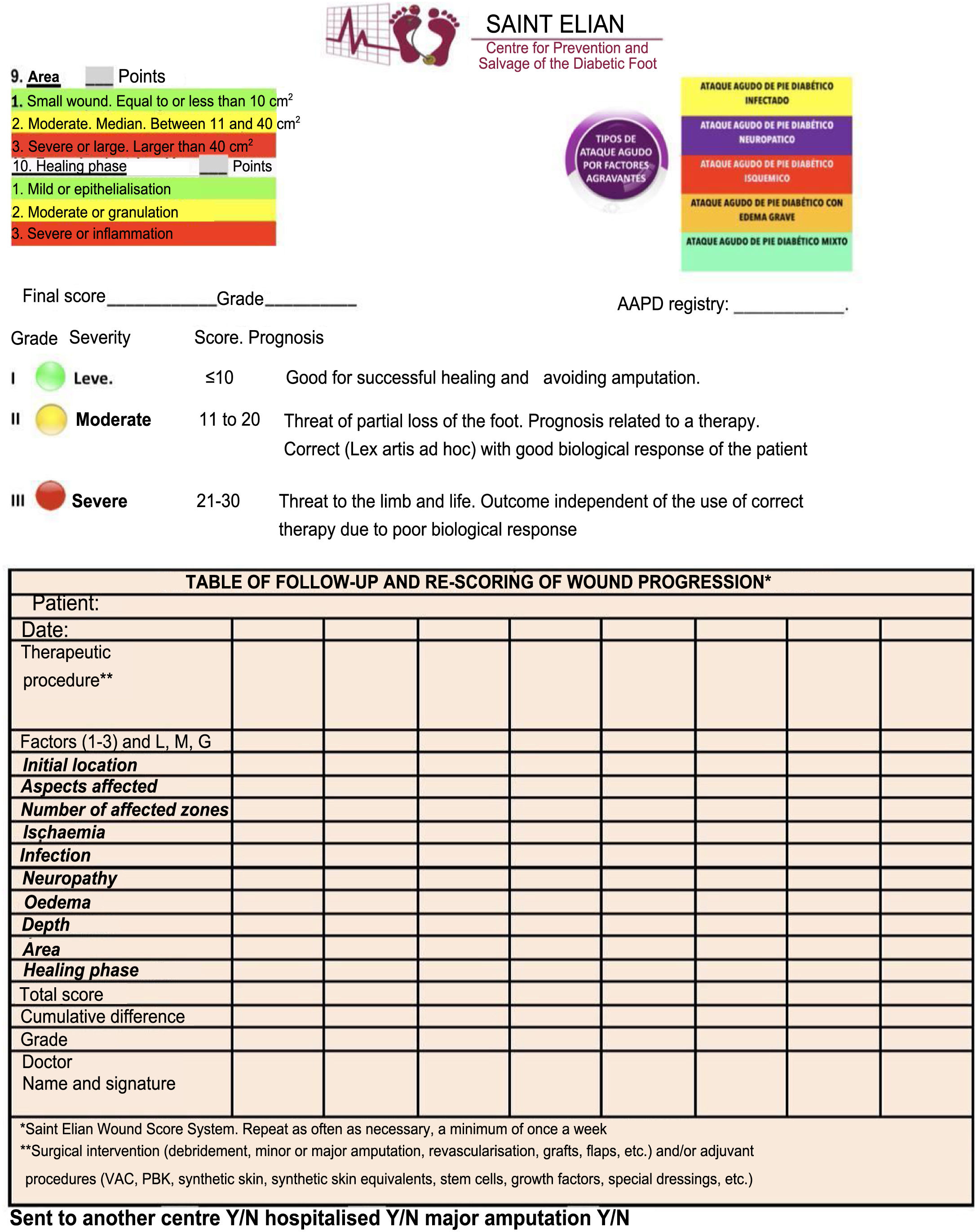
DF classification: Latin American Saint Elian SystemThe Saint Elian system (Saint Elian Center for Prevention and Salvage of the Diabetic Foot, Veracruz, Mexico) includes diagnosis, classification, treatment, monitoring, and prevention of DF.63–66 Since classification is based on the location of foot injuries and their severity, it includes 10 variables, which are divided into mild, moderate, and severe. (See Tables 1 and 2).67–69
The final score obtained will suggest the following- 1.
Grade I (mild, good prognosis for successful healing)
- 2.
Grade II (moderate, partial risk, results depend on the application of adequate treatment and the patient's biological response)
- 3.
Grade III (severe, the affected extremity and the patient's life is at risk) (See Table 3)
Table 3.Saint Elian Wound Score results.
Grado de gravedad Puntaje Pronostico I Leve <10 Bueno para cicatrización exitosa y evitar amputación. (Éxito 9/10) II Moderado 11 a 20 Amenaza de perdida parcial del pie pronostico relacionado a una terapéutica correcta con una buena respuesta biológica del paciente. (Éxito 7/10) III Grave 21 a 30 Amenaza la extremidad (amputación mayor) y la vida. Resultado independiente. Del uso. De una terapéutica correcta por mala respuesta biológica. SAINT ELIAN WOUND SCORE, SEVERITY GRADE
Reasons for using the Saint Elian Classification:
- 1.
For reclassifying DM foot injuries
- 2.
For visualizing improvements in the clinical conditions of Grades II and III
- 3.
For reassessing wounds to visualize improvement in healing
- 4.
For gaining information regarding failed procedures that may lead to amputation/death
- 5.
For analyzing the patient in a comprehensive and not so specific way (considering that the foot is a part of the body)
(See Tables 4 and 6).
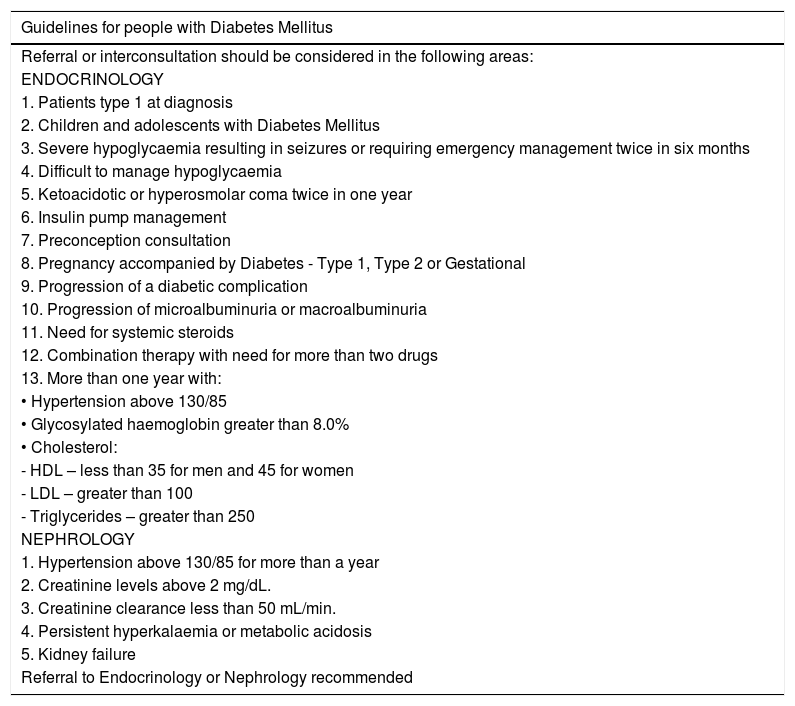
Criteria for remission while receiving outpatient care:These are the state of the art criteria in the care of patients with DM. We are aware that our physicians, paramedics, and specialized professionals under training have different levels of expertise in different medical fields. Although strict rules regarding patient referral cannot be established, these criteria should always be considered when one of the goals set for a patient cannot be achieved. Moreover, even experts should consider consulting another specialist when a goal cannot be achieved. (See Table 5).
Outpatient referral criteria for patients assessed for diabetic foot or diabetic foot disease.
| Guidelines for people with Diabetes Mellitus |
|---|
| Referral or interconsultation should be considered in the following areas: |
| ENDOCRINOLOGY |
| 1. Patients type 1 at diagnosis |
| 2. Children and adolescents with Diabetes Mellitus |
| 3. Severe hypoglycaemia resulting in seizures or requiring emergency management twice in six months |
| 4. Difficult to manage hypoglycaemia |
| 5. Ketoacidotic or hyperosmolar coma twice in one year |
| 6. Insulin pump management |
| 7. Preconception consultation |
| 8. Pregnancy accompanied by Diabetes - Type 1, Type 2 or Gestational |
| 9. Progression of a diabetic complication |
| 10. Progression of microalbuminuria or macroalbuminuria |
| 11. Need for systemic steroids |
| 12. Combination therapy with need for more than two drugs |
| 13. More than one year with: |
| • Hypertension above 130/85 |
| • Glycosylated haemoglobin greater than 8.0% |
| • Cholesterol: |
| - HDL – less than 35 for men and 45 for women |
| - LDL – greater than 100 |
| - Triglycerides – greater than 250 |
| NEPHROLOGY |
| 1. Hypertension above 130/85 for more than a year |
| 2. Creatinine levels above 2 mg/dL. |
| 3. Creatinine clearance less than 50 mL/min. |
| 4. Persistent hyperkalaemia or metabolic acidosis |
| 5. Kidney failure |
| Referral to Endocrinology or Nephrology recommended |
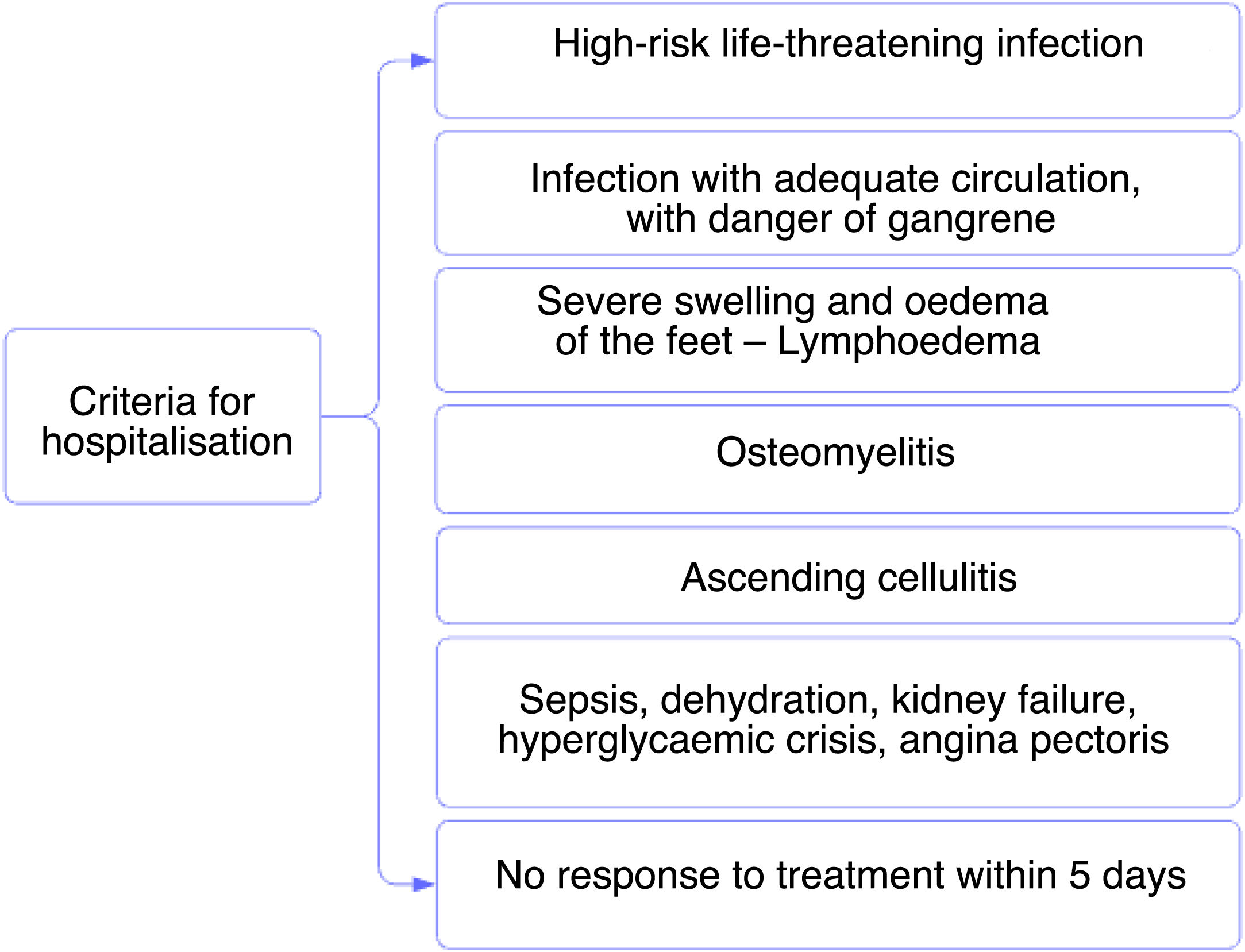
Hospitalization criteria are defined in Tables 7 and 8.
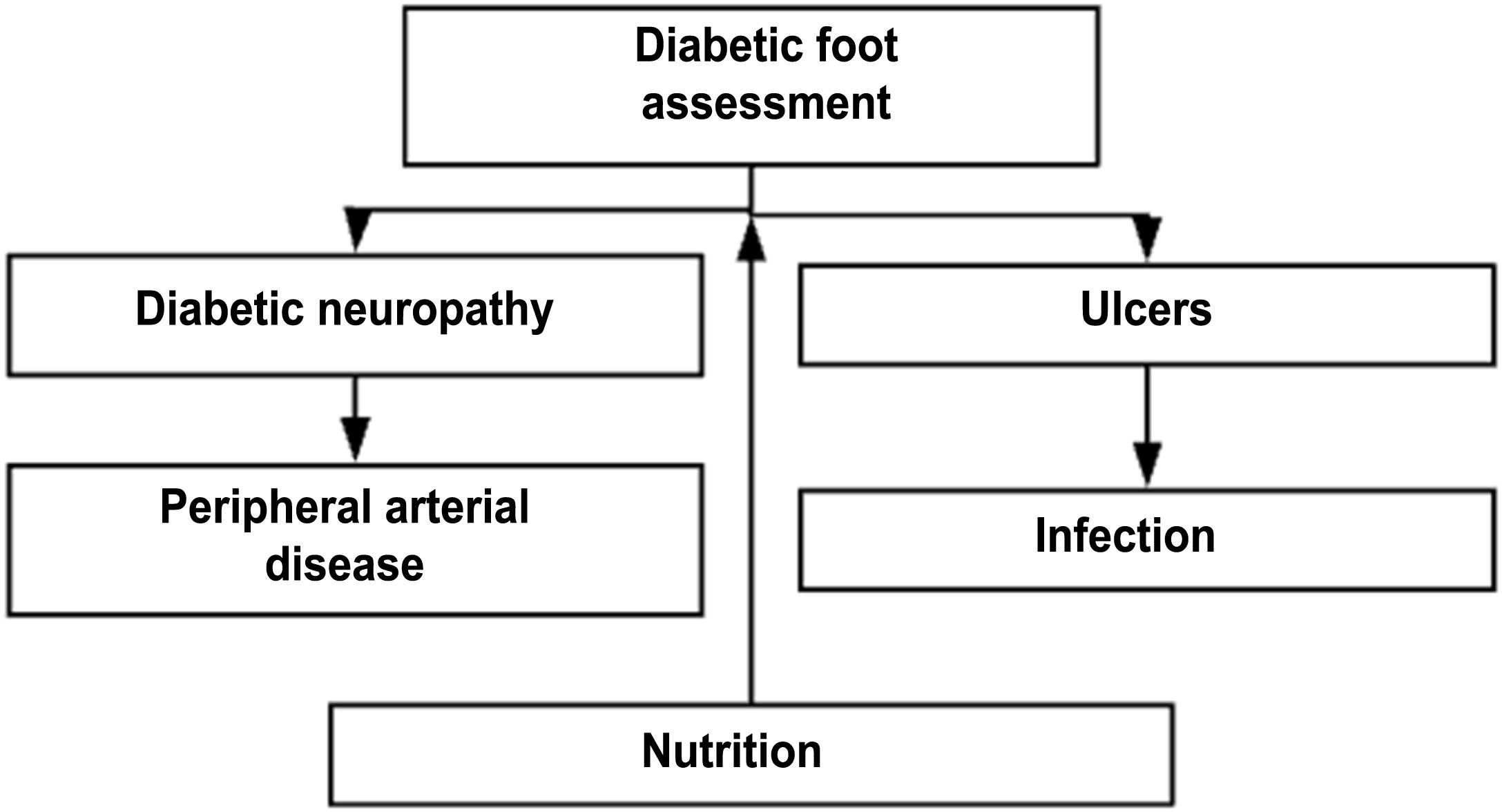
DF assessmentTable 7. Flow diagram for the comprehensive assessment of diabetic foot
Steps for the comprehensive care of a patient with DF receiving outpatient care or being admitted into the hospital or the emergency unit
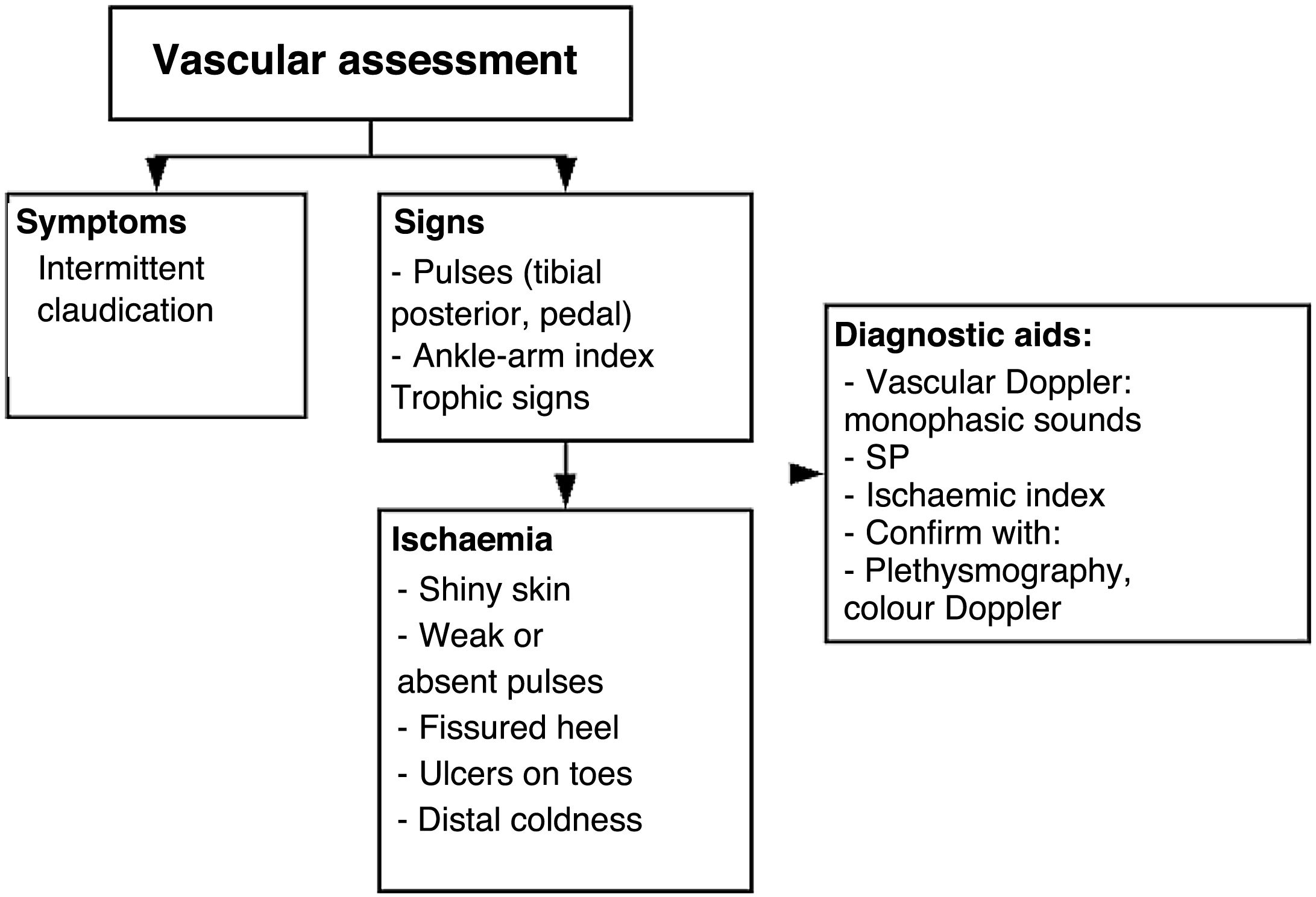
Vascular surgery assessmentTable 8. Flow diagram for the comprehensive management of a patient with diabetic foot receiving outpatient care or being admitted into the hospital or the emergency unit
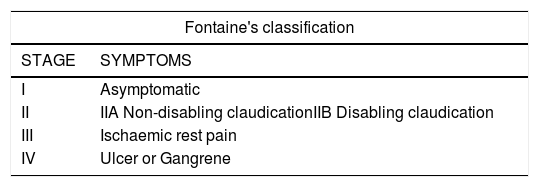
Fontaine classificationIt is important to consider that severe phase I patients, despite showing no symptoms, are elderly individuals with multiple comorbidities and reduced mobility (masked occlusive peripheral arterial disease); therefore, patients’ gait and the presence of neuropathy should be assessed before evaluating the presence of pain when walking. (See Table 9).
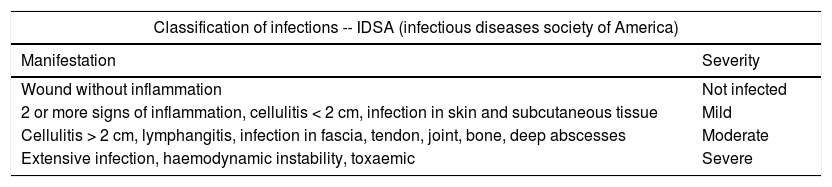
InfectionClassification of infections: International Federation of Infection Control (IDSA) Table 10
When to consider vascular or orthopedic surgery: Table 11
Classification of infections.
| Classification of infections -- IDSA (infectious diseases society of America) | |
|---|---|
| Manifestation | Severity |
| Wound without inflammation | Not infected |
| 2 or more signs of inflammation, cellulitis < 2 cm, infection in skin and subcutaneous tissue | Mild |
| Cellulitis > 2 cm, lymphangitis, infection in fascia, tendon, joint, bone, deep abscesses | Moderate |
| Extensive infection, haemodynamic instability, toxaemic | Severe |
Defining criteria for amputations
- 1.
Cold thermal sensation of the skin Table 12
- 2.
Absence of pedal pulse even when assessed using Doppler
- 3.
Presence of large-artery occlusion as per arterial eco-Doppler
- 4.
Ankle–brachial index <0.5, severe damage
- 5.
Plethysmography, if possible
- 6.
Anemia <11g/dL
- 7.
Assessment of ulcers and their comorbidities based on Saint Elian criteria.
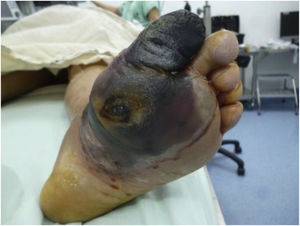
Dysregulated endothelial function caused by endothelial resistance has been identified as an early vascular damage indicator for the development of prediabetes and DM2. The progression of these changes in the lower limb (from microvascular to macrovascular) may be evidenced by the onset of reactive hyperemia and can be stratified by different tests, such as the ankle–brachial index, pletismography, or eco-Doppler. A high correlation between reactive hyperemia and high HbA1c levels has been described. A comprehensive approach should be adopted by combining assessments performed in the vascular and orthopedic surgery departments to define the level of amputation required or the necessity of revascularization. When choosing amputation, its level should be determined based on the involvement of the vascular system and the viability of angiosomas surrounding the ulcer or necrosis site. The decision to amputate should be made based on the patient's clinical state and supported by test results, such as the degree of vessel occlusion by eco-Doppler, 0.9 microvascular damage and 0.5 severe damage values obtained by the ankle–brachial index,70 or the degree of occlusion as shown by pletismography. On the other hand, patients with anemia and hemoglobin <11g/dl show a significant correlation with amputation outcomes mainly caused by the ulcer progression factor .71 Regarding the level of amputation, experience shows that above-the-knee amputations (AK) are preferable to prevent secondary skin necrosis; however, this has been changing substantially since it has been evidenced that patients’ survival changes considerably following suprapatellar amputations. Decisions should be made on an individual basis, and there are no general rules that may be applied at a population level based on scientific evidence.
UlcersGoals for the management of a nonulcerated DF with adequate diabetes control Table 13:
- 1.
Correction of deformities associated with hyperkeratosis that may result in pressure ulcers. Surgical correction is not ruled out if the disease is monitored adequately. Whether minimally invasive surgical techniques have benefits or not is currently under discussion. When the disease is not monitored adequately, corrections should be made through the shoe with total contact orthosis .72,73
- 2.
Nociceptive and proprioceptive rehabilitation: Physiotherapy with surface stimulation and lymphatic drainage should be conducted in order to recover 10-g sensitivity (Semmes Wenstein 5.07 monofilament), and patients should follow a home-based exercise plan for the rest of their lives. The exercise plan includes corner and circuit proprioceptive exercises (race box and Figure 8) in which patients must walk with the aim of maintaining proprioception levels of>11 s in the modified Romberg test. Since mechanical fatigue (stress) is involved in the pathophysiology of Charcot arthropathy, proprioceptive rehabilitation will be similar to the one performed for ankle and foot fractures (race box and Figure 8; depending on the patient's age, backwards walking or jogging and carioca exercises may not be included). Proprioceptive physiotherapy rehabilitation is usually prescribed by an orthopedist, physiatrist, or foot surgeon based on platform stability (stable or unstable), visual feedback (with or without visual feedback), and support pattern (single foot or both feet). However, the prescription itself and its execution should be adjusted as per the modified Romberg test result. With 0- to 1-s values, proprioceptive rehabilitation should be performed on a stable platform with visual feedback and supported by both feet; with 2- to 11-s values, it can be performed on a stable platform with no visual feedback and supported on a single foot, including corner exercises; and when values are>12 s, rehabilitation should be performed on an unstable platform without visual feedback and supported on a single foot. From 15 s onward, home-based circuit exercises can be included (race box and Figure 8). Rehabilitation does not end upon the physiotherapist's or treating physician's decision; the purposes of proprioceptive rehabilitation must be met according to the modified Romberg test result.
- 3.
Skin and skin appendage management: Hydration creams should be applied, avoiding application in interdigital spaces .74,75 In hyperkeratosis and ungual mycosis cases, magnesium sulfate (Epsom Salt) should be used to soften skin appendages and hyperkeratosis. In severe ungual deformities, onychomycosis should be treated orally with itraconazole or terbinafine .76,77 (See Table 13).
Fungal infections eventually result in nail deformities, which are a risk factor for DF. Moderate deformities can be managed by patients and do not require oral antifungal drugs.
Severe deformities, usually involving one or two nails, are best managed by the patient or a foot care specialist and do not tend to require oral medication. In some cases, nail ablation (permanent nail removal) is necessary when the aforementioned steps do not work.
Patients with diabetes having fungal infections in multiple nails and severe deformities can take oral medications under the following circumstances:
- •
When they show clinically significant peripheral neuropathy or peripheral vascular disease
- •
When their nails have received specialized care at least for 2 months
- •
When they are using shoes of adequate depth and with the necessary corrections
- •
When they show potential risk of injuries due to mechanical debridement, even when performed by a specialist.
Due to its effects on cytochrome p450, itraconazole causes hypoglycemia by increasing oral hypoglycemic drug levels in the blood. Terbinafine is metabolized in the liver and does not affect the blood sugar levels of patients under oral hypoglycemiant drugs. None of these drugs affects blood sugar levels of patients receiving insulin .78,79
Many studies show that the use of itraconazole pulse therapy is as effective as daily terbinafine administration. Itraconazole is used 7 days a month over 3 months (42 capsules), whereas terbinafine is used daily over 12 weeks (84 tablets).
Many studies show no cost–benefit relationship for the use of fungal culture before initiating this treatment; therefore, it is not a recommended practice .80,81
Diabetes control in patients with nonulceratedDFIn addition to specific pathology control in patients with nonulcerated DF, the minimum global disease control goals should be considered and monitored periodically by the physiatrist, podologist, orthopedist, or foot and ankle surgeon (See Table X) to comprehensively manage the systemic pathology and prevent adverse outcomes such as deformities and ulcers.82–84Table 14.
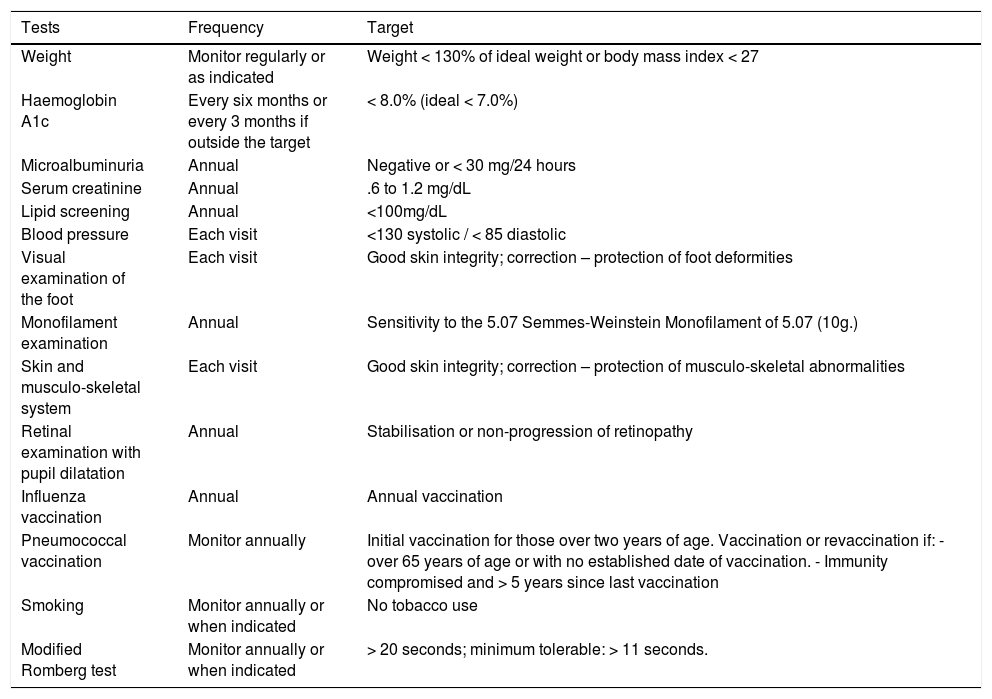
Targets in the management of diabetes in the patient with non-ulcerated diabetic foot.
| Tests | Frequency | Target |
|---|---|---|
| Weight | Monitor regularly or as indicated | Weight < 130% of ideal weight or body mass index < 27 |
| Haemoglobin A1c | Every six months or every 3 months if outside the target | < 8.0% (ideal < 7.0%) |
| Microalbuminuria | Annual | Negative or < 30 mg/24 hours |
| Serum creatinine | Annual | .6 to 1.2 mg/dL |
| Lipid screening | Annual | <100mg/dL |
| Blood pressure | Each visit | <130 systolic / < 85 diastolic |
| Visual examination of the foot | Each visit | Good skin integrity; correction – protection of foot deformities |
| Monofilament examination | Annual | Sensitivity to the 5.07 Semmes-Weinstein Monofilament of 5.07 (10g.) |
| Skin and musculo-skeletal system | Each visit | Good skin integrity; correction – protection of musculo-skeletal abnormalities |
| Retinal examination with pupil dilatation | Annual | Stabilisation or non-progression of retinopathy |
| Influenza vaccination | Annual | Annual vaccination |
| Pneumococcal vaccination | Monitor annually | Initial vaccination for those over two years of age. Vaccination or revaccination if: - over 65 years of age or with no established date of vaccination. - Immunity compromised and > 5 years since last vaccination |
| Smoking | Monitor annually or when indicated | No tobacco use |
| Modified Romberg test | Monitor annually or when indicated | > 20 seconds; minimum tolerable: > 11 seconds. |
Charcot neuropathy, or Charcot arthropathy, is a complex pathology that is hard to manage in patients with an associated chronic metabolic pathology (DM), which can also cause different injuries in target organs and may result in severe morbidities in patients .85,86 (See Table 13). It is a diagnostic and therapeutic challenge. Charcot arthropathy was described more than 140 years ago by the French neurologist Jean Marie Charcot as an arthropathy associated with foot and ankle syphilis within the natural history of the disease, which may progress into tertiary syphilis (neurological involvement). Even though it has been more than 140 years, Charcot neuroarthropathy still represents a challenge in terms of understanding its pathophysiology; therefore, its management still focuses more on deformity correction rather than prevention or articular rehabilitation in early stages.87,88 In 1936, WR Jordan described a causal association between the changes observed in Charcot neuroarthropathy and DM. Nowadays, DM is the main cause of Charcot neuroarthropathy. Initially, this pathology was associated with different neurological diseases that generate peripheral denervation and degenerative neuroarthropathy under any condition. As antibiotic therapy evolved and the syphilis epidemic ended, it was found that other conditions associated with peripheral neuropathy could also result in similar articular involvement. As life expectancy increased and diseases associated with nutrition and modern lifestyle appeared, DM incidence became higher and it was found that the peripheral neuropathy associated with it could also lead to an articular pathology similar to that caused by syphilis.
The risk of Charcot arthropathy in patients with DM is represented by its prevalence of 0.08%–13% in high-risk populations; however, these incidence and prevalence rates as well as other epidemiological data may be underevaluated because diagnosis is usually late or confounded by infectious processes.
Regarding the etiology and pathophysiology of the disease, several theories accounting for its natural history have been proposed. The neurotraumatic theory describes a decrease or loss of protective sensitivity, which is associated with repetitive microtrauma and results in progressive intracapsular effusion at the articular level. Since it occurs gradually and persistently, it causes ligamentous laxity and articular instability. Persistent and progressive instability in turn results in progressive destruction of articular surfaces, which barely accounts for part of the development of neuroarthropathy. For example, the presence of neuropathy in bed-bound patients who do not suffer any trauma cannot be explained, but they still develop associated deformities.
Additionally, there is the neurovascular reflex theory, which suggests hyperemic bone resorption due to blood flow increase as a cause of this pathology. The blood flow increase associated with endothelial dysfunction occurs due to the loss of smooth endothelial muscle tone. Eventually, this increase will result in spongy bone osteolysis, and progressive lysis decreases contact between articular surfaces due to bone resorption. As a result, instability increases progressively until a deformity is developed in different articular segments of the foot.
This condition is nowadays seen as having multifactorial etiologies, i.e., a combination of the aforementioned theories. In other words, it is caused by repetitive microtrauma in an insensitive foot with decreased proprioception (modified Romberg <11 s), although if well perfused, it could result in Charcot arthropathy. When patients have no sensitivity, they cannot feel trauma and bone damage increases when walking. Bone edema appears first, followed by capsular ligament injury and elongation. Finally, joint destruction occurs followed by articular deformation of the foot.
Charcot neuropathy classificationAnder and Frykberg patterns:
Injury patterns depending on joint location.
Pattern 1: Forefoot: Metatarsals, phalanges
Pattern 2: Lisfranc joint
Pattern 3: Midtarsal and intercuneiform joints
Pattern 4: Ankle and subtalar joints
Pattern 5: Calcaneus (calcaneal insufficiency avulsion fracture)
Natural history of Charcot arthropathyDuring initial consultation, patients report an edema that presents as an obstacle when wearing shoes; this edema may or may not be the result of trauma. It is thought that 75% of patients may feel pain, which undoubtedly means that some patients with initial edema and local heat do not feel pain. In a patient with diabetes and no injury, Charcot neuropathy should always be suspected instead of infection. It is important to consider that sometimes some specialists that are not aware of DF pathophysiology may find inflammation and suggest surgical drainage to treat what they think is an infection or osteomyelitis, when it is actually Charcot arthropathy. Although Charcot arthropathy can be diagnosed through conventional ankle and/or foot radiological series, additional studies are mandatory before making any decisions.89 CT scans should always be performed in addition to gammagraphy with labeled leukocytes if possible. Alternatively, single-photon emission computed tomography (SPECT) can also be conducted before taking the decision to perform surgery 90
As a general rule, peripheral irrigation is not significantly affected and patients’ pulses are evident, unless the edema is so severe that it cannot be located correctly. Patients with Charcot neuroarthropathy tend to preserve perfusion with no further implications. A relevant finding may be an increase in the temperature of one foot but not of the other (3%–8% higher). Skin is erythematous and response to warmth as well as sensitivity is reduced, including proprioception and response towards vibration and pain. Two-point sensitivity should always be assessed using 10-g monofilaments (Semmes Wenstein 5.07). In addition, atrophy of the intrinsic muscles of the foot may be observed, although this sign is usually masked by the patient's edema. These signs can be found in the active and initial stage of the disease, which is the inflammatory stage. At that point, neither deformities nor ulcerations can be seen. Eichenholtz described the following three stages: development, coalescence, and reconstruction. This classification is based on the radiological status of the disease, but it also describes an acute or active stage and a sequel stage in which permanent changes in the foot shape as well as deformities resulting in ulceration and, if not managed properly, infections occur. In the development stage, fractures, bone debris, and fragmentation of the cartilages and subchondral bone are observed. In addition, capsular distension, ligamentous laxity, and articular subluxation can be observed. In the coalescence stage, the disease progresses. This is a noninflammatory stage in which debris absorption, edema reduction, and fracture consolidation can be observed. Finally, in the reconstructive stage we can see articular ankylosis, bone hypertrophic proliferation, and severe bone disintegration. This stage can be confounded with bone lysis, which occurs in infections.
The acute stage is clinically characterized by edema, osteopenia, multiple fractures, loose bodies, luxation, or subluxation. These occur along with well-known changes such as diabetic osteolysis, which is a series of atrophy in the metatarsal heads and phalanges. Severe osteolysis can occur in the forefoot, especially in the ankle and subtalar joints. Ankle fractures coexist with these changes, although they sometimes can trigger the pathology.
The reconstruction stage that follows the inflammatory stage shows changes such as hypertrophy, sclerosis, bone fragmentation, remineralization, and edema reduction. In this stage, the consequences of the disease can be observed in the form of clubfoot, rocker bottom foot, dropped cuboid, etc. These deformities cause ulcerations, which are generally associated with infections. Unfortunately, many patients are diagnosed at this stage. Extensive surgical interventions are required at this stage, and complications that lead to amputation may arise.91
Charcot neuroarthropathy should be suspected if a patient with diabetes shows edema, insensitivity, and increased temperature, with or without the presence of trauma or foot injury history. In addition, three-projection X-rays with support should be requested to look for the aforementioned findings described for the acute phase.92,93
In case of ulcerations, performing X-rays is not enough to differentiate arthropathy from osteomyelitis; therefore, the presence of acute phase reactants, which are useful for identifying an acute infection process, should be tested. Differentiating between Charcot neuropathy and an infectious process is generally difficult in these patients. Orthopedists should use as many diagnostic tools as possible to diagnose these pathologies accurately and treat them adequately, decreasing sequelae as much as possible.94
Bone biopsy may be considered the gold standard for diagnosis. If it shows bone and soft tissues wrapped in synovial layers, it is a sign of Charcot arthropathy. However, the benefit–risk ratio of this procedure, in which an external pathogen may be introduced to a healthy patient and a severe infectious process may be triggered, does not allow for its use in a routine basis.
MRI can be useful in initial stages, but differentiating Charcot arthropathy from osteomyelitis using MRI can be challenging; therefore, SPECT can be used instead. It can detect Charcot arthropathy in its early stages with up to 95% sensitivity and specificity when labeled leukocytes (99mTc-HMPAO) and 3D reconstruction are used.
In the first stages of the disease, a conservative approach can be adopted to prevent the development of associated deformities. Some conservative strategies are stress reduction and immobilization of the affected limb. Immobilization is achieved by asking the patient to not stand on the foot for 8–12 weeks, the time necessary for the active phase to finish and the inactive phase to start. During this process, a plantigrade foot should be preserved as the acute bone fragmentation and articular damage phase comes to an end. The use of crutches may increase stress in the contralateral extremity, which may result in neuroarthropathy associated with the same metabolic disease (DM) in the contralateral limb. Orthosis-mediated immobilization (brace) or the use of rigid ankle walker boot are good options; the use of total contact cast is decreasing because its unpleasant smell makes it uncomfortable for patients. However, it is still useful in cases wherein monitoring can be difficult, such as rural areas. Over time, literature has reported good clinical results related to the use of nonremovable devices, such as total contact cast for the treatment of plantar ulcers in Charcot arthropathy, and limited evidence of positive results related to the use of removable devices. However, regardless of the unloading device used for prevention or treatment, the main goal should be to conduct strict monitoring and achieve adequate patient adherence to treatment because even the best unloading device will not be effective if it is not adequately used. Regardless of the device chosen, it should reduce plantar pressure in the foot area at risk for ulceration, which is the main factor for the prevention of plantar ulcer development in patients with neuropathy.
Total contact and support casts, if chosen, should be changed every week, given that the edema reduces in size and the foot is adequately shaped.
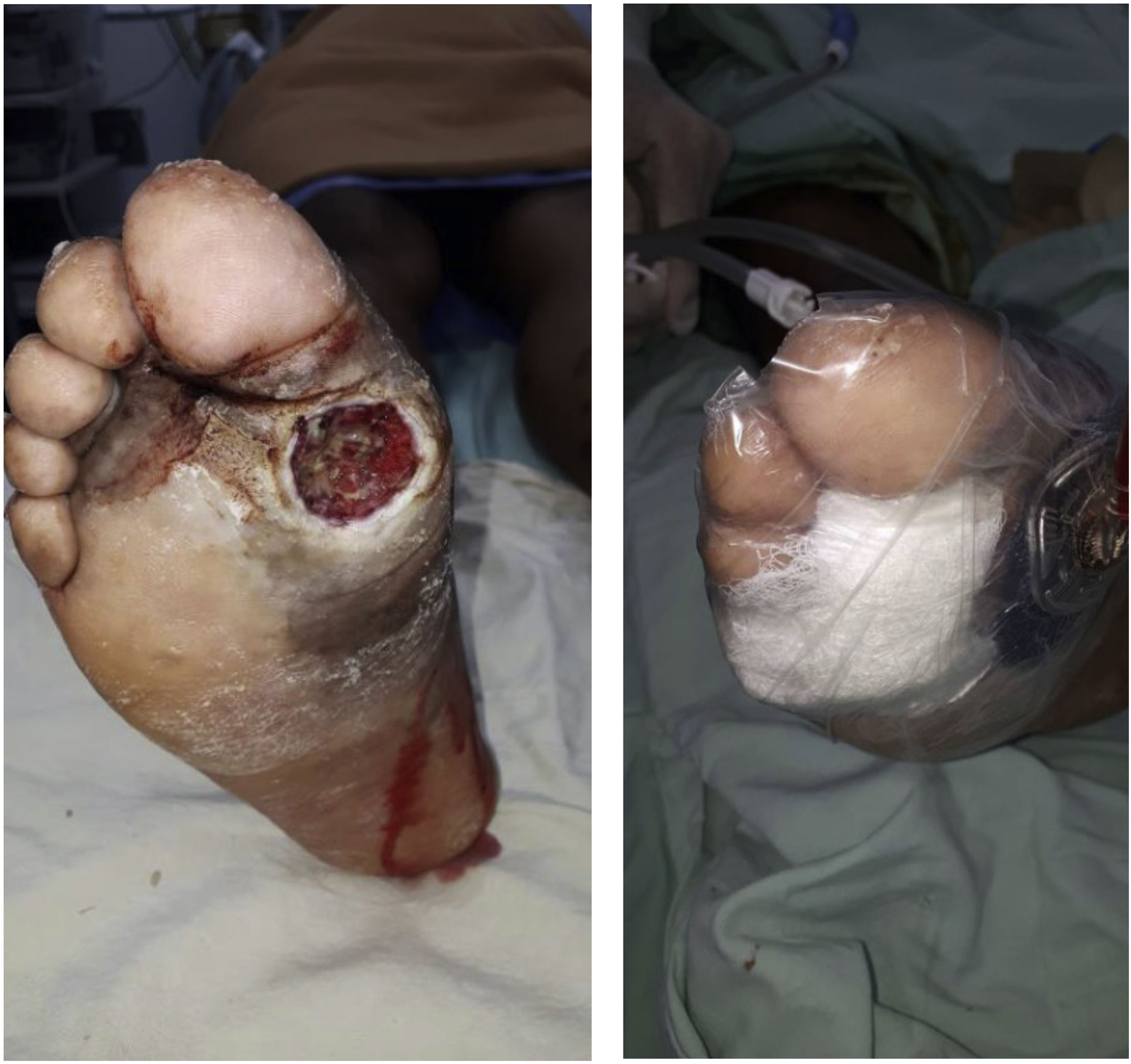
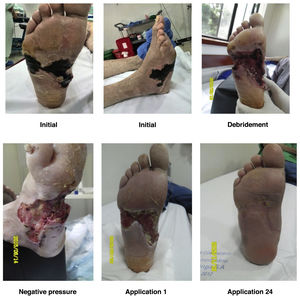
In case of ulcerations, they should be debrided and its progression should be carefully monitored. Any necessary number of debridements should be performed until the ulceration is fully clean. Usually, these ulcerations can get infected; therefore, they should be managed carefully and clinical monitoring should be performed continuously.
Load on the affected limb should be reduced and the same should be maintained for 3–6 months. Entering the reparative phase is extremely important so that the edema can decrease with as little residual deformity as possible. The success of this practice depends directly on the level of bone destruction observed in the inflammatory phase. Radiographic monitoring should be performed to detect reparative changes and assess disease progression, which will help us assess the prognosis and predict the possible need for additional surgical management.
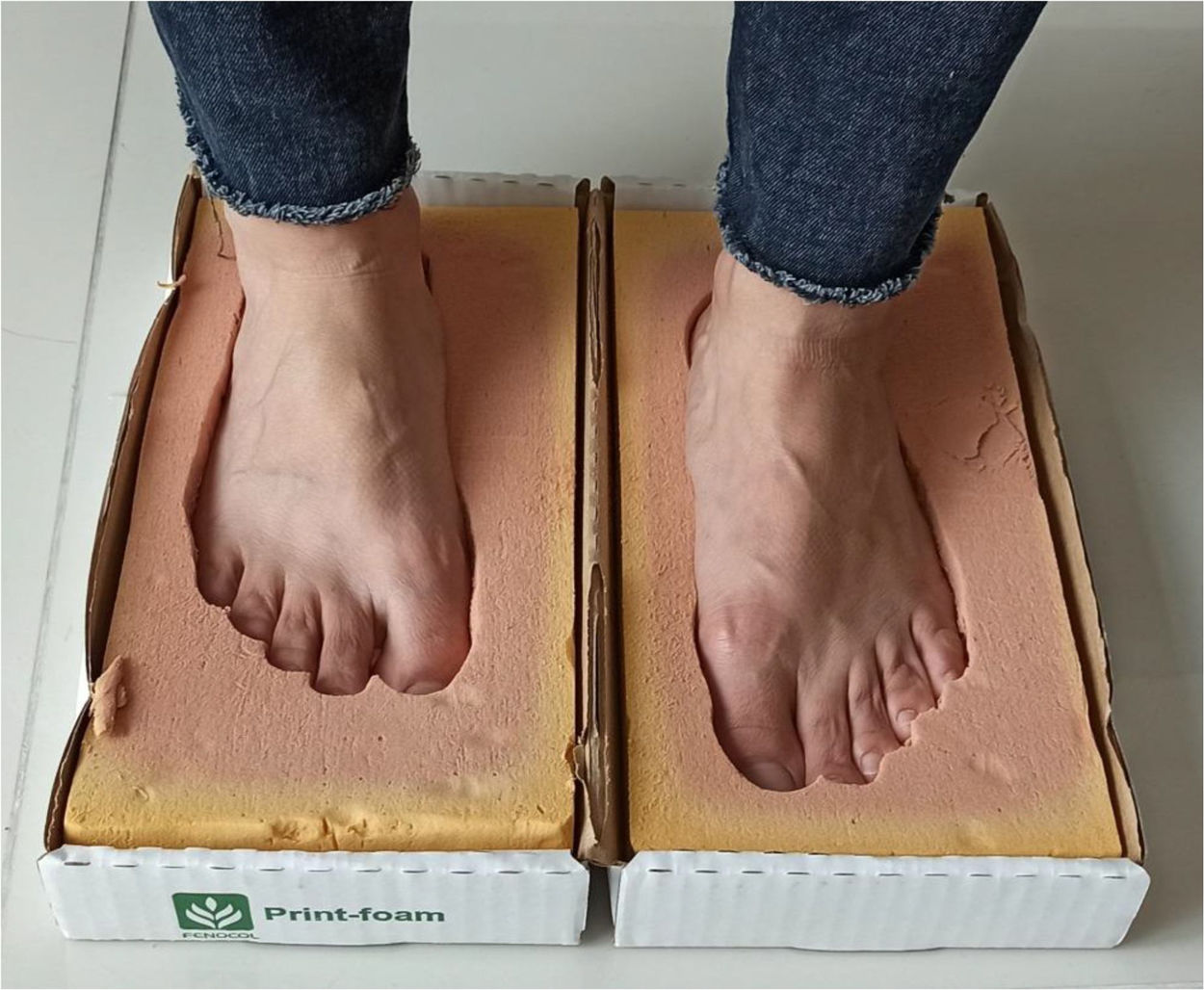
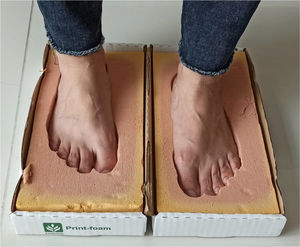
Multiple ways of immobilization can be employed—use of total contact cast, walker boot with no articulation, bivalve cast, braces that ensure safe walking and fracture consolidation. In case of severe deformities of the midfoot, surgical correction or the use of tailored shoes that adapt to the new shape of the foot (inserts or total contact insoles preferably made of plastazote and based on a foot cast) in order to prevent ulcerations and potential infections may be necessary. Plantar impression is taken on foam, and shoes should be made based on the forefoot circumference measured at the level of the metatarsal heads and 6cm (See Figure 6) near this area (midfoot circumference) so that the shoe space in which the total contact insert is to be placed is sufficiently wide.
Regarding ankle joint involvement, if the joint is unstable but does not have any significant deformity, tailored shoes can be used along with orthosis. Highly unstable or misaligned hindfoot requires floor-reaction ankle–foot orthosis, which decreases load in the hindfoot by 32%.
In patients with associated bone demineralization, bisphosphonate use has been proposed; however, no changes in clinical or radiographical progression have been evidenced. It is considered as an adjuvant therapy aimed at reducing the time required to reach the inactivity phase. Studies with improved epidemiological design are required to show its actual utility.
New therapies for arthropathy management have been developed. However, as with everything new, there are not enough studies to support its use; therefore, there is a lack of consensus or accurate indications. However, worth mentioning are therapies such as low-intensity ultrasound and electrical bone stimulation, which is an adjuvant therapy indicated for bone nonunions and even for acute fractures because it promotes consolidation. Further studies with improved methodology are warranted to assess their potential benefits. Nevertheless, these are low-risk therapies and therefore can be introduced as useful tools for the management of this complex pathology.
Initially, nonsurgical management alternatives should be employed to change the disease status from active to inactive and reparative. If these strategies fail, surgical management should be considered. Therefore, surgical interventions should only be performed when all the other conservative strategies aimed at maintaining a stable plantigrade foot have failed or when bone destruction and ulceration persist despite having all loads removed. This is observed when a patient's foot shows persistent instability, severe deformity, and progressive destruction, in spite of orthopedic management, as well as persistent ulcerations associated with bone prominences.
Surgical management of Charcot footThe first and most important rule is “refrain from conducting surgical procedures in the acute phase”; not following this rule can worsen bone atrophy and resorption. The second equally important rule is “procedures should take place in the inactive or reparative stage.” Some studies have shown positive results for surgeries performed in early stages of disease for the prevention of subsequent deformities if conservative strategies have failed. Nonetheless, more large-scale studies are required to recommend these practices on a routine basis.
Patients’ age and general medical conditions should be considered before opting for surgical management. A benefit–risk balance of wide reconstructive surgeries in patients aged 60–70 should always be evaluated. In elderly patients, the following question should always be considered: is the patient suitable for undergoing major reconstructive surgery? Another way to assess patient's suitability for undergoing major reconstructive surgery is to consider if arthrodesis is the best option to make the patient's prognosis, and surgical outcome more predictable.
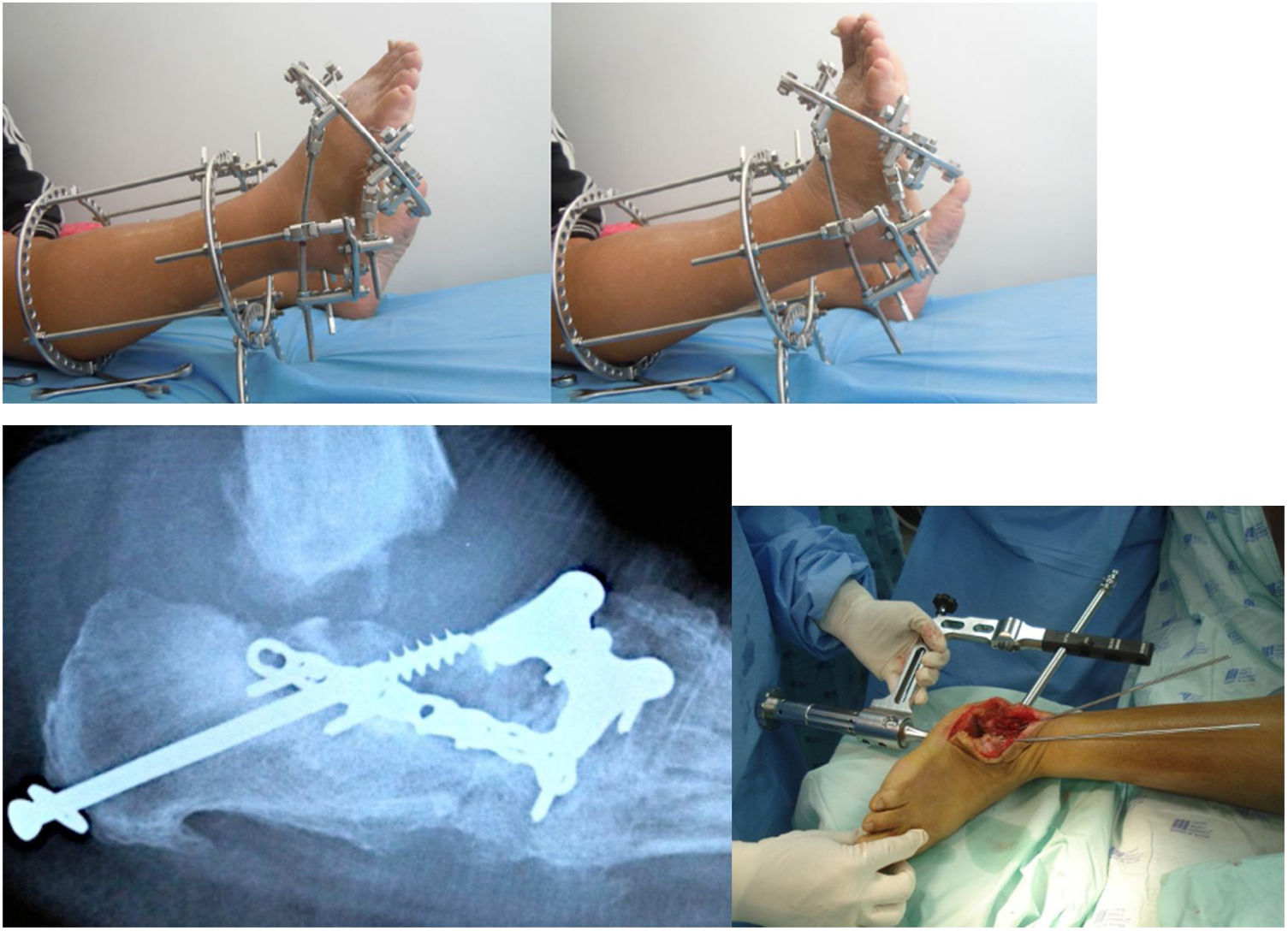
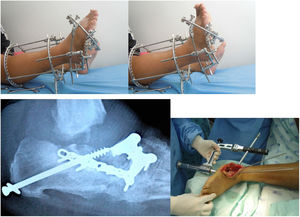
Multiple surgical procedures can be performed, including bone resections, osteotomies, major bone reconstruction, arthrodesis and, if the former fail, amputation. Moreover, surgical management of associated infectious processes requiring surgical debridement should be considered until the infection is healed (See Figure 7).
In Charcot arthropathy, the most usual procedure is the ostectomy of plantar bone prominences in case of frequent ulcerations. The procedure involves three ellipsoidal incisions as well as excision of associated plantar ulcers. Better prognosis and progression have been reported for the resection of bone prominences in the middle column, and healing has been reported in up to 86% of lateral column ulcer cases.
Regarding the management of fractures associated with Charcot arthropathy, nondisplaced fractures of the ankle joint can be managed conservatively with 3-month immobilization. Over that period, the fracture and the affected joint should be subjected to careful clinical and radiographical follow-up. On the other hand, displaced fractures should be treated individually, and generally require open reduction and internal fixation. In some cases, closed reduction can be performed, although secondary displacement rates are not low. In such cases and when the patient has some degree of arthrosis, arthrodesis is the best option. Owing to the pathological conditions of the bone, the displacement rate is high even when the patient is immobilized for 3 months. Avascular necrosis is also frequent, and fusion is necessary to stabilize the joint.
Regarding trauma and forefoot fractures, or residual deformity, arthrodesis may be considered if the subtalar or transverse tarsal joint is affected. In case of avulsion fractures of the calcaneal tuberosity, surgical management is rarely needed. Midfoot deformities are generally managed with exostosis resection. In case of severe deformity and ulceration, arthrodesis should be considered. In case of clubfoot, Achilles tendon lengthening or midfoot osteotomies, such as Cole midfoot osteotomy, may be considered.
When fixing a fracture via arthrodesis or osteotomy, the use of superconstructs is preferred nowadays, which are based on the bridge principle and have rigid fixation elements throughout the osteolytic area. Although healthy joints may be sacrificed, it shows remarkable improvements in terms of stability and outcomes compared to conventional techniques. Fusion covers the region beyond the injured area up to noninvolved joints, increasing the stability of the fixation. Sometimes, additional bone resection may be needed to reduce soft tissue tension caused by the fixation. The most rigid material should be used to provide as much stability as possible. Materials are chosen with the aim to obtain the highest mechanical advantage; depending on the deformity, different kinds of constructs have been designed. Each construct has an advantage that improves its stability. Some techniques are worth mentioning, such as the plantar plaque technique, which is the use of plantar plaques for rocket bottom deformities in the midfoot, or the use of transarticular blocked plaques. It is known that these plaques offer higher axial stability; therefore, the unaffected bone can be used. The reduction loss rate can be decreased by using transarticular axial screws, which consist of cannulated screws fixed to bone structures with less involvement. The aim of this is to increase construct stability and conduct a less invasive procedure to reduce infection rates. Another alternative is performing external fixation once or twice, which is useful if concomitant management of an infectious process is needed. This has shown improvements in the progressive correction of deformities and management of the involved soft tissue. All these constructs are used to obtain the most functional plantigrade foot, prevent falls, and decrease amputation risk (See Figure 8).
Finally, we should always remember that joint replacements of the ankle and foot are contraindicated in Charcot arthropathy given the lack of neuromuscular control caused by the decrease in or absence of proprioception.95
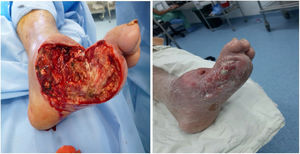
Ulcerated DFUlcer management in patients with DFUlcers occurring in a DF with no wide necrotic areas can be managed with different outpatient techniques. In a few words, there are different techniques that can be used to achieve local bacterial control and stimulate the healing process. As a general rule, the injury will not heal if the bacteria or infection are not locally controlled; therefore, techniques to control local contamination or infection are essential to facilitate adequate skin healing and ulcer resolution. However, local control of the contamination or infection is not enough to achieve adequate skin healing. Therefore, ulcer management can be divided into the following two stages: local infection and contamination control and healing stimulation.96
Local infection control in an ulcerated DF: In order to achieve local control of bacterial contamination and/or ulcer infection in a DF, the physiological and pathophysiological mechanisms underlying ulcer development—microangiopathy and associated neuropathy—should be studied first. A diabetic ulcer will not occur if there is no microangiopathy with associated neuropathy. If these two are present, it is considered that the natural skin barrier has been overcome and local bacterial colonization in the ulcer is inevitable. In other words, as it happens in every wound, once the underlying integumentary tissues have been exposed for 6hours, the wound is considered contaminated and closure by secondary intention should be performed. Since the DF ulcer is a contaminated and hypovascular wound with a neuropathic factor and closure will be achieved by secondary intention, each of the factors responsible for ulcer development should be corrected.97 First, diabetes should be controlled. If diabetes cannot be controlled via outpatient care, the ulcer cannot be treated in an outpatient basis. On the contrary, if diabetes can be controlled via outpatient care, the ulcer can probably be treated in an outpatient basis. The first factor that facilitates the improvement of vascularity in an ulcerated DF is underlying disease control. When there is no local or generalized gangrene, critical microangiopathy is absent. Moreover, the foot angiosomes are likely to supply other adjacent angiosomes during a physiological process that lasts 7–21 days. Based on these two principles, we could state that if local control of the wound contamination is achieved, clinically significant healing signs will be observed after 21 days.
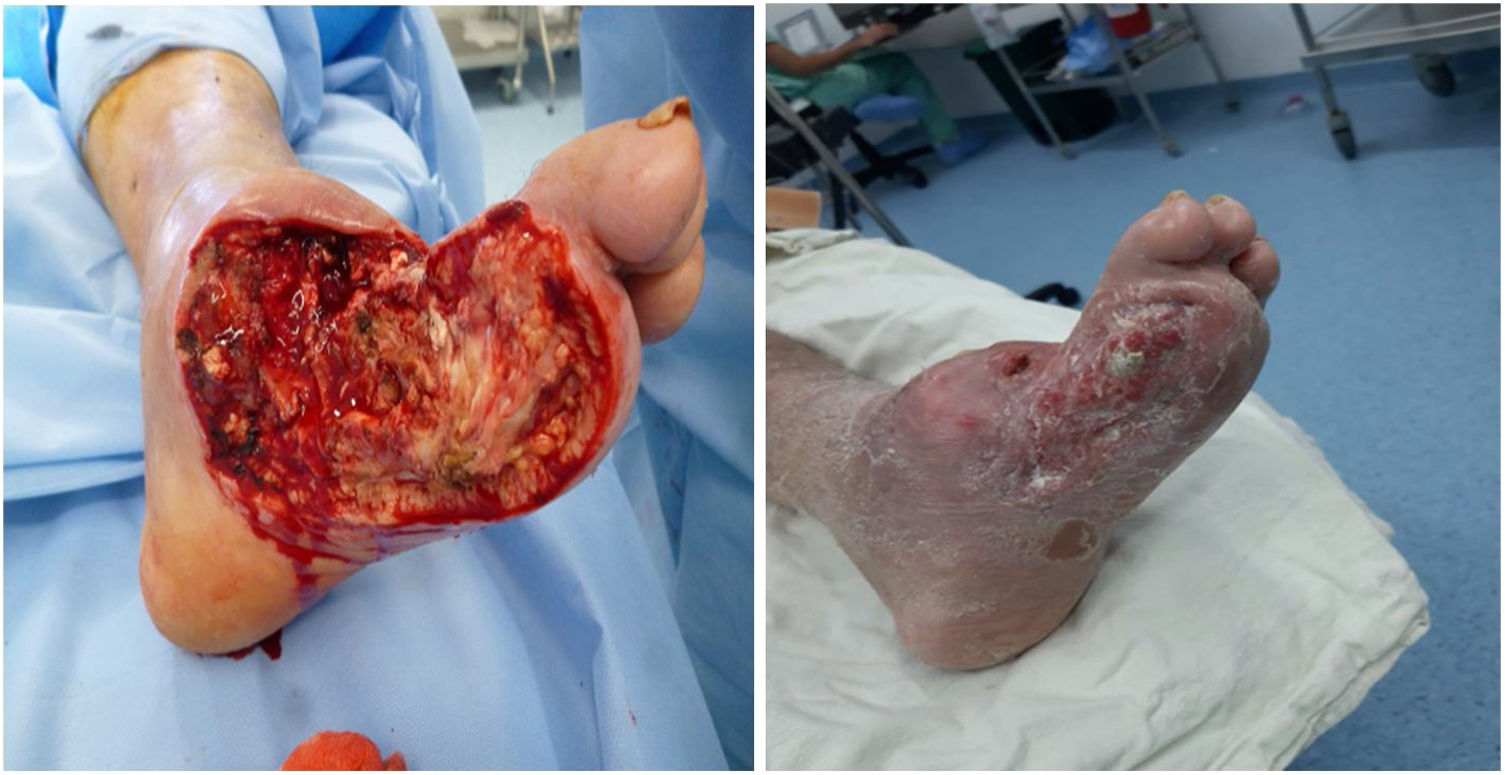
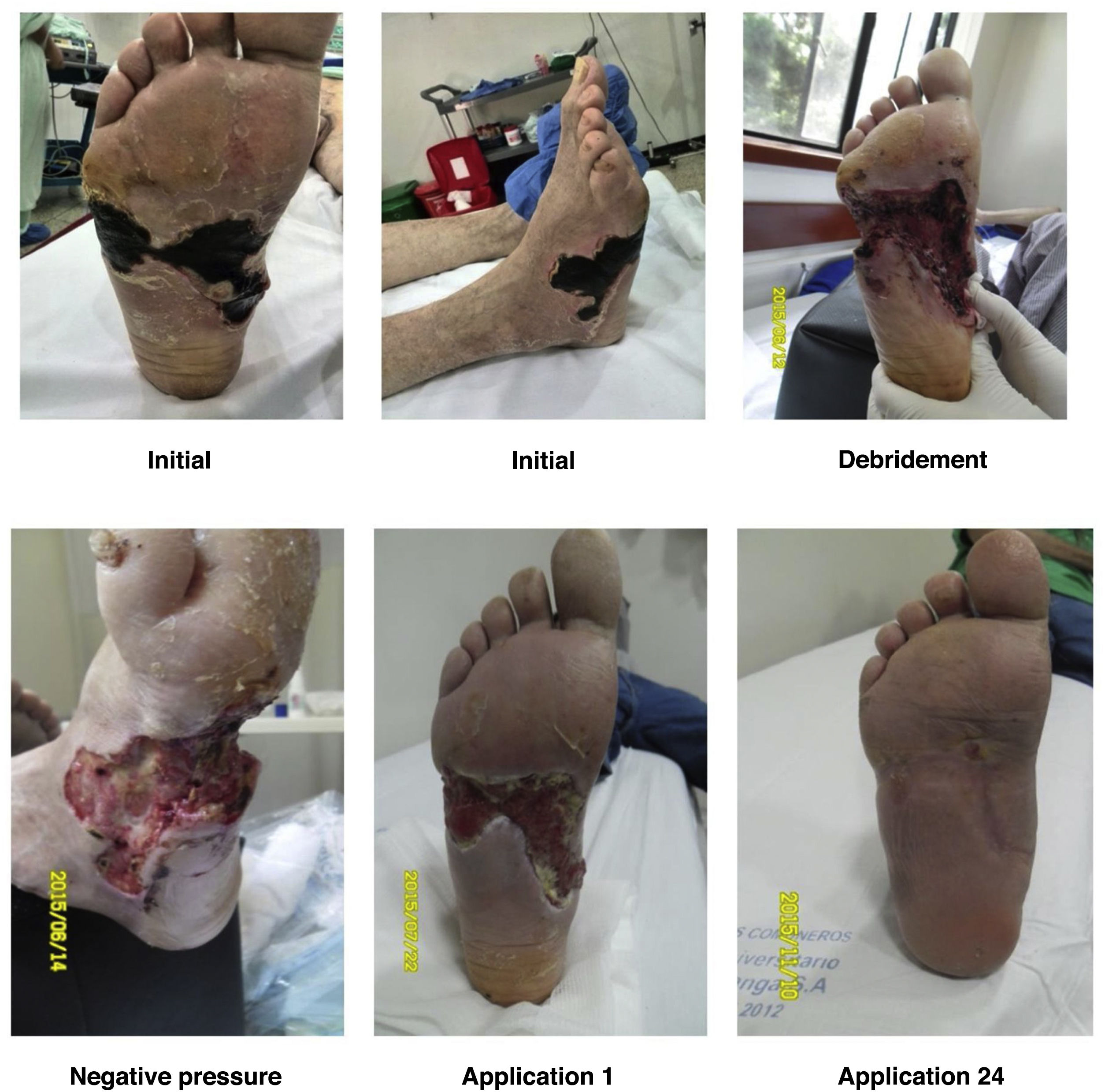
For a normal foot, a surgical wound is thought to be infected if serous drainage persists after 21 days or after 14 days in the proximal third of the leg and near the proximal area. Unlike other wounds, foot wounds, especially in a DF, need a considerably long time to heal, which is why the methodology reported in the literature for performing clinical trials of healing stimulation techniques is confusing. As a general rule, debridement of necrotic tissue should be performed before applying healing stimulators. This does not mean that healing stimulators may not be used as platelet or recombinant growth factors (See Figures 9 and 10).
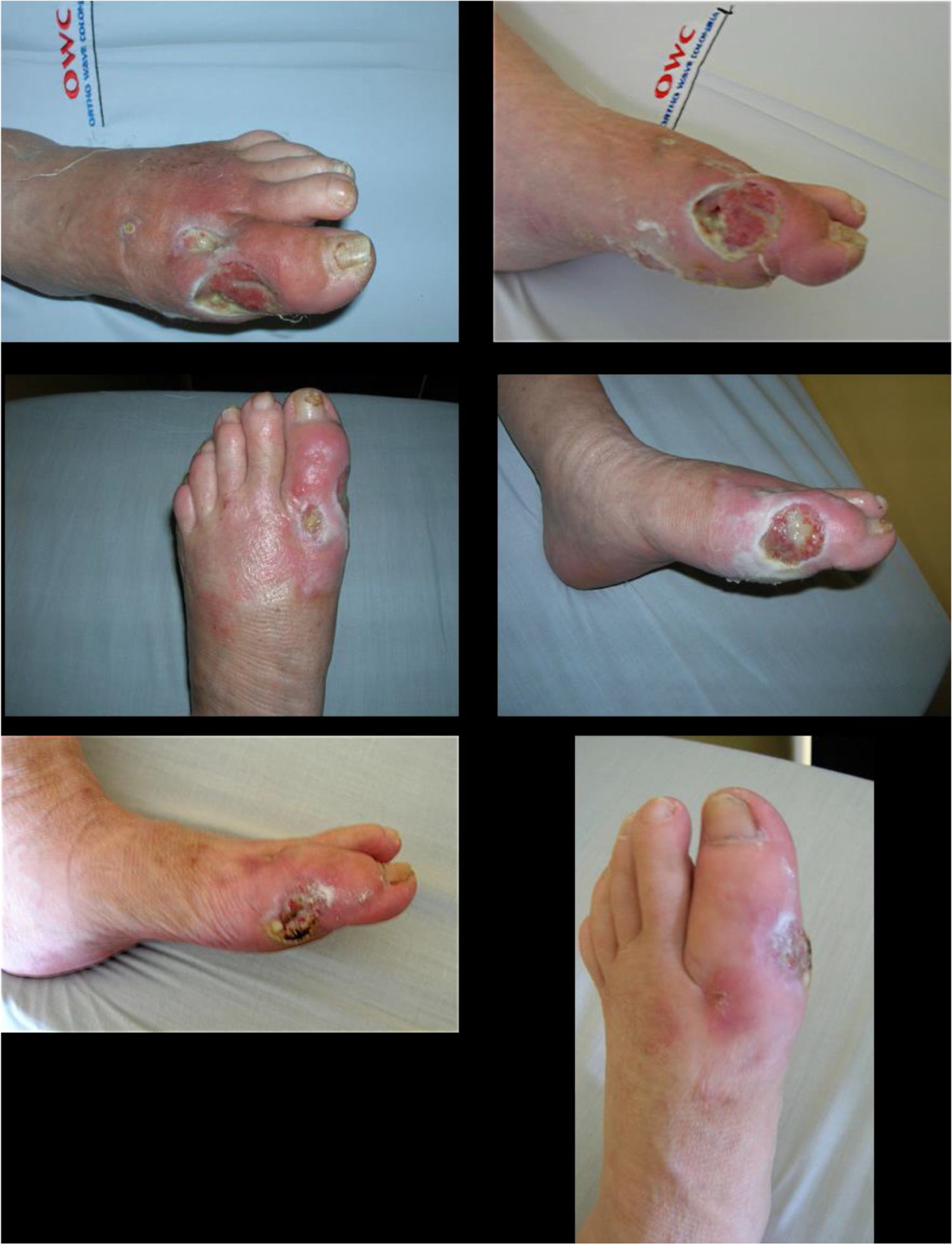
Recombinant Human Epidermal Growth Factor (rhEGF): It acts as an adjuvant agent for complex ulcer healing and can be applied within and around the wound. It controls and stimulates cell migration through the proliferation of fibroblasts, keratinocytes, and vascular endothelial cells; decreases oxidative stress; promotes cell differentiation and survival against metabolic events or exacerbations. It is applied after careful debriding of dead tissues and adequate control of the infection and arterial perfusion.
Its safety profile is adequate and should be carefully used in patients with uncompensated coronary disease, untreated type III heart blockages, and acute DM complications. It is contraindicated in patients with active malignancies. It should be administered by a health care professional trained for the management of its possible adverse events, which include pain in the administration area, chills, and pruritus. These can be controlled with support therapy such as antipyretic, analgesic, and antihistamine use. Following its use, a considerable decrease in healing times of wounds, whose infection has been controlled, has been observed in patients with complex ulcers.
Using 75μg of nepidermin (Epiprot ®) three times a week and following the treating physician's instructions improves granulation and decreases healing time, which results in excellent outcomes for the closure of complex ulcers, such as the ones observed in a DF, by secondary intention.
Experienced professionals should administer this local medication. Once adequate metabolic and infectious control and debridement of dead tissues have been performed, it is considered as an adjuvant agent in the management of ulcers (See Figure 11).
Antiseptics: There are few local antiseptics that can be used when abnormal fibroblast proliferation due to low local oxygen tension (O2) is observed. Elements containing iodine, hydrogen peroxide (oxygenated water), and ethyl and isopropyl alcohol should be avoided because they not only kill bacteria but also damage the lipid layer of fibroblasts, reducing their amount and delaying healing. Ideal options include antiseptics with 4%–20% acetic acid since it acts as a bactericide and bacteriostatic agent as it decreases pH to levels below 4 (bacteria cannot survive at a pH below 4 and fibroblasts can survive at a pH above 2.8). Therefore, fibroblasts can use this anionic gap to proliferate and favor healing in a bacteria-free environment. However, its use is not recommended for patients with considerable neuropathy since it tends to be extremely painful. Commercial chlorhexidine is a local antiseptic and bactericide highly efficient for infection control. However, its use in alcohol and soap solutions should be avoided given the effect of these substances on fibroblasts. For this reason, it can be difficult to obtain. Hypochlorous acid is a bacteriostatic and bactericidal agent that causes cellular degradation and is commonly produced by neutrophils (intracellularly) in a conventional inflammatory reaction; neutrophils use molecular O2 to produce hydrogen peroxide through NADPH oxidase. Neutrophils then take extracellular azurophilic granules loaded with myeloperoxidase and release it intracellularly in order to catalyze the intracellular hydrogen peroxide–chlorine reaction aiming to produce hypochlorous acid and degrade the pathogen intracellularly. Hypochlorous acid is highly powerful inside neutrophils, but at the extracellular level, it leads to the degradation of the fibroblast membrane and any surrounding living cell. It is hardly found in antiseptic commercial preparations, and it is important to mention that it is not the same as sodium hypochlorite because the latter has the same effects but also causes chemical burns at the site of application. Quaternary ammonium (a cationic tensoactive substance) can also be used as a low-potency local bactericide. However, it is associated with high mortality of healing fibroblasts; therefore, it keeps the wound clean but also prevents healing. For this reason, it is mainly used for viral capsid denaturalization. Polyhexamethylene biguanide (PHMB) is a bactericide used to treat acute and chronic wound infections and acts on fungi and gram-positive and gram-negative bacteria. Due to its mechanism of action, it does not cause bacterial resistance. When applied in the inner part of dressings, it limits the occurrence of antimicrobial reactions in the bed of the ulcer, balancing the inflammatory response. Collagenases have no bactericidal or bacteriostatic effect; therefore, their use for keeping a diabetic ulcer clean is not recommended. Collagenase is only used to remove the necrotic tissue of the ulcer in a quick manner; however, bacterial flora does not decrease with its use.
The actual problem with local antiseptics is that they can be bought in a supermarket. Given the Latin American culture, patients tend to confound medical prescriptions with local practices adopted and adapted by the general population (use of calendula, elderflower, red lyrium, coffee plasters, panela plasters, local bicarbonate application, iodated or non-iodated salt, sanctified salt, mineral water, etc.).98 Given that 90% of Latin American physicians are not familiar with the pharmacodynamics or pharmacokinetics of the antiseptic soaps they use to wash their hands every day, if they do not take the necessary time to educate patients to differentiate between a medical prescription and a local practice, patients will replace or adapt the medical prescription even before getting home because of a suggestion made by their cousin, uncle, neighbor, mother in law, or acquaintance who had the same wound in, for example, “their back.”
PHMB (Vulcosan® PHMB) dressings with internal action: the PHMB antimicrobial complex is a sterile and ready-to-use dressing impregnated with a liquid solution of PHMB complex*. It is used for infection decontamination, as prophylaxis against microorganisms, and for preventive protection against bacterial and fungal pathogens in acute or chronic wounds. This highly effective combination dissolves biofilm, cleans the wound, and creates an optimal environment, favoring the formation of granulation tissue.99
Antibiotic therapy: For patients with DF who receive outpatient care, antibiotic therapy should be administered after obtaining specific information regarding the germ or group of germs that are infecting the diabetic ulcer; such information may be collected using an antibiogram. In general, antibiotic use in a patient with gangrene should be planned considering that its bioavailability will change due to microangiopathy. When prescribing antibiotics, the minimum inhibitory concentration obtained in the antibiogram result should be considered, and antibiotic mixtures and their doses should be adjusted accordingly. If the bioavailability of the antibiotic cannot be ensured enterally and parenteral administration is required, the patient should not receive outpatient care.100,101
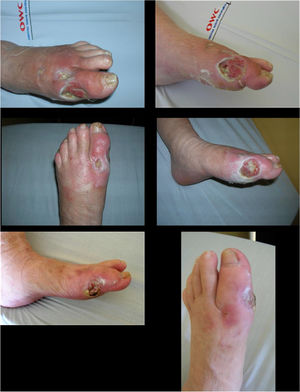
In some experiments, dilutions of antibiotics have been used locally and weekly based on the results of the antibiogram performed for cultures collected directly from the ulcer during surgery (in a sterile surgical room). These dilutions were administered by means of platelet-rich plasma (PRP), which has shown effective bacterial inhibition in in vitro studies. Additionally, the consistency of PRP as a local delivery system for antibiotics has been reported. Complete clinical studies are required to determine the feasibility of performing routine PRP applications with antibiotic dilutions in the outpatient management of these cases and establish accurate indications about when to use this treatment based on the Saint Elian risk and severity classification 102,103 (See Figure 12). However, both PRP and antibiotics have been shown to be safe; despite the lack of evidence level I and II studies, in some special cases its use can be supported in the way described above and at doses determined using antibiograms.
a and b: diabetic ulcer surgically debrided with medial and dorsolateral joint exposure of the left hallux MP; c and d: progression of the ulcer after 4 weeks of weekly application of PRP with antibiotic dilution and selection of the antibiotic according to the antibiogram; e and f: progression of the ulcer after 8 weeks of local application of PRP with antibiotic dilution and selection of the antibiotic according to the antibiogram.
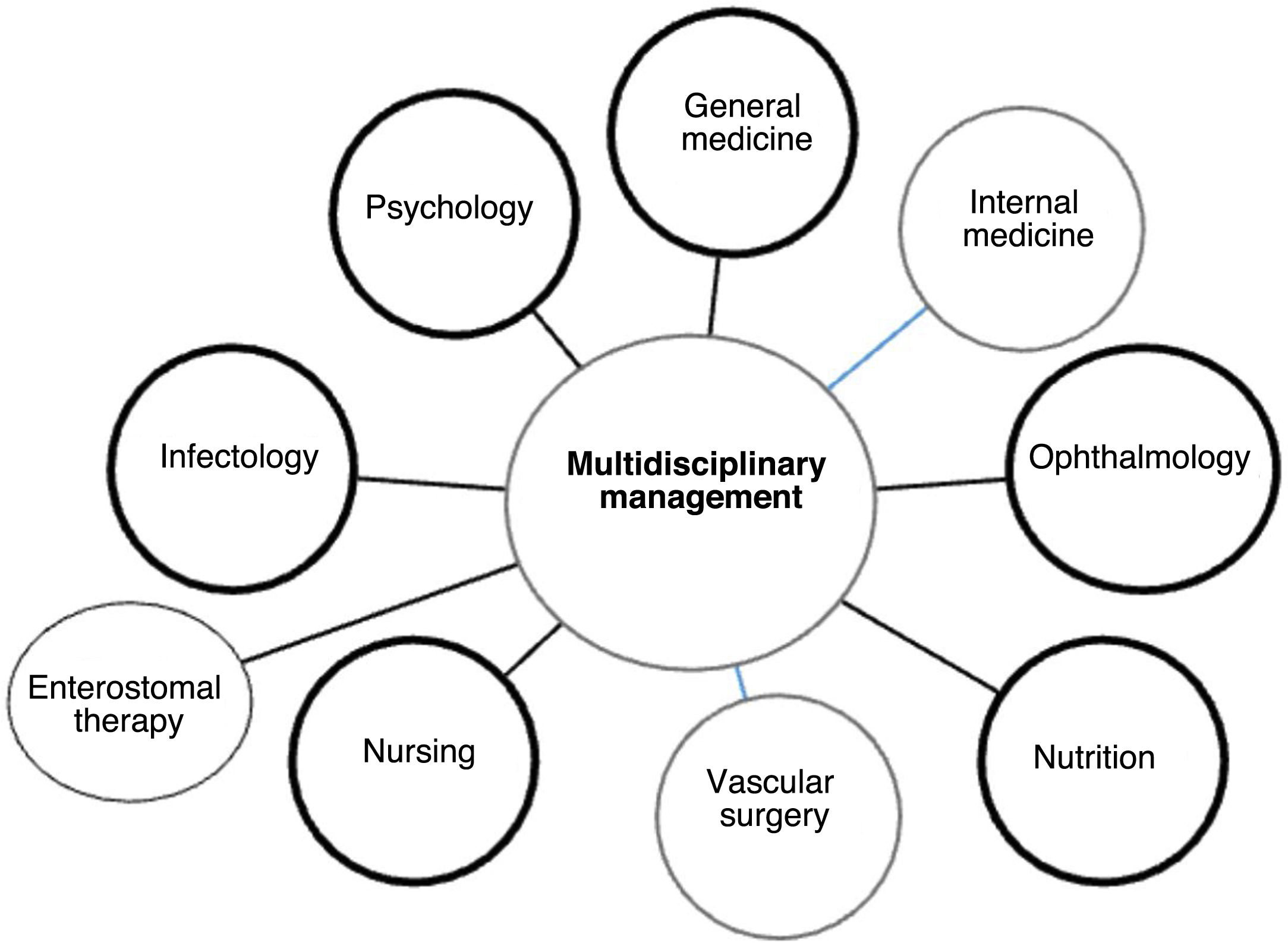
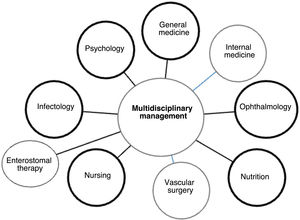
Once a patient with diabetic ulcer is admitted to the hospital, usually referred by a primary health physician, diabetes specialist, or an emergency unit, the first assessment is performed by a general physician who, depending on the severity of the case, may require the assistance of the orthopedic unit. This unit will ensure coordination between different specialties [internal medicine, infectology, vascular surgery, ophthalmology (depending on the involvement), nutrition, nursing/enterostomal therapy, psychology], to manage and assess the patient's injury 104 (See Figure 13).
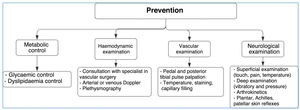
PreventionNurses as well as general and specialized physicians should educate and ask patients to carry out specific foot care activities such as:
- -
Daily feet examination (look for wounds, dryness, infection, interdigital injuries), preferably using a mirror to observe the foot or with someone's help.
- -
Application of moisturizing creams to prevent dry skin.
- -
Daily foot washing with warm water (temperature should be tested before in order to prevent burns).
- -
Careful foot drying (interdigital areas); the feet should never be wet, and talcum powder should be used to prevent moisture development.
- -
Strong antiseptics (iodine) or callus removers should not be used.
- -
If blisters or infections occur, a physician should be consulted immediately.
- -
Ergonomic shoes must be used to maintain the natural foot posture. Shoes should also be tailored based on the length of the first or second toe and should be as long as the longest toe so that deformities and scratches can be avoided.
- -
Use of cotton or wool socks to prevent wounds or scratches caused by seams.
- -
Avoid walking without shoes.
- -
Avoid active or passive smoking.
Routine performance of proprioceptive exercises in order to maintain the modified Romberg test result of>11 s (See Figure 14).
The following aspects should always be considered:
- •
Glycemic control.
- •
Extensive control of associated cardiovascular risk factors (Arterial hypertension, dyslipidemia, smoking habits, overweightness, etc.). Patients should know what to do at home and physicians should talk to their families, i.e., indications should be shared.
- •
Assessment by an orthopedist/traumatologist, vascular surgeon, infectologist, and nutritionist.
- •
Following discharge from the DF unit, the patient's progression should be followed up.
- •
With regard to primary health care, the patient's consulting physician should assess DF progression, educate the patient, and perform any necessary treatments in cooperation with nurses and enterostomal therapists. Precisely, one of the purposes of primary care is the control, follow-up, and enhancement of an individual's independence in taking care of their chronic disease.
- •
At all times, the relay of feedback between different health professionals should be ensured. It is important to consider that patients with diabetes may have coronary diseases with no symptomatology; therefore, more cardiological assessments should be performed (angiography).
- •
One of the most important aspects to be considered in order to enhance healing is to avoid initial foot standing. Most ulcers appear on bone prominences and are the result of walking. Shoes, sandals, dressings, pads, splints, or adhesive felt can be used to achieve equal pressure distribution and decrease impact and shearing motion forces (insoles). A vast majority of patients with DF and bone deformities may need to be referred to external orthopedists for consultation regarding surgical treatments. Physiotherapy for neurological stimulation of the nonulcerated limb should be performed both in an inpatient and outpatient bases (the contralateral limb has the same disease, and 50% of patients will present with injuries in the contralateral limb over the next 3 years).
In 2007, the World Health Organization recommended hypocaloric nutrition for patients with diabetes, under nutritionist observation, as well as the reduction of saturated fat, sugar, and sodium consumption and increase of fruit and vegetable consumption to enhance weight loss (see Table 15).
Nutritional parameters.
| Paraclinical:Total protein, albumin, globulin, vitamin B12, vitamin D3 |
| Remember:Metformin blocks intestinal absorption of vitamin B12.Vitamin D3 promotes intestinal absorption of calcium for bone formation and restoration.Diabetic patients have age-related vasculopathies and other comorbidities such as osteoporosis |
The use of vitamin B12 supplementation as an adjuvant in DF management is currently a topic of research and debate. Several studies report vitamin B12 deficiency in up to 50% of patients. This deficiency is associated with autonomic peripheral neuropathic injuries, which can accelerate DN. Moreover, metformin (an oral hypoglycemiant drug frequently used by patients with DM) causes vitamin B12 deficiency, which is apparently associated with a decrease in the absorption carried out by the terminal ileum that can appear just 4 months after starting treatment.105 In some studies, vitamin B12 supplementation has been used along with hypoglycemic agents in patients with poor DM control. In these patients, symptoms associated with DN sometimes seem to improve. Ideally, it is recommended to monitor vitamin B12 levels in the blood when vitamin B12 supplementation is being used.
In this regard, a study was conducted in 2021 by Didangelos et al 106 in which vitamin B12 was administered for a year to patients with neuropathy and adequate control of glycemia at the beginning of the study. Results of sural nerve conduction test, response to vibratory stimuli, quality of life test, and analogous pain scale were compared. All the parameters of the test group improved compared to those of a parallel group managed with placebo since the beginning of the study, and patients showed no pain. Conversely, patients who received placebo showed progressive deterioration in all the tested parameters.
Based on these findings and the biological feasibility of peripheral neurological degenerative changes observed with vitamin B12 deficiency and the well-known secondary effect of metformin and its deficit, vitamin B12 supplementation and periodic follow-up of its blood levels are recommended to prevent or delay the progression of DM-associated neuropathy.
FundingStudy funded with authors’ own resources.
Conflict of InterestThe authors declare no conflict of interest.