A high proportion of depressive disorders are accompanied by anxious manifestations, just as depression and anxiety often present with many painful manifestations, or conversely, painful manifestations cause or worsen depressive and anxious expressions. There is increasingly more evidence of the pathophysiological, and neurophysiological and technical imaging similarity of pain and depression.
MethodsNarrative review of the pathophysiological and clinical aspects of depression and chronic pain comorbidity. Research articles are included that emphasise the most relevant elements related to understanding the pathophysiology of both manifestations.
ResultsThe pathological origin, physiology and clinical approach to these disorders have been more clearly established with the latest advances in biochemical and cellular techniques, as well as the advent of imaging technologies. This information is systematised with comprehensive images and clinical pictures.
ConclusionsThe recognition that the polymorphism of inflammation-related genes generates susceptibility to depressive manifestations and may modify the response to antidepressant treatments establishes that the inflammatory response is not only an aetiopathogenic component of pain, but also of stress and depression. Likewise, the similarity in approach with images corroborates not only the structural, but the functional and pathophysiological analogy between depression and chronic pain. Knowledge of depression-anxiety-chronic pain comorbidity is essential in the search for effective therapeutic interventions.
Una alta proporción de los trastornos depresivos se acompañan de manifestaciones ansiosas, así como la depresión y la ansiedad cursan frecuentemente con dolor. En otro sentido, las manifestaciones dolorosas causan o empeoran los síntomas depresivos y ansiosos. Cada vez hay más evidencia sobre la similitud fisiopatológica, imagenológica y neurofisiológica del dolor y la depresión.
MétodosRevisión narrativa de los aspectos fisiopatológicos y clínicos de la comorbilidad depresión y dolor crónico. Se incluyen los artículos de investigación que enfatizan los elementos relevantes relacionados con la comprensión de la fisiopatología de ambas manifestaciones.
ResultadosCon los más recientes avances en técnicas bioquímicas y celulares y el advenimiento de tecnologías imagenológicas de avanzada, se ha podido considerar cada vez más claramente la aproximación etiopatogénica, fisiopatológica y clínica de estos trastornos. Se sistematiza esta información en imágenes y cuadros comprensivos.
ConclusionesEl reconocimiento de que el polimorfismo de los genes relacionados con la inflamación genera susceptibilidad a las manifestaciones depresivas y puede modificar la respuesta a los tratamientos antidepresivos establece que la respuesta inflamatoria no solo es un componente etiopatogénico del dolor, sino del estrés y la depresión. De igual manera, la similitud en la aproximación con imágenes corrobora la analogía no solo estructural, sino también funcional y fisiopatológica, entre la depresión y el dolor crónico. El conocimiento de la comorbilidad depresión-ansiedad-dolor crónico es importante en la búsqueda de intervenciones terapéuticas eficaces.
In 1989, Hudson and Pope proposed that a number of chronic medical and psychiatric conditions may be part of a family of related disorders that share a common pathophysiology, referring to them as affective spectrum disorders.1 These disorders share features such as depressive and anxiety symptoms, they often respond to antidepressants and they are frequently associated with clinical conditions with painful physical symptoms such as fibromyalgia, chronic fatigue syndrome, migraine, irritable bowel syndrome and premenstrual dysphoric disorder.2
In primary care, physical symptoms are a common reason for consultation, but the underlying reason is depression. More than 50% of patients with depression report somatic complaints and at least 60% of these symptoms are pain-related.3 Consulting with physical complaints reduces recognition of depression and anxiety, as the primary care physicians assume that all physical symptoms are caused by an underlying medical condition.4 Numerous complaints of pain are associated with greater severity of the depression, while more severe pain is predictive of poor prognosis for the treatment of the depression.1 The aetiopathogenic relationship between depression and anxiety states, pain and somatic disorders has become increasingly clear, and this has reinforced the concept of affective spectrum disorders. Patients with fibromyalgia, for example, are twice as likely to suffer from other psychiatric disorders, especially depression or anxiety, as patients without fibromyalgia.5
Different classification systems in psychiatry either do not include chronic pain among the symptoms of depression or give it scant relevance. This has led to generalised acceptance of the idea that depression has very little association with chronic pain.3 Pain does not feature as a symptom of any mood or anxiety disorder, and complaints of depression are marginal in the list of required symptoms among criteria for chronic pain disorder. Recent medical research indicates that this separation in terms of disease classification and symptoms is not consistent with the clinical and neurobiological reality.5,6
MethodsThis article is the result of a narrative review of the pathophysiological and clinical aspects of the relationship between depression, anxiety and chronic pain. We included research articles that gave prominence to data on major depression and chronic pain comorbidity, extracting the most relevant elements relating to understanding of the pathophysiology of the two conditions. We organised the abundance of information into a comprehensive text and considered a common psychopathological outcome, depression-anxiety-pain, which we have formulated into figures and tables.
ResultsDepressive disorder, anxiety disorder and painNot only is there pathophysiological correlation between anxiety disorders and depressive disorders, there is also clinical correlation. One of the first meta-analyses to be carried out looking for comorbidity between anxiety and depression5 found that 58% of depressed patients had some type of anxiety, and that concomitant depression and anxiety had occurred in 52.2% of cases in the previous year. Conversely, 56% of patients with anxiety had depression. There is evidence that depression and anxiety are associated with chronic pain and not only that they can be clinically concomitant, but also that chronic pain is considered a predictor of major depression or worsening of anxiety symptoms6–10; a relationship has been found between the magnitude of the complaint of pain and the intensity of the depressive and anxiety symptoms.9
An analysis of a two-dimensional model of anxiety and depression found that the scores for each of the syndromes correlated significantly with the intensity and severity of the pain.11,12 An epidemiological study showed that painful physical symptoms are a common feature in patients suffering from generalised anxiety disorder and even more so in patients with comorbid depressive disorder. An association has been demonstrated in patients with anxiety disorder with or without depressive comorbidity between painful physical symptoms and functional impairment at work, and impairment in social aspects and family functioning.11
Among primary care patients with chronic musculoskeletal pain, depression and anxiety have independent and cumulative effects on pain intensity, pain interference, functional limitations, days of disability and quality of life.13 Other studies have shown that 65.8% of primary care patients requiring antidepressant treatment reported headaches and muscle pain as concomitant complaints.14,15
A review of the literature in search of the relationship between depression and concurrent pain revealed the prevalence of pain symptoms in patients with depression to be from 15% to 100% (approximate mean 65%).3 In general, it is considered that 30–60% of patients with depression have some type of significant pain symptoms.13 Just as chronic pain conditions are common in patients with major depressive disorder, pain increases the frequency and severity of seven depressive symptoms: depressed mood, loss of interest, agitation or psychomotor inhibition, weight gain, insomnia, fatigue and problems concentrating.8 The author of that study proposes that pain should be considered as one of the clinical manifestations of major depressive disorder. It is then suggested that the number of complaints related to pain, which generally increases according to the severity of the depression, should be considered both in the diagnosis of depression and in the planning of treatment strategies and measurement of the results.16
In a three-year longitudinal study,17 the presence of painful symptoms substantially reduced the probability of recovery from depression in a group of elderly patients; they had a remission rate of 9% compared to 47% among patients who did not have concomitant pain symptoms. A Latin American study18 in depressed patients reported that 72.6% of people classified as having painful physical symptoms had greater severity and duration of depressive manifestations, indicating the need for a holistic approach to the emotional and physical symptoms of depression. An association was then identified between depression, anxiety and somatisation, especially painful, in a primary care population in Colombia, with independent effects of each syndrome that increased disability and suicidal ideation.19 This type of patient frequently consults persistently, more for somatic symptoms of pain than for anxiety, depression or insomnia, which highlights the need to screen patients attending both primary and specialised care services.20,21
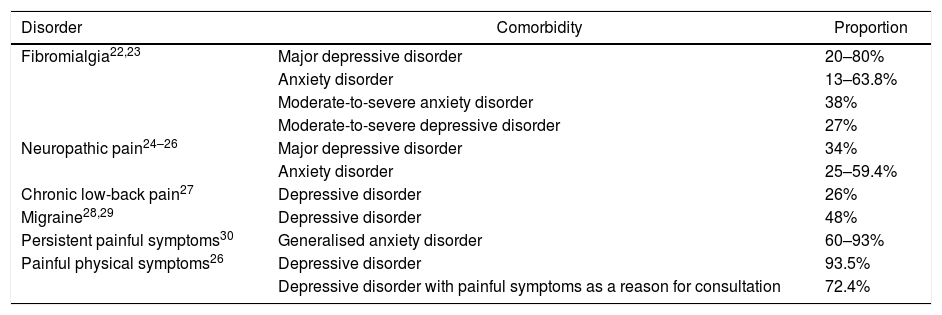
A significant association has also been described between depressive and anxiety disorders and specific chronic types of pain such as fibromyalgia, neuropathic pain, chronic low-back pain, migraine, painful physical symptoms and persistent painful symptoms (Table 1). Patients who experience anxiety and pain are more likely to be more aware of bodily sensations and to detect physical symptoms, report more intense pain, have less tolerance to pain and report more anxiety and catastrophic thoughts than patients with painful symptoms without anxiety.31,32
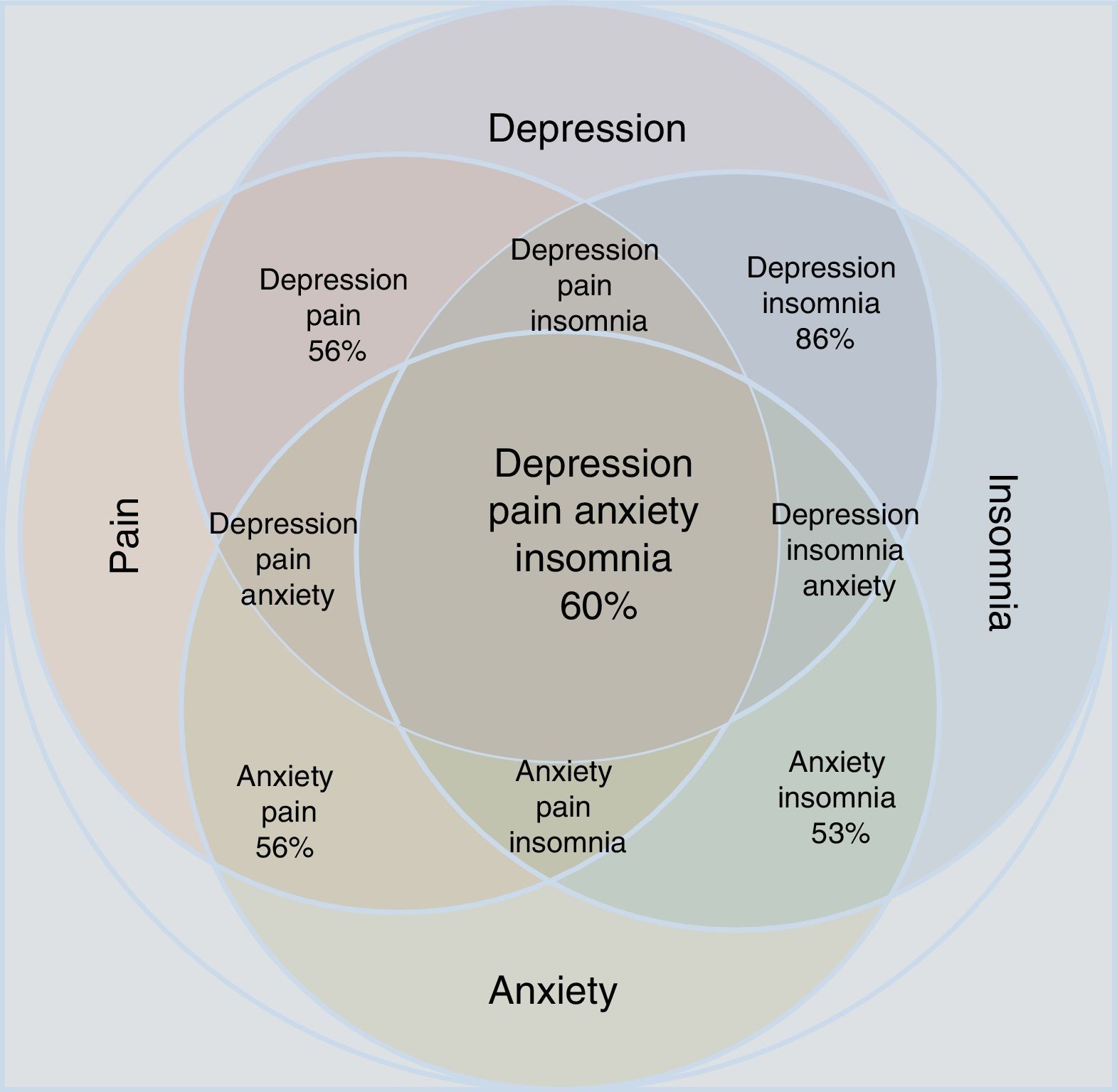
Proportion between the comorbidity of depression and anxiety with chronic painful syndromes.
| Disorder | Comorbidity | Proportion |
|---|---|---|
| Fibromialgia22,23 | Major depressive disorder | 20–80% |
| Anxiety disorder | 13–63.8% | |
| Moderate-to-severe anxiety disorder | 38% | |
| Moderate-to-severe depressive disorder | 27% | |
| Neuropathic pain24–26 | Major depressive disorder | 34% |
| Anxiety disorder | 25–59.4% | |
| Chronic low-back pain27 | Depressive disorder | 26% |
| Migraine28,29 | Depressive disorder | 48% |
| Persistent painful symptoms30 | Generalised anxiety disorder | 60–93% |
| Painful physical symptoms26 | Depressive disorder | 93.5% |
| Depressive disorder with painful symptoms as a reason for consultation | 72.4% |
Insomnia is a very prevalent complaint in the general population, with the prevalence of chronic insomnia as high as 9% and that of occasional insomnia 30%. The incidence rates reported in longitudinal studies range from 3% to 20%,33 varying according to the population studied, the period analysed and the definition of insomnia. From 65% to 89% of patients with chronic pain assessed in specialised treatment centres have at least one sleep complaint or describe themselves as having non-restorative sleep.34,35 Patients who complain of pain and have insomnia report greater intensity of pain, difficulty falling asleep, frequent wakening, fewer hours of sleep and less restorative sleep than patients with pain without insomnia or sleep disturbances even with pain.34,36
The emotional rating of pain and anxiety were the best predictors of the severity of insomnia, representing 30% of the total variance, even when the pain intensity was controlled. It has been concluded that the affective manifestations associated with pain are a significant predictor of the severity of insomnia.37 A research group analysed a cross-sectional sample of 118,336 participants aged over 18 with pain caused by arthritis in comparison to people without arthritis38; in the arthritis group, 45.8% reported arthritic pain, 24.8% insomnia and 11.9% non-restorative sleep, compared to 11.7, 10.6 and 6.1% respectively in the non-arthritis group.
Pain can significantly interfere with getting to sleep and staying asleep, but there is also evidence that insomnia significantly increases pain intensity. A clinical study39 provided the first evidence of an exaggerated increase in pain symptoms after sleep loss in patients with rheumatoid arthritis compared to control subjects. After partial sleep deprivation, the pain symptoms reported by patients with rheumatoid arthritis in the morning were more severe than those of the controls. In addition, partial sleep deprivation increased the number of painful joints and the intensity of pain. It was found that sleep loss, as opposed to sleep fragmentation, has a unique role in the differential induction of pain symptoms in patients with rheumatoid arthritis. Moreover, the quality of sleep has been found to be an important contributory factor in the deficit in inhibitory conditioned pain modulation in fibromyalgia, and it is therefore possible that better treatment of sleep disturbances would result in an improvement in the painful symptoms in fibromyalgia.40
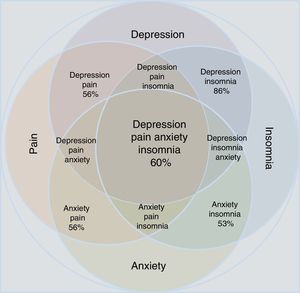
Statistical reports show that anxiety and depression exacerbate painful manifestations, and this triad worsens insomnia.34–37 Insomnia and pain, in turn, increase depressive and anxiety symptoms. The comorbidity depression-anxiety-pain and the clinical manifestation of insomnia form a clinical complex in which all the possible combinations can be found (Table 1 and Fig. 1). Clinicians have to take all aspects of the depression-anxiety and insomnia-pain relationships into account in order to adequately evaluate the therapeutic options and any improvement in a patient's condition.
Approximation according to the epidemiological data of the concomitance, overlap and comorbidity of depressive disorder and anxiety disorder in relation to the manifestations of pain and insomnia. Note that individual conditions occupy less area than the merged set. All possible combinations are shown; the percentages refer to epidemiological estimates of comorbidity.
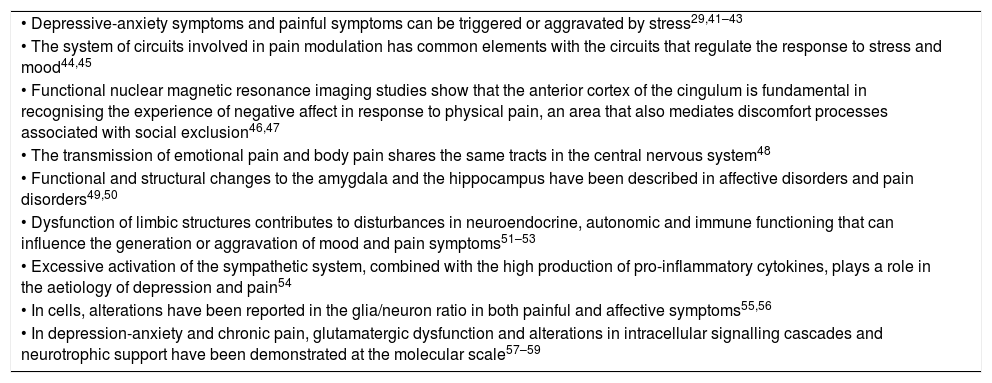
Advances in biomedical research in recent years indicate that depressive and anxiety disorders share biological and environmental aspects with chronic painful disorders and insomnia. Among the biological factors, genetic determinants have been found that include genes that regulate neurotransmitter and neurotrophic and inflammatory cytokine signalling. Among the environmental risk factors are psychosocial stress, especially when it is continuous, and disease in general which, in vulnerable individuals, promote changes in the sensitivity of glucocorticoid receptors in the nervous system and other organs, dysfunction of the hypothalamic–pituitary–adrenal axis, alterations in autonomic functions and increase in the production and release of pro-inflammatory cytokines; all of which converge to form structural and functional variations in the nervous system (Table 2). The common pathophysiological pathway involves alterations in neurotrophic support and neuron–glia interaction, which cause a phenomenon of central sensitisation to pain, associated through the same pathway with depressive and anxiety manifestations and sleep disturbances. The concomitance of the four clinical components acquires a synergistic biological character that is associated with the progressive nature of these disorders and their tendency to perpetuate themselves, especially when not adequately treated.
Aetiological, functional and structural similarities between chronic pain, depression and anxiety.
| • Depressive-anxiety symptoms and painful symptoms can be triggered or aggravated by stress29,41–43 |
| • The system of circuits involved in pain modulation has common elements with the circuits that regulate the response to stress and mood44,45 |
| • Functional nuclear magnetic resonance imaging studies show that the anterior cortex of the cingulum is fundamental in recognising the experience of negative affect in response to physical pain, an area that also mediates discomfort processes associated with social exclusion46,47 |
| • The transmission of emotional pain and body pain shares the same tracts in the central nervous system48 |
| • Functional and structural changes to the amygdala and the hippocampus have been described in affective disorders and pain disorders49,50 |
| • Dysfunction of limbic structures contributes to disturbances in neuroendocrine, autonomic and immune functioning that can influence the generation or aggravation of mood and pain symptoms51–53 |
| • Excessive activation of the sympathetic system, combined with the high production of pro-inflammatory cytokines, plays a role in the aetiology of depression and pain54 |
| • In cells, alterations have been reported in the glia/neuron ratio in both painful and affective symptoms55,56 |
| • In depression-anxiety and chronic pain, glutamatergic dysfunction and alterations in intracellular signalling cascades and neurotrophic support have been demonstrated at the molecular scale57–59 |
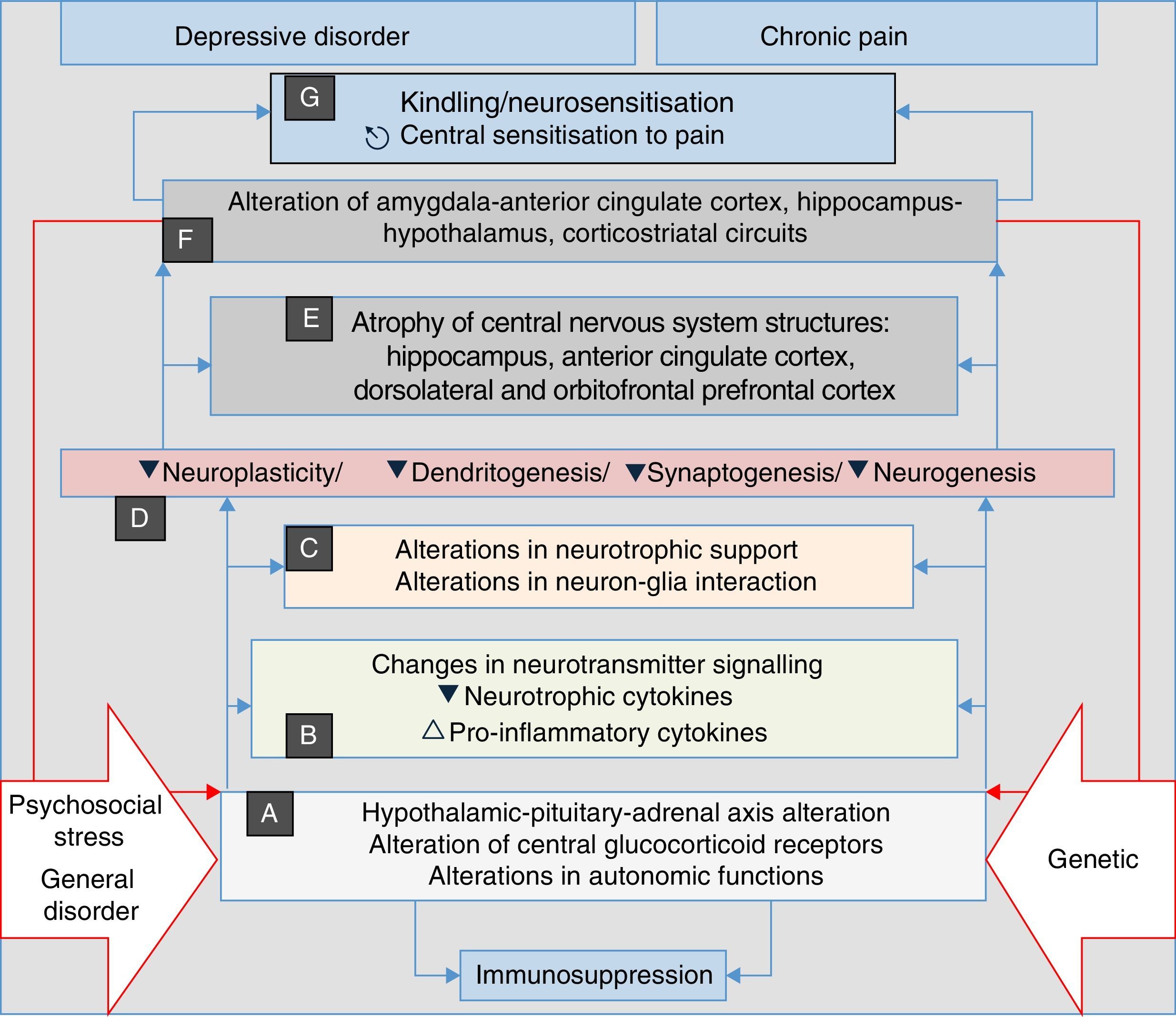
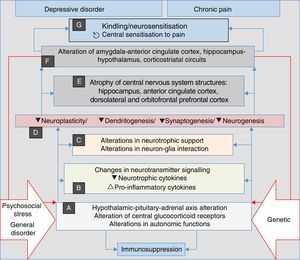
Recent research has discovered that neurosensitisation processes develop in depression, anxiety and chronic pain. This is a phenomenon analogous to the kindling described in epilepsy, which consists of a persistent increase in neuronal reactivity. In both depression and chronic pain, these processes have the same neurobiological origins, such as neuroplasticity and changes in gene expression. Some authors consider neurosensitisation to be the basis of a common aetiology of chronic pain, depression and anxiety disorders60–63; in all cases, a pathophysiological process is generated in which the manifestations are mainly associated with endogenous circumstances, and external stressors such as trauma, painful disorders and/or psychosocial stress, factors which initially trigger the process in a genetically vulnerable subject, are less relevant (Fig. 2). In both depression and pain, neurosensitisation makes clinical manifestations increasingly spontaneous, persistent and severe.60,63
Stress, general illness and genetic factors are the terrain in which the processes that converge into clinical manifestations of depression, anxiety, pain and insomnia are triggered. They begin with autonomic alterations and in the neuroendocrine axis (A) that trigger neurochemical changes (B) and changes in the trophic support (C), which generates microstructural (D) and later macrostructural (E) and functional (F) alterations, establishing a pathological complex (G) that can be common to the four clinical manifestations. Note the perpetuation of the psychopathological phenomenon resulting from the influence of (F) on (A) (double red lines).
The first hypothesis relating to the pathogenesis of depression, based on changes in neurogenesis in the hippocampus, was proposed in 2000.64 Subsequently, similar changes were observed in circumstances of anxiety and chronic pain,65–67 and a common clinical and pathophysiological substrate was proposed (Table 2). In all the cases, the changes have been related to alterations in substances such as brain derived neurotrophic factor (BDNF) and other substances such as serotonin that support neuronal and glial vitality. Considering these aspects, it is striking that in both major depression and chronic pain disorders, there is a gradual progression towards cognitive impairment possibly related to structural changes in the brain, especially in the medial prefrontal cortex, the anterior cingulate cortex and the hippocampus.68
In general terms, it has been established that the genes involved in mental illness do not determine the development of a specific disease, but that endophenotypic traits increase the risk of psychiatric morbidity.69–71 It has been shown in different studies that the short allele of the 5-HTTLPR promoter gene increases the likelihood of depression and suicidal ideation only if people are exposed to environmental stressors; if the environmental factor is minimal, the person may not develop the disorder.71,72 In major depressive disorder and suicide, the Val66Met allele manifests itself with decreased BDNF activity and has been associated with structural brain changes such as reduction in grey matter in the dorsolateral prefrontal cortex, the orbital lateral prefrontal cortex, and the hippocampus. A relationship has been identified between the BDNF gene and the modulation of cell recovery capacity, neuroplasticity and neurogenesis.73–75 A relationship has also been described between the manifestations of chronic pain and the bilateral decrease in the size of the hippocampus in chronic back pain and complex regional pain syndrome.76 In both depression and chronic pain, the changes potentiate the risk of alterations in the limbic circuit of the amygdala-anterior cingulate cortex and in the hypothalamic–pituitary–adrenal axis.77,78 The genetic mechanism that could link the alterations in these circuits is epistasis, a phenomenon that involves the interaction between alleles present in different loci and occurs when two pairs of genes affect the same characteristic.
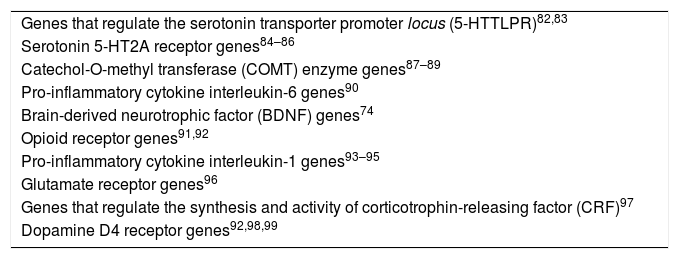
The genetic polymorphism of depression has been related to serotonin receptors and to the catechol-O-methyltransferase enzymes, pro-inflammatory cytokines, opioid receptors, glutamate receptors, corticotrophin-releasing factor and dopamine receptor genes; the same genes have been implicated in the genesis of pain symptoms.79–81Table 3 summarises the connection between the genetic aspects of major depressive disorder and acute and chronic pain.
Genes involved in vulnerability to major depressive disorder and acute and chronic pain.
| Genes that regulate the serotonin transporter promoter locus (5-HTTLPR)82,83 |
| Serotonin 5-HT2A receptor genes84–86 |
| Catechol-O-methyl transferase (COMT) enzyme genes87–89 |
| Pro-inflammatory cytokine interleukin-6 genes90 |
| Brain-derived neurotrophic factor (BDNF) genes74 |
| Opioid receptor genes91,92 |
| Pro-inflammatory cytokine interleukin-1 genes93–95 |
| Glutamate receptor genes96 |
| Genes that regulate the synthesis and activity of corticotrophin-releasing factor (CRF)97 |
| Dopamine D4 receptor genes92,98,99 |
It has been suggested that fibromyalgia is related to a deficit in the internal modulation of pain, especially of the inhibitory mechanisms.100,101 The neurobiology of pain involves the regulation of endogenous processes of inhibition and excitation that include the inhibitory conditioned pain modulation; it is postulated that in this phenomenon the nociceptive stimulation cancels out another nociceptive stimulus if it occurs at a body site distant from the painful surface. This system involves serotonergic, noradrenergic and opioidergic inhibitory pathways and causes a reduction in diffuse pain throughout the body with the associated emotional effect. Some experimental studies have shown that the inhibitory conditioned pain modulation is deficient in fibromyalgia.101–103 Fibromyalgia is thought to be related to central and peripheral hyperexcitability of the nociceptor system that manifests as multiple painful tender points, hyperalgesia and allodynia.
In depressed patients, imaging alterations are described in the sector of the dorsal anterior insula where changes usually occur in patients with chronic pain; this may have a role in what is known as “emotional allodynia”, a concept related to the pain experienced by people with major depression in response to stimuli which would not normally be painful.104 It should be noted that people with bipolar disorder have also been found to have comorbidity with fibromyalgia105–109 and migraine.110–112 Functional imaging studies reveal structural changes in the amygdala and the prefrontal cortex and support the fact that bipolar depression, suffering from pain and suffering from rejection share the same biological circuits.113,114 Bipolarity and chronic pain could be related to the fact that some mood-modulating drugs have clear effects in controlling pain such as trigeminal neuralgia, neuropathic pain and migraine.115
ConclusionsThe multiple symptoms of depression aggravate and accentuate the pain symptoms and, vice versa, similar potentiation occurs with insomnia and anxiety symptoms. The relationship between pain, sleep disturbances and cognitive complaints constitutes a syndrome similar to depression which makes it difficult to distinguish between the different psychopathological components.116,117 All these circumstances make therapeutic intervention complex and, given the overlap in symptoms, the efficacy of the medications is frequently not adequate for one single manifestation. Knowledge of the comorbidity of depression-anxiety-chronic pain-insomnia is fundamental in the search for effective and assertive therapeutic interventions.
As with insomnia, because of their high rate of manifestation in depression, painful symptoms should be considered as cardinal expressions of depressive disorders. The recognition that polymorphism of the inflammation-related genes generates susceptibility to depressive symptoms and can modify the response to antidepressant treatments establishes that the inflammatory response is not only an aetiopathogenic component of depression, but also of stress and pain. Moreover, the similarity in the imaging approach indicates that the analogy is not only structural, but also functional and pathophysiological.118,119
FundingResearch resources from the Fundación Valle del Lili and Universidad Icesi de Cali, Colombia.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Arango-Dávila CA, Rincón-Hoyos HG. Trastorno depresivo, trastorno de ansiedad y dolor crónico: múltiples manifestaciones de un núcleo fisiopatológico y clínico común. Rev Colomb Psiquiat. 2018;47:46–55.