“Thou shalt randomize”1
Martin A. Weinstock
With this scientific command, researchers illuminate the rest of the world with the most rigorous and reliable type of evidence about the usefulness of therapies and other interventions for patient care and public health. However, randomization poses a mathematical challenge widely known and described in probability more than a hundred years ago as the Bertrand paradox, Count Buffon needle problem (Geoeges-Louis Leclerc) and several others. Therefore randomization “per se” does not imply that the evidence is better, more rigorous, or more reliable. In fact, when the randomization method is not correctly described in probability, a default bias is created. Ultimately, this bias makes a study neither reproducible nor controversial, events that are both indisputable pillars of the scientific method.
In order for both reviewers and readers to adequately assess critical pillars of the scientific method and decision-making in patients based on the evidence, the structure and findings of each randomized trial must be presented to them as clearly as possible. Frequently, medical journals and manuscript authors do not present all the details of randomized trials clearly and transparently. In fact, important details are often omitted from the randomized experiment, creating a perceptual bias that may also be even amplified in meta-analysis.
To critically asses published randomized manuscript, readers need complete, clear, and transparent information about the methodology and findings of that clinical trial. The same occurs with peer reviewers during the manuscript acceptance process. With the intention of correcting this problem, several journal editors made what is known as the CONSORT statement (Consolidation of Standards Of Reporting Trials).2,3
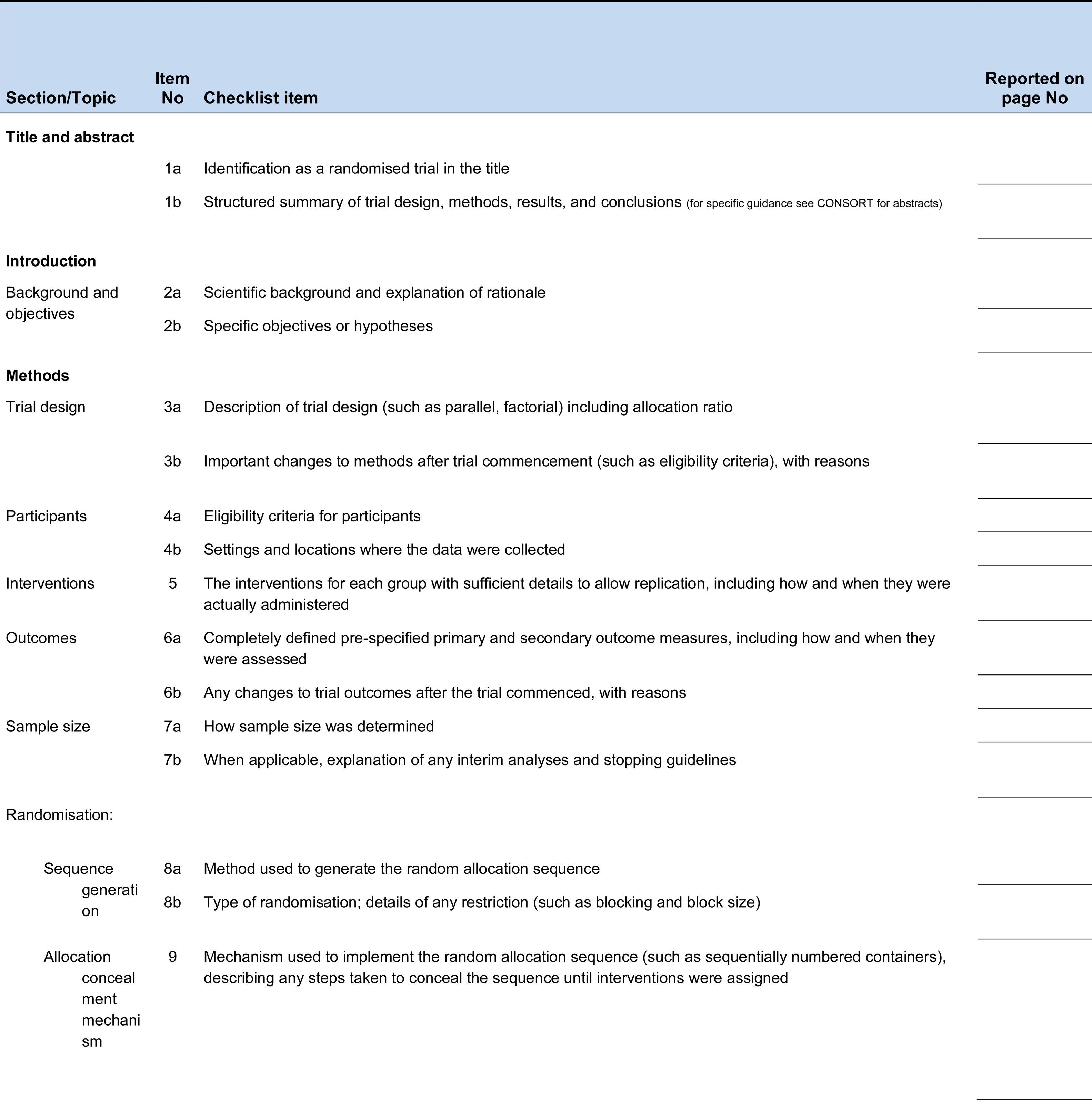
Although the journal (Revista Colombiana de Ortopedia y Traumatología) includes the suggestion to follow the recommendations of the CONSORT statement in its guidelines for authors since 2013, as of July 1, 2021, it will be mandatory for authors of Randomized trials reports to include a complete checklist of the key elements of a randomized trial report, and indicate for each element the page on which that element is documented in the manuscript according to with the 2010 revision of the CONSORT statement. (Fig. 1) The checklist (verification) will not be published, but will be included in the material provided to the reviewers of the manuscript.
CONSORT 2010 checklist of information to include when reporting a randomised trial*.
*We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see www.consort-statement.org.
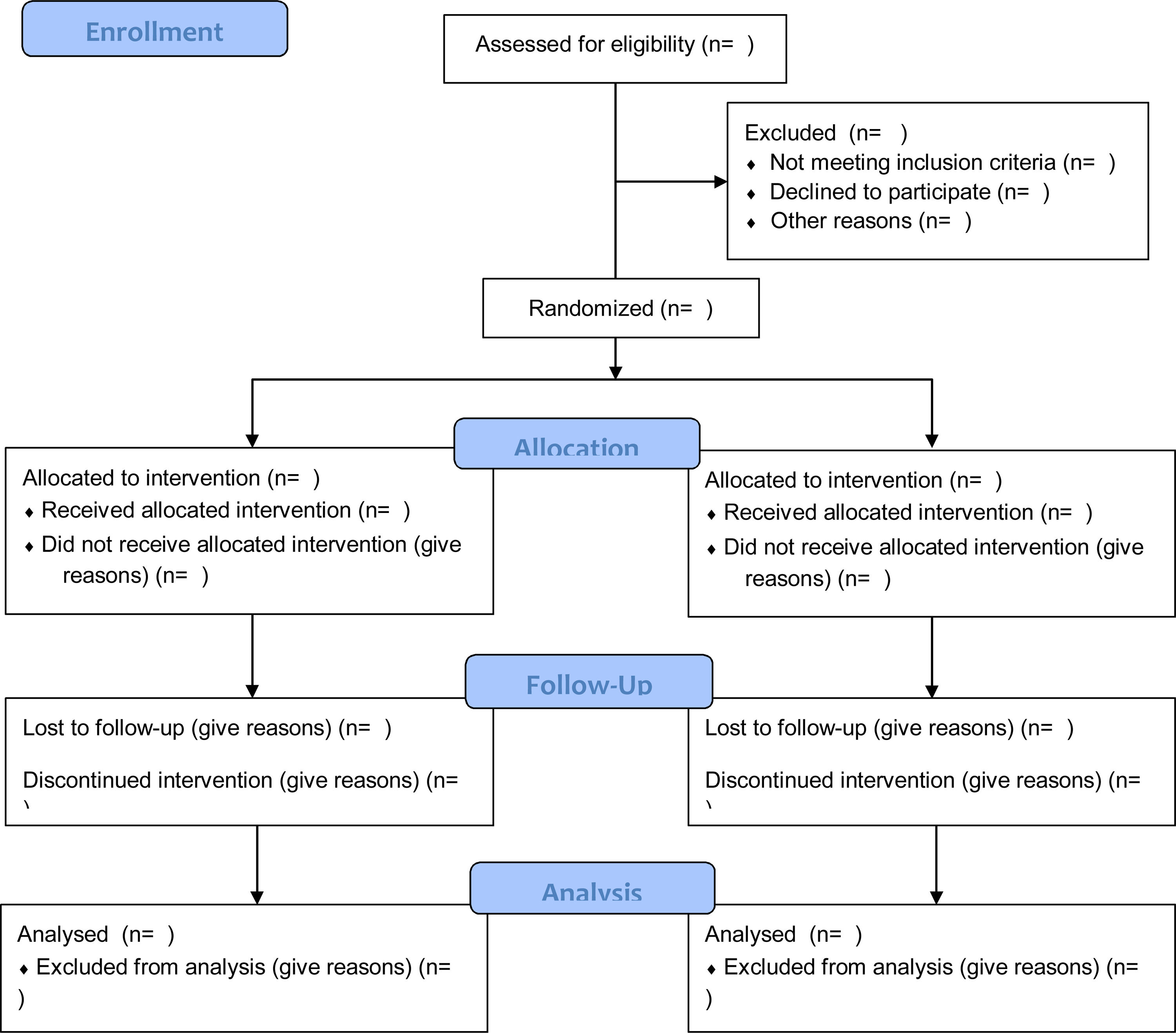
Authors also will be required to include a flow chart in the manuscript that clearly documents the number of patients who were eligible, recruited, randomized, operated on, withdrawn, or lost to follow-up, and the number evaluated at the end of the study test. (Fig. 2) Based on this, all readers can better interpret the value and applicability of the results in the publication.
CONSORT 2010 Flow Diagram.
This policy will apply to all manuscripts that are reported as randomized trials, regardless of the section of the journal in which they appear or whether they appear in a sponsored supplement. Authors can obtain a copy of the checklist and sample flowchart in English by downloading it from the following link: http://www.consort-statement.org.
You can also obtain the official translations of the checklist (verification) and the sample flow diagram in other languages, including Spanish, by downloading them from the following link:
http://www.consort-statement.org/downloads/translations