Bipolar disorder (BD) has been associated with a decrease in white matter integrity. Diffusion tensor imaging (DTI) studies have enabled these changes to be elucidated with higher quality. Due to BD’s high heritability, some studies have been conducted in relatives of BD patients looking at white matter integrity, and have found that structural connectivity may also be affected. This alteration has been proposed as a potential BD biomarker of vulnerability. However, there are few studies in children and adolescents.
ObjectiveTo conduct a review of the literature on changes in white matter integrity determined by DTI in high-risk children and adolescents.
ResultsBrain structural connectivity in the paediatric population is described in studies using DTI. Changes in the myelination process from its evolution within normal neurodevelopment to the findings in fractional anisotropy (FA) in BD patients and their high-risk relatives are also described.
ConclusionsStudies show that both BD patients and their at-risk relatives present a decrease in FA in specific brain regions. Studies in children and adolescents with a high risk of BD, indicate a reduced FA in axonal tracts involved in emotional and cognitive functions. Decreased FA can be considered as a vulnerability biomarker for BD.
El trastorno afectivo bipolar (TAB) se ha asociado con una disminución de la integridad de la sustancia blanca. Los estudios con imágenes con tensor de difusión (DTI) han permitido elucidar con una mayor calidad estos cambios. Debido a la gran heredabilidad del TAB, se han realizado estudios en familiares de pacientes con TAB acerca de la integridad de la sustancia blanca, y se ha encontrado que la conectividad estructural también puede estar afectada. Dicha alteración se ha propuesto como un potencial biomarcador de vulnerabilidad a este trastorno. Sin embargo, los estudios en niños y adolescentes son pocos.
ObjetivoRevisar la literatura sobre los cambios en la integridad de la sustancia blanca determinados mediante DTI en niños y adolescentes con alto riesgo.
ResultadosSe describe la conectividad estructural cerebral en la población pediátrica en estudios que utilizaron DTI. Se describen los cambios en el proceso de mielinización desde su evolución dentro del neurodesarrollo normal hasta los hallazgos en la anisotropía fraccional (AF) en pacientes con TAB y los familiares en alto riesgo.
ConclusionesLos estudios demuestran que tanto pacientes con TAB como sus familiares en riesgo presentan disminución de la AF en regiones cerebrales específicas. Los estudios en niños y adolescentes con riesgo familiar de TAB señalan una AF reducida en tractos axonales implicados en funciones emocionales y cognitivas. La disminución de la AF puede considerarse como un biomarcador de vulnerabilidad al TAB.
Bipolar disorder (BD) negatively impacts on the functionality and quality of life of individuals who suffer from it; it is one of the 20 leading causes of disability in the world and ranks twelfth among the most common causes of years of healthy life lost due to disability in the Latin American population, ahead of diabetes, cerebrovascular disease and HIV.1 BD generally has its onset in adolescence or early adulthood, between the ages of 15 and 24.2 Early-onset BD has been associated with a worse prognosis, more comorbidity with other psychiatric disorders, a high level of dysfunction, and a greater risk of affective disorders in first-degree relatives.3
The major impact on quality of life, the burden on the patients’ caregivers, and the high costs generated by the dysfunction have led to an interest in research into the aetiology and pathophysiology of this disorder. The aetiological factors of BD include genetic factors, given great relevance due to its heritability (as high as 78%4), and environmental factors, which interact with the genetic factors to produce the disorder.5 In addition to these factors, recent studies have shown abnormalities in structural connectivity detected by diffusion tensor imaging (DTI), a magnetic resonance imaging technique which provides a better definition of white matter.6
DTI studies have provided extensive information about the structure of the white matter of patients with BD. However, these findings have to be interpreted with caution because of certain factors which may influence the results, such as the effects of medication, the different clinical phases of the disorder and age. The ideal could be to assess the connectivity in an asymptomatic population without medications. Still, as that is not always possible, one option would be to study it in the relatives of patients at high risk of acquiring BD, given its high heritability.7 As the onset of symptoms is during adolescence or early adulthood, the ideal population would be children or adolescents. Therefore, this article aims to review the literature on changes in white matter integrity determined by DTI in child and adolescent relatives of patients with BD.
MethodsSearch strategyWe searched through PubMed for studies with DTI which included children or adolescents related to individuals with BD. No date limitation was placed on the articles found and only articles published in English were included. We searched for the following keywords: “bipolar disorder, diffusion tensor imaging, white matter, first-degree relatives, offspring”.
DTI, basic conceptsMRI works by measuring the proton signals from the water molecules making up much of the human body. In the brain, these molecules circulate in a space restricted by axons and membranes, leading to different diffusion patterns. The images of these patterns are what enable us to visualise the structure of brain tissue and its axonal organisation.8
The need to obtain greater sensitivity in water diffusion signals, to enable better definition of structural connectivity, the group of connections between neuronal units, has led to the development of new magnetic resonance imaging techniques. One of these is DTI. This method uses different measurements9:
- •
Fractional anisotropy (FA): determines the degree to which water diffusion is restricted to one direction with respect to other directions. It is scored from 0 to 1, with 0 being fully isotropic (homogeneous) diffusion of water in all directions and 1 being diffusion restricted to one direction only. In the brain, this property depends on the integrity of the axon tracts.
- •
Axial diffusivity (AD): reflects the rate of movement of water molecules parallel to the direction of the axons and is related to the diameter and organisation of the fibres.10
- •
Radial diffusivity (RD): reflects the rate of movement of water molecules perpendicular to the direction of the axons. Damage to the myelin sheaths causes an increase in RD.11
- •
Mean diffusivity (MD): determines the average magnitude of the direction of diffusion of the water.9 Axon degeneration and demyelination alter the direction of water diffusion and ultimately lead to an increase in MD values.12
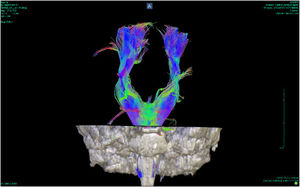
DTI uses a symmetric tensor field to define the diffusion profile of water in brain tissue. With this field, the extent of diffusion of water molecules in different directions is measured. Employing tractography, the white matter tracts are plotted in three dimensions (according to the direction in which the water molecules move). These tracts can be located on three different axes, one from right to left (x), one anterior-posterior (y) and the other rostral-caudal (z). According to their location, the tracts are plotted in different colours: red for the x axis, green for the y axis and blue for the z axis8 (Figs. 1 and 2).
There are two main limitations to white matter visualisation by DTI: the measurement of the behaviour of the water molecules, which is an indirect measurement of neuroanatomy, and the low resolution of the images. In view of these limitations, the conclusion is that DTI resonance only provides a macroscopic view of the tracts and their anatomical limits are only poorly defined. Microscopic or histological techniques would be necessary for more precise definition. Despite these limitations, however, DTI has meant an advance in the definition of white matter and provides information which is difficult to obtain using histological techniques.13
Normal development of white matter in childhood and adolescence and correlation with DTI findingsThe early stages of life involve major transformations in the human brain which contribute to neurodevelopment, but also represent a risk for the onset of some mental disorders.14 The psychological, physical and social spheres undergo a series of changes during this period which seem to be mediated by neurobiological variations.15
The different structural changes in the white matter are best characterised by DTI. This technique has made it possible to follow the development of white matter from birth to adulthood.16
Childhood is a crucial stage for brain development, especially in the first two years of life. The brain increases in size by 101% in the first year and 15% in the second. The initial growth is mainly of grey matter, with only a minimal increase in the white matter in the first two years and much slower growth.9
Changes in the development of white matter over the course of life have been inferred from DTI measurements, primarily from FA and MD. In general, an increase in FA has been found from childhood to adulthood, with a peak between the ages of 20 and 42, and then a slow, gradual decline.10 MD tends to decrease until it reaches its lowest values between the ages of 18 and 41, and then increases in late adulthood; RD and AD measurements follow a similar pattern to DM.10
The myelination process begins in the embryonic period and extends into adulthood. DTI studies have identified three stages in the development of white matter. In the first, an increase in RD and AD can be seen by the progressive organisation of the nerve fibres, as well as an increase in the AD of water, which reflects the increase in oligodendrocytes in the premyelination stage. In the second, there is a decrease in the diffusivity of water due to an increase in the density of the membrane caused by growth of the cytoskeleton and the proliferation of glial cells. Finally, the third stage shows an increase in FA which corresponds to the covering of axonal fibres by oligodendrocytes.9 Studies with DTI have shown that this increase in FA is greater in the first 24–36 months of life, and is faster in the anterior than in the posterior structures, although the anterior structures are less developed in the neonatal stage.17
Myelination occurs first in the most proximal and central pathways, the sensory pathways, the occipital pole and the projection fibres.9 The cerebellar pons and cerebellar peduncles are the first brain structures to become myelinated, and this happens during pregnancy. At three months of age, the posterior limb of the internal capsule, the splenium of the corpus callosum and the optic radiation are myelinated. Subsequently, at the age of six months, myelination occurs in the genu of the corpus callosum and the anterior branch of the internal capsule.9 The development in early stages of the corpus callosum, along with other structures such as the fornix and the inferior longitudinal fasciculus, seems to be explained by the basic functions they fulfil; the corpus callosum is involved in the interhemispheric connection processes, the fornix, in memory, and the inferior longitudinal fasciculus, in visual processing.
The cingulum and the uncinate and superior longitudinal fasciculi, important tracts connecting the temporal and frontal regions, develop slowly during childhood and adolescence.10 In the cingulum, FA values have been found only to reach their maximum peak after the age of 40, and then have slower reductions as a person gets older. A similar trend has been found in the uncinate and superior longitudinal fasciculi.10 The corticospinal tract has been found to reach its maximum peak of increase in FA before the peak of the frontotemporal tracts and it resembles the pattern of the anterior limb of the internal capsule.10
During adolescence, significant changes in DTI measurements have been found in the superior longitudinal fasciculus, corona radiata, thalamic fibres, inferior fronto-occipital fasciculus and internal capsule.18 The increase in FA in these tracts may be mediated by a decrease in RD, which in turn reflects an increase in axon calibre and myelination.19
The progressive increase in FA values during child and adolescent brain development seems to be correlated with the structural formation of microtubules, the organisation of neurofilaments, the increase in axon diameter, and dendritic arborisation. All these processes involve the maturation and organisation of the white matter and also therefore, the development of more complex cognitive and executive capacities.17 A correlation has been found between working memory, visual processing, IQ and reading skills with increased FA in the different areas related to these functions.19
DTI can provide important information on the microstructure and development of white matter. These findings need to be taken into account when interpreting the images of individuals who are in stages of growth and brain development.9
Findings in the DTI of children and adolescents with BDOne meta-analysis20 found an estimated prevalence of BD in children and adolescents of 1.8%, while in another study,21 the estimated prevalence for the entire bipolar spectrum was 2.06%. In some studies, the figures suggest that 66% of adult subjects with BD had their first episodes before the age of 1822 (Table 1).
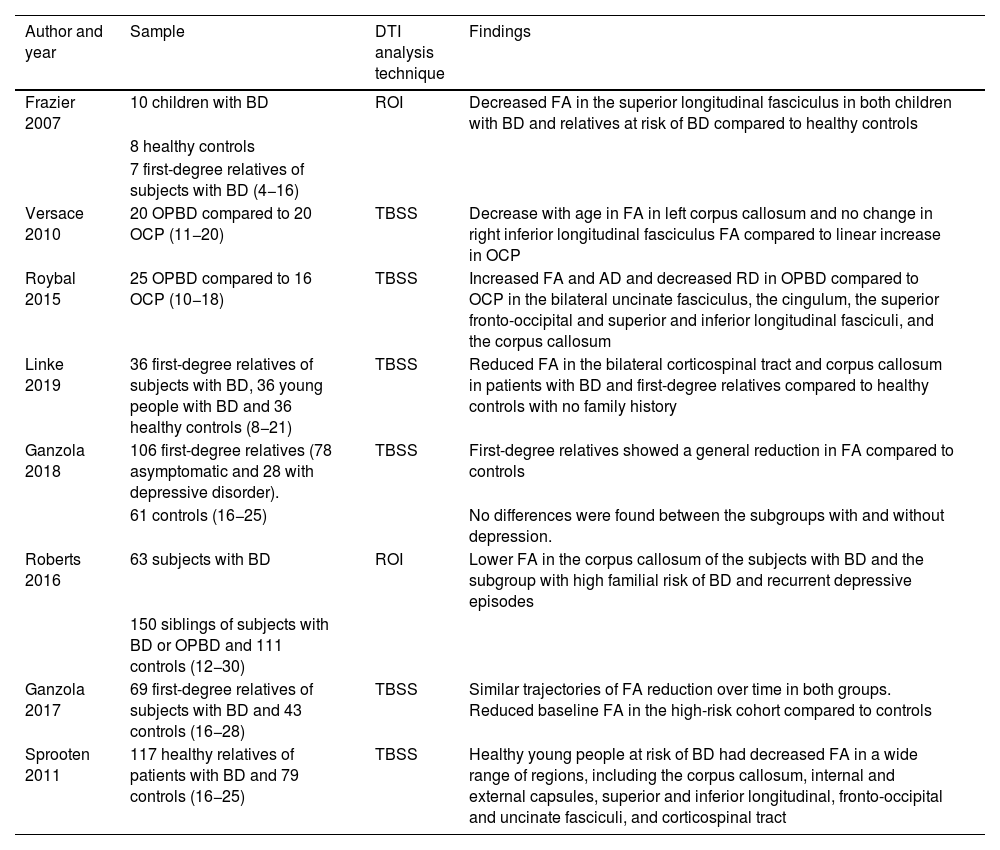
Studies with DTI in the paediatric population at risk of BD (offspring and siblings).
| Author and year | Sample | DTI analysis technique | Findings |
|---|---|---|---|
| Frazier 2007 | 10 children with BD | ROI | Decreased FA in the superior longitudinal fasciculus in both children with BD and relatives at risk of BD compared to healthy controls |
| 8 healthy controls | |||
| 7 first-degree relatives of subjects with BD (4−16) | |||
| Versace 2010 | 20 OPBD compared to 20 OCP (11−20) | TBSS | Decrease with age in FA in left corpus callosum and no change in right inferior longitudinal fasciculus FA compared to linear increase in OCP |
| Roybal 2015 | 25 OPBD compared to 16 OCP (10−18) | TBSS | Increased FA and AD and decreased RD in OPBD compared to OCP in the bilateral uncinate fasciculus, the cingulum, the superior fronto-occipital and superior and inferior longitudinal fasciculi, and the corpus callosum |
| Linke 2019 | 36 first-degree relatives of subjects with BD, 36 young people with BD and 36 healthy controls (8−21) | TBSS | Reduced FA in the bilateral corticospinal tract and corpus callosum in patients with BD and first-degree relatives compared to healthy controls with no family history |
| Ganzola 2018 | 106 first-degree relatives (78 asymptomatic and 28 with depressive disorder). | TBSS | First-degree relatives showed a general reduction in FA compared to controls |
| 61 controls (16−25) | No differences were found between the subgroups with and without depression. | ||
| Roberts 2016 | 63 subjects with BD | ROI | Lower FA in the corpus callosum of the subjects with BD and the subgroup with high familial risk of BD and recurrent depressive episodes |
| 150 siblings of subjects with BD or OPBD and 111 controls (12−30) | |||
| Ganzola 2017 | 69 first-degree relatives of subjects with BD and 43 controls (16−28) | TBSS | Similar trajectories of FA reduction over time in both groups. Reduced baseline FA in the high-risk cohort compared to controls |
| Sprooten 2011 | 117 healthy relatives of patients with BD and 79 controls (16−25) | TBSS | Healthy young people at risk of BD had decreased FA in a wide range of regions, including the corpus callosum, internal and external capsules, superior and inferior longitudinal, fronto-occipital and uncinate fasciculi, and corticospinal tract |
FA: fractional anisotropy; AD: axial diffusivity; BD: bipolar disorder; DTI: diffusion tensor imaging; OCP: offspring of control parent; OPBD: offspring of parent with bipolar disorder; RD: radial diffusivity; ROI: brain regions of interest; TBSS: tract-based spatial statistics.
The main changes in structural connectivity found by DTI in this population are discussed below:
- •
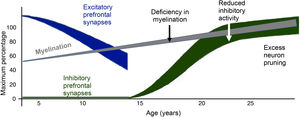
Decreased FA in the anterior corpus callosum: a study carried out on 15 adolescents with BD and psychosis showed a reduction compared to healthy subjects. Prefrontal interhemispheric tracts pass through the corpus callosum, reaching complete myelination at the age of 20; in other words, lower FA may correspond to a delay in myelination compared to healthy individuals of the same age.23
- •
Decreased FA in the genu of the corpus callosum and anterior commissure: Saxena et al.24 examined these specific areas and found lower FA in 10 adolescents with BD compared to controls.
- •
Decreased FA in the left anterior limb of the internal capsule: Lu et al.25 conducted a study on 35 patients with a first episode of BD who had not taken medication and found significantly lower values in patients with early-onset BD than in the population with adult onset.
- •
Decreased FA in the right anterior cingulum: Gao et al.26 detected lower FA in a group of 18 adolescents with a manic episode compared to the control group. In the study by Frazier et al.,27 FA values in this brain area belonging to the limbic system were also lower in 10 children with BD compared to eight healthy controls.
- •
Decreased FA in the hippocampus and cingulum: Kafantaris et al.28 demonstrated that 18 adolescents with BD had lower FA in this region involved in emotional regulation compared to controls. They also found an increase in FA in the left region of the hippocampus and the cingulum after treatment with lithium for four weeks, indicating that this drug may act on abnormalities in the microstructure of the white matter and thus have an effect on emotional regulation.
- •
Decreased FA in the anterior corona radiata: Pavuluri et al.29 conducted a study with DTI in 13 adolescents with BD, 13 with attention deficit hyperactivity disorder (ADHD) and 13 controls. They found lower FA in both BD and ADHD patients compared to the controls, implying impaired myelination in both disorders.
- •
Decreased FA in the right orbitofrontal cortex: Kafantaris et al.30 found that this region was altered in 26 patients with BD I compared to 26 healthy controls, and this finding also corresponded to slower visual-motor speed in the group with BD (Fig. 3).
There have been few DTI studies in relatives of patients with BD. Most of them have been carried out with adults and differences have been shown in FA measurements comparing patients with BD and healthy individuals with no genetic predisposition (Table 2). Emsell et al.31 found a decrease in FA in the uncinate and superior longitudinal fasciculi of subjects with familial risk of BD, and Chaddok et al.,32 although finding no significant differences in FA between at-risk relatives and controls, did find that these individuals had intermediate FA values, somewhere between patients with BD and controls, in the corpus callosum and the left and right superior longitudinal fasciculi.
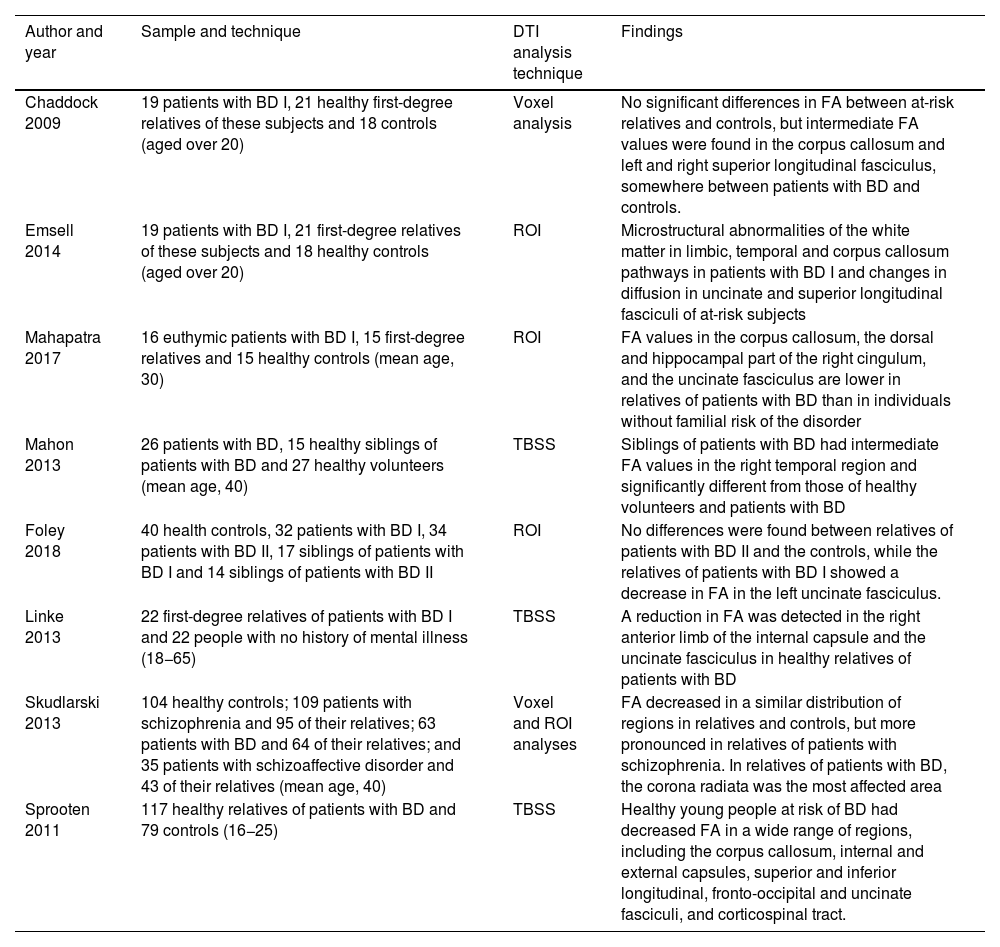
Studies with DTI of relatives of individuals with BD.
| Author and year | Sample and technique | DTI analysis technique | Findings |
|---|---|---|---|
| Chaddock 2009 | 19 patients with BD I, 21 healthy first-degree relatives of these subjects and 18 controls (aged over 20) | Voxel analysis | No significant differences in FA between at-risk relatives and controls, but intermediate FA values were found in the corpus callosum and left and right superior longitudinal fasciculus, somewhere between patients with BD and controls. |
| Emsell 2014 | 19 patients with BD I, 21 first-degree relatives of these subjects and 18 healthy controls (aged over 20) | ROI | Microstructural abnormalities of the white matter in limbic, temporal and corpus callosum pathways in patients with BD I and changes in diffusion in uncinate and superior longitudinal fasciculi of at-risk subjects |
| Mahapatra 2017 | 16 euthymic patients with BD I, 15 first-degree relatives and 15 healthy controls (mean age, 30) | ROI | FA values in the corpus callosum, the dorsal and hippocampal part of the right cingulum, and the uncinate fasciculus are lower in relatives of patients with BD than in individuals without familial risk of the disorder |
| Mahon 2013 | 26 patients with BD, 15 healthy siblings of patients with BD and 27 healthy volunteers (mean age, 40) | TBSS | Siblings of patients with BD had intermediate FA values in the right temporal region and significantly different from those of healthy volunteers and patients with BD |
| Foley 2018 | 40 health controls, 32 patients with BD I, 34 patients with BD II, 17 siblings of patients with BD I and 14 siblings of patients with BD II | ROI | No differences were found between relatives of patients with BD II and the controls, while the relatives of patients with BD I showed a decrease in FA in the left uncinate fasciculus. |
| Linke 2013 | 22 first-degree relatives of patients with BD I and 22 people with no history of mental illness (18−65) | TBSS | A reduction in FA was detected in the right anterior limb of the internal capsule and the uncinate fasciculus in healthy relatives of patients with BD |
| Skudlarski 2013 | 104 healthy controls; 109 patients with schizophrenia and 95 of their relatives; 63 patients with BD and 64 of their relatives; and 35 patients with schizoaffective disorder and 43 of their relatives (mean age, 40) | Voxel and ROI analyses | FA decreased in a similar distribution of regions in relatives and controls, but more pronounced in relatives of patients with schizophrenia. In relatives of patients with BD, the corona radiata was the most affected area |
| Sprooten 2011 | 117 healthy relatives of patients with BD and 79 controls (16−25) | TBSS | Healthy young people at risk of BD had decreased FA in a wide range of regions, including the corpus callosum, internal and external capsules, superior and inferior longitudinal, fronto-occipital and uncinate fasciculi, and corticospinal tract. |
BD: bipolar disorder; DTI: diffusion tensor imaging; FA: fractional anisotropy; ROI: brain regions of interest; TBSS: tract-based spatial statistics.
Some studies did find alterations. In the study by Mahapatra et al.,33 lower FA values were found in the corpus callosum, the dorsal and hippocampal part of the right cingulum, and the uncinate fasciculus in relatives of patients with BD than in individuals without familial risk of the disorder. Another study34 carried out on 15 healthy siblings of patients with BD found that they had white matter alterations in the right temporal region, which although significant compared to healthy controls, were not as large as in the group of patients with BD; in fact they lay in between. Linke et al.35 found decreased FA in the right anterior limb of the internal capsule and in the right uncinate fasciculus in both patients with BD and first-degree relatives, while the corpus callosum was only affected in the patients with BD. This finding could indicate that alteration of the corpus callosum is a later marker in the course of the disease.
Changes in structural connectivity in the relatives of patients with BD seem to be different depending on which type the patient has: BD I or BD II. Foley et al.36 found no differences between relatives of patients with BD II and controls, while the relatives of patients with BD I showed a decrease in FA in the left uncinate fasciculus.
Some DTI studies in patients with BD have found changes in the integrity of the white matter similar to those in patients with schizophrenia. When comparing the relatives of patients with schizophrenia with those with psychotic BD, they found a decrease in FA in a similar distribution of regions with respect to controls, but more pronounced in the relatives of patients with schizophrenia. In this last group, the area with the greatest differences compared to controls was the genu of the corpus callosum. A in relatives of patients with BD it was the upper region of the left posterior corona radiata. In the group of patients and relatives with BD, the differences were noticeable in young subjects, but were not found in older subjects. This may indicate that decreases in FA occur at early stages but do not progress later over the course of the disorder.37
Of the first-degree relatives, the child and adolescent offspring of patients with BD (offspring of parent(s) with BD [OPBD]) are a vulnerable group, which may even have subsyndromal symptoms of mania and other early psychiatric disorders.38 The presence of psychopathology in this population seems to be related to different findings than in the offspring of control parents (OCP).
A study conducted in 20 asymptomatic OPBD found a decrease over time in FA values in the left corpus callosum and no change in FA in the right inferior longitudinal fasciculus, compared to the linear increase in FA in OCP in these two areas.39 Another study,40 which expanded its sample to individuals with a first-degree relative or two second-degree relatives, showed that healthy young people at risk of BD had lower FA in a wide group of regions, such as the corpus callosum, the internal and external capsule, the superior and inferior longitudinal, fronto-occipital and uncinate fasciculi, and the corticospinal tract.
The findings of studies conducted in OPBD with psychiatric symptoms have been conflicting. A study conducted in 25 young OPBD with associated emotional dysregulation found that these patients had higher FA in the corpus callosum, the cingulum, and the superior and inferior longitudinal, superior fronto-occipital and uncinate fasciculi.41 Frazier et al.27 showed a decrease in FA in the superior longitudinal fasciculus both in paediatric OPBD with ADHD, behavioural disorders and anxiety disorders and in paediatric OPBD diagnosed with BD. However, the FA values were much lower in the second group, mainly in the cingulate and paracingulate gyri, which could indicate that these areas are more involved in the disease state rather than in the risk state. Lastly, a study42 conducted on 108 young people aged 8−21 found a reduction in FA in the bilateral corticospinal tract and the corpus callosum in BD patients and first-degree relatives, compared to healthy subjects with no family history of BD. However, in the group of relatives, corticospinal tract alterations were greater in individuals diagnosed with ADHD, suggesting that this finding is not very specific for BD.
Two studies in young relatives of patients with BD looked for an association between depressive episodes and imaging findings. In their longitudinal study, Ganzola et al.43 found no differences in the subgroups of individuals at high risk between those who suffered from depression and those who did not. Roberts et al.44 found that the subgroup of participants with a high familial risk of BD and recurrent depressive episodes had lower FA in the corpus callosum than those who did not have these episodes.
Finally, a longitudinal study in young individuals with familial risk of BD versus a control group found that the trajectories of FA reduction were similar in both groups. However, they also detected reduced baseline FA in the subjects of interest versus the controls, which may indicate that alterations in structural connectivity do not arise close to the onset of the disease but much earlier, from birth or early childhood.45
DiscussionThe available literature on changes in white matter integrity determined by DTI in child and adolescent relatives of patients with BD is limited, and there is little consensus from one study and another. However, most show that healthy young people with familial risk of BD do have differences compared to those without a genetic predisposition.
Studies show that both BD patients and at-risk relatives have lower FA in specific brain regions. Most of these alterations are shared by both groups in the corpus callosum,24,33 the cingulum,27 the superior27 and inferior longitudinal fasciculi, and the fronto-occipital and uncinate fasciculi.33,39 However, some studies have shown that, compared to controls, FA is much lower in patients with BD and the FA of their relatives is at an intermediate point between the two groups.27,34 This could mean that the changes in FA are markers of vulnerability.
Lower FA in regions such as the internal capsule,25 the cingulate and paracingulate gyri,40 the corona radiata46 and the right orbitofrontal cortex30 has only been described in studies of patients with BD and not in OPBD, which may suggest that these tracts have a more direct association with the disease state or the environmental factors which influence its development.
There is heterogeneity among the populations studied, patients and relatives, which makes it difficult to interpret the findings and make conclusions about the changes in the structure of the white matter. Some studies include family members with personality disorders, ADHD or anxiety disorders, making it difficult to establish whether FA changes correspond to specific markers of vulnerability to BD or can be explained by other disorders. The administration of medications for BD may also generate changes in the white matter, as is known with lithium; it seems to affect axon integrity, which counteracts alterations in structural connectivity in these patients.47 The different techniques in image processing may also have a hand in the inconsistency of the findings. Finally, the variety in the age of the relatives included in the studies makes it difficult to formulate hypotheses, as the DTI measurements change continuously from birth to late adulthood.10
ConclusionsDespite the above limitations, the findings of reduced FA in different axonal tracts involved in emotional and cognitive functions in children and adolescents with familial risk of BD may be a good starting point for a better understanding of the pathophysiological bases and identifying new biomarkers of vulnerability to BD.
FundingThis project was funded by Colciencias No. 111577757629: “Características clínicas, marcadores genéticos, y factores de adversidad psicosocial que predicen Trastorno por Déficit de atención con Hiperactividad (TDAH) en hermanos de alto riesgo” [Clinical characteristics, genetic markers, and psychosocial adversity factors that predict attention deficit hyperactivity disorder (ADHD) in high-risk siblings]; and also by CODI2017-16250: “Factores de adversidad psicosocial, genética y clínica asociada al TDAH en hermanos en alto riesgo” [Factors of psychosocial, genetic and clinical adversity associated with ADHD in siblings at high risk]. Co-funded by the University of Antioquia and the CES University.
Conflicts of interestThe authors declare that they have no conflicts of interest.





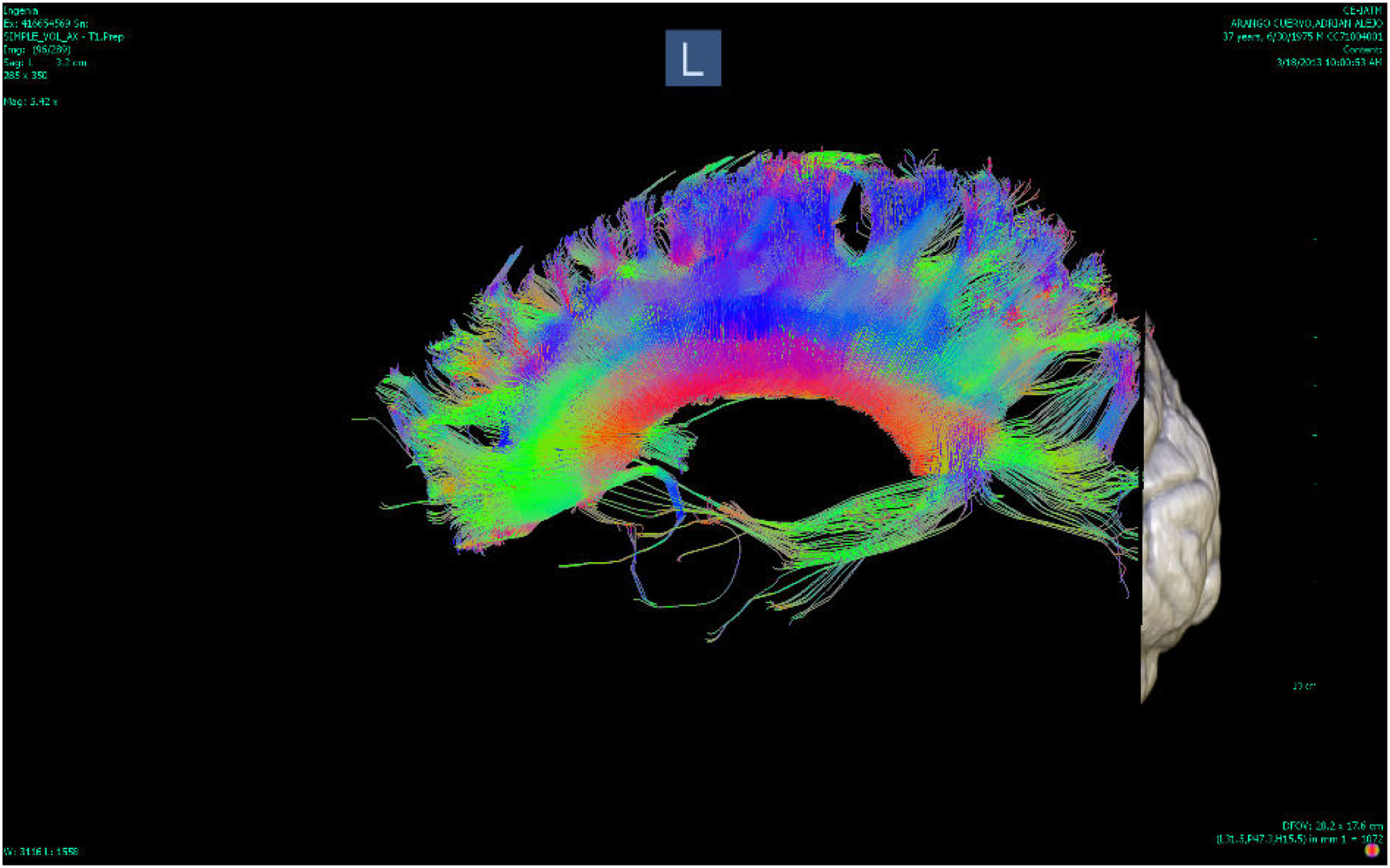
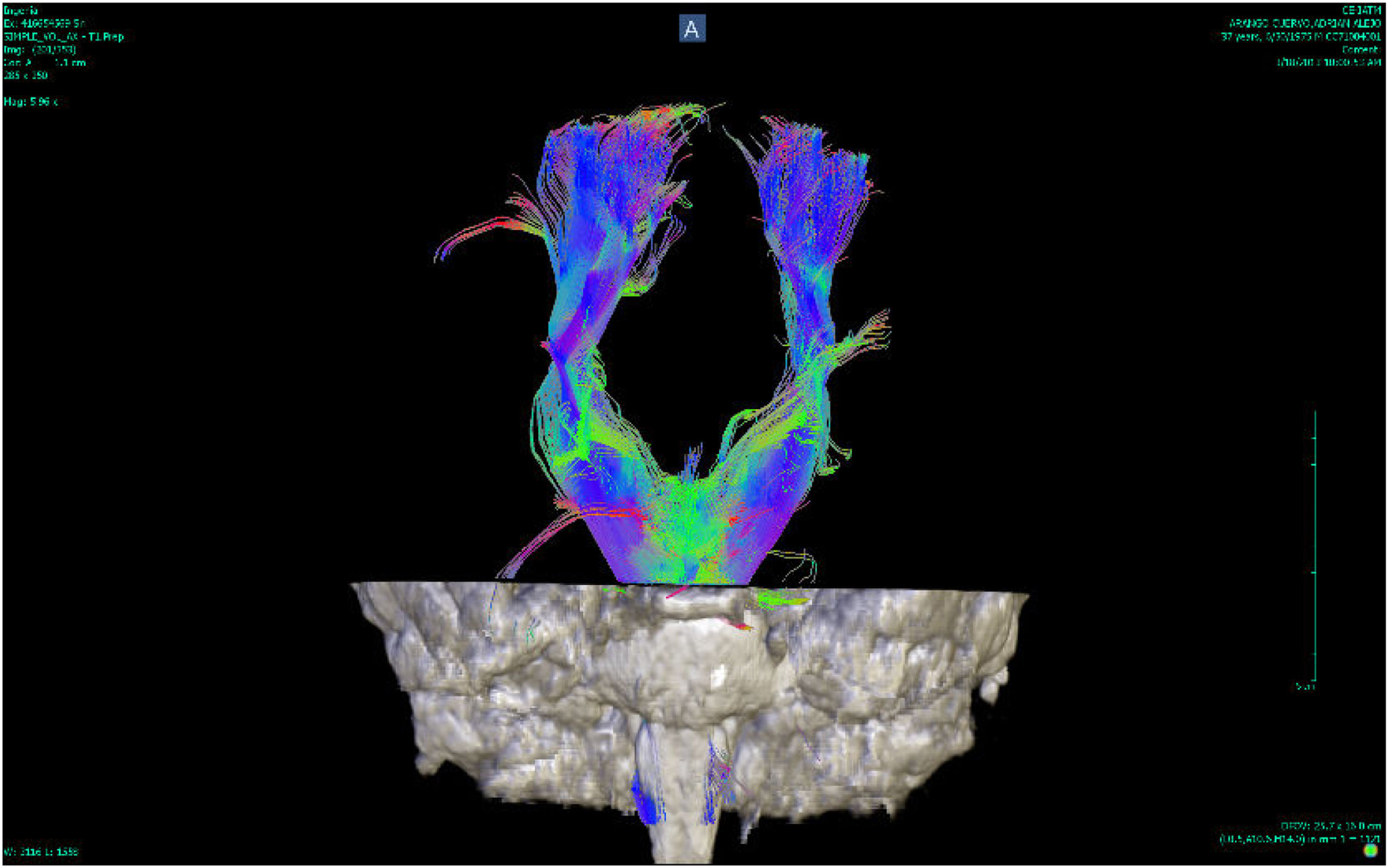

![Tractography of the corpus callosum of a patient with bipolar disorder (Instituto de Alta Tecnología Medica-Grupo de Investigación en Psiquatría; IATM-GIPSI [Institute of High Medical Technology-Psychiatry Research Group]). Tractography of the corpus callosum of a patient with bipolar disorder (Instituto de Alta Tecnología Medica-Grupo de Investigación en Psiquatría; IATM-GIPSI [Institute of High Medical Technology-Psychiatry Research Group]).](https://static.elsevier.es/multimedia/25303120/0000005200000002/v1_202307261121/S2530312023000231/v1_202307261121/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)