There are several clinical and laboratory features for lupus nephritis diagnosis; however, renal biopsy remains as the gold standard. Different series have tried to establish the relationship between these findings, with conflicting results.
ObjectiveTo describe the correlation between clinical and laboratory variables with histological biopsy-proven lupus nephritis.
MethodsAn analytical cross-sectional study was conducted, between January, 2004 and December, 2012. Qualitative variables were described using absolute and relative frequencies, while quantitative variables were assessed by medians with interquartile range. The relationship with clinical findings was explored using chi-square maximum likelihood test, adjusted standardized residuals, hierarchical Kruskal–Wallis test, homogeneity of variance in data, post hoc Dunn's test, Spearman's correlation coefficient, and Mann–Whitney test.
Results132 patients were included. Proliferative lupus nephritis was the most frequent (74%). The most common clinical condition was nephritic syndrome (46%); proteinuria was observed in 80%. No relationship was found between clinical syndromes and histological types; only statistically significant differences were observed between proliferative and non-proliferative forms regarding hematuria (72.1 vs. 46.7%; p=0.012), C3 hypocomplementemia (70.9 vs. 43.3%; p=0.007), 24-h proteinuria (2560 vs 741mg; p=0.001), and serum creatinine (1 vs. 0.77mg/dL; p=0.006). We found positive correlations between activity index and serum creatinine values, 24-h proteinuria, C3 hypocomplementemia, along with positive anti-DNA antibodies.
ConclusionThere is a clinicopathological relationship within proliferative types and certain laboratory features (hematuria, elevated 24-h protein excretion, serum creatinine level, and C3 hypocomplementemia) in a mestizo population with lupus nephritis; nonetheless, no association was found with any other variables.
Existen varias características clínicas y de laboratorio para el diagnóstico de la nefritis lúpica; sin embargo, la biopsia renal sigue siendo el estándar de oro. Diferentes series han tratado de establecer la relación entre estos hallazgos, con resultados contradictorios.
ObjetivoDescribir la correlación entre variables clínicas y de laboratorio con los hallazgos histológicos de nefritis lúpica comprobados por biopsia.
MétodosSe realizó un estudio transversal analítico entre enero de 2004 y diciembre de 2012. Se describieron las variables cualitativas utilizando frecuencias absolutas y relativas, mientras que las variables cuantitativas fueron evaluadas por medianas con rango intercuartílico. La relación con los hallazgos clínicos se exploró utilizando: prueba de probabilidad máxima de Chi-cuadrado, residuos estandarizados ajustados, prueba jerárquica de Kruskal-Wallis, homogeneidad en los datos de la varianza, prueba de Dunn post hoc, coeficiente de correlación de Spearman y prueba de Mann-Whitney.
ResultadosSe incluyeron 132 pacientes. La nefritis lúpica proliferativa fue la más frecuente (74%). La condición clínica más común fue el síndrome nefrítico (46%); Se observó proteinuria en el 80%. No se encontró relación entre los síndromes clínicos y los tipos histológicos; solo se observaron diferencias estadísticamente significativas entre las formas proliferativa y no proliferativa con respecto a: hematuria (72,1 vs. 46,7%; p=0,012), hipocomplementemia C3 (70,9 vs. 43,3%; p=0,007), proteinuria de 24h (2.560 vs. 741mg; p=0,001) y creatinina sérica (1 vs. 0,77mg/dl; p=0,006). Se encontraron correlaciones positivas entre el índice de actividad y los valores de creatinina sérica, proteinuria de 24h, hipocomplementemia C3, junto con anticuerpos anti-DNA positivos.
ConclusiónExiste una relación clínico-patológica entre los tipos proliferativos y ciertos hallazgos paraclínicos (hematuria, elevación de 24h de proteína, nivel de creatinina sérica e hipocomplementemia C3) en una población mestiza con nefritis lúpica; sin embargo, no se encontró asociación con ninguna otra variable.
Systemic lupus erythematosus (SLE) is a chronic, complex, multisystem autoimmune disease with protean clinical manifestations, which are modulated by genetic, environmental, hormonal, and immunological factors.1,2 Renal involvement accounts for 51–60% of SLE patients3–5; it is one of the leading causes of morbidity and mortality,6 with about 10–30% of subjects progressing to end-stage renal disease (ESRD), despite current treatment.7–10 Considering these aspects, early diagnosis and treatment of lupus nephritis (LN) are mandatory, which is one of the main recommendations of the “treat to target” strategy in Systemic Lupus Erythematosus (T2T/SLE) International Task Force.11
Renal biopsy (RB) is considered the gold standard to diagnose LN.12–15 Biopsy findings are classified according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS), issued in 2003. This classification includes six types of nephritis established through light, electronic, and immunofluorescence microscopy findings. Besides type VI, which denotes an irreversible stage of fibrosis, the histological types can be broadly dichotomized into non-proliferative and proliferative lupus nephritis (NPLN and PLN, respectively). PLN is constituted by types III and IV, which are characterized by endothelial proliferation which damages the glomerulus and can rapidly progress to ESRD, requiring immediate and aggressive immunosuppression. In addition to current classification, RB provides information about vascular, tubular, and interstitial affection and estimates the degree of activity and chronicity; both indices are important from a therapeutic and prognostic point of view.16
Despite all its benefits, RB is not usually available at the moment of defining immunosuppression as it requires specialized equipment and is associated with significant clinical risks; thus, it is contraindicated in individuals with certain clinical conditions, such as anemia or thrombocytopenia. Consequently, the physician must rely on clinical aspects which can help determine the diagnosis, such as proteinuria, abnormal urinary sediment, and an increase in serum creatinine, supported by markers of lupus activity, such as anti-ds DNA high titers17 or hypocomplementemia.18 From a pragmatic point of view, the important decision is to define the presence of PLN. Taking these considerations into account, different authors have tried to establish the relationship between clinical, laboratory, and histological findings in both adult19–26 and pediatric27,28 population, with conflicting results. To our knowledge, in Latin America, there is only one study that uses the current LN classification.19 For this reason, we decided to establish the clinicopathological relationship in patients with LN, emphasizing the difference between PLN and NPLN in Colombian patients.
Materials and methodsWe conducted an analytical cross-sectional study, which included all patients with SLE according to the 1997 American College of Rheumatology revised criteria29 with biopsy-proven LN in agreement with the 2003 ISN/RPS classification,16 who were treated at two reference centers in the city of Medellín, Colombia, between 2004 and 2012. Subjects with kidney transplantation, coexistence of other autoimmune diseases, or medical records with missing data on the main variables of interest were excluded. Treatment and time of renal biopsy were in consonance with usual clinical practice.
After receiving approval from the ethics committees of each institution, the information was retrieved from the patients’ medical records at the time of renal biopsy and was registered in a Microsoft Excel® 2010 worksheet, containing the following variables: demographic (age at diagnosis of SLE, gender); clinical (time from onset of symptoms and LN diagnosis, arterial hypertension, nephritic and nephrotic syndromes, acute and chronic renal failure, as defined by the KDIGO guidelines30,31; paraclinical (hematuria, pyuria, cylindruria, serum creatinine, positive anti-DNA antibodies, C3 and C4 hypocomplementemia – values below 90 and 10mg/dL, respectively) and histopathological classification of LN, along with activity and chronicity indices.16
Statistical analysesQualitative variables were described using absolute and relative frequencies, and quantitative variables by medians with interquartile range (IQR) due to non-compliance of normality assumption. In individuals with a single histological type, the relationship with clinical findings was explored using the chi-square or exact Fisher's test for qualitative variables, initially comparing each class (from I to V) separately and later grouped in proliferative (III and IV) vs. non-proliferative types (I–II–V). The difference of proportions between the comparison groups and their respective confidence intervals at 95% was determined.
Mann–Whitney test was used for comparison of quantitative variables. The relationship between activity and chronicity indices with complement values, 24-h proteinuria, and serum creatinine was determined using Spearman's correlation coefficient. In addition, activity index values were compared according to the presence or absence of anti-DNA antibodies, using the Mann–Whitney test. For all analyses, a p value of <0.05 was considered statistically significant. The processing of information was carried out in the statistical package SPSS version 22.
Bias controlPopulation selection bias decreased by including all patients with LN that met eligibility criteria during the study period. To control information bias by observers, a comprehensive review of the multiple information sources was performed, as well as the standardization of the collection process before researchers began to review medical records. In addition, information bias was controlled by excluding incomplete medical records or those with doubtful results in the variables of interest. Additionally, both centers referred the RB to the same pathologist for interpretation, which minimizes the possibility of interobserver variability.
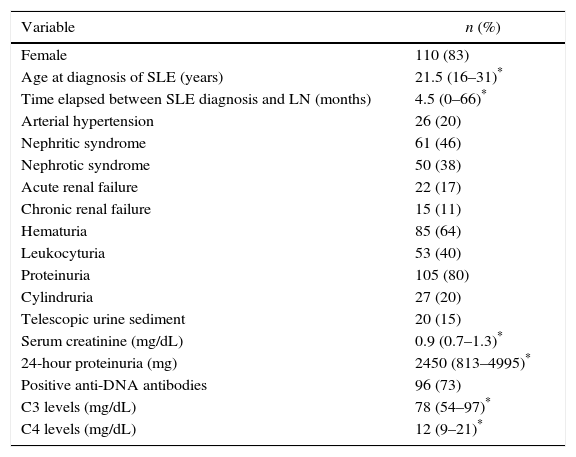
ResultsA total of 132 patients were included, of whom 110 (83%) were female. The median age was 24 years (IQR: 19–37) and the median elapsed time between diagnosis of SLE and LN was 4.5 months (IQR: 0–66). The median serum creatinine was 0.9mg/dL (IQR: 0.7–1.3). Ninety-six (73%) patients had positive anti-DNA antibodies; 85 (64%) and 44 (33%) had C3 and C4 hypocomplementemia, respectively. Proteinuria was observed in 105 individuals (80%), while hematuria was the most common urine sediment alteration, which was present in 85 subjects (64%). Regarding the clinical presentation, nephritic syndrome was the most common, followed by nephrotic syndrome with 46 and 38%, respectively (Table 1).
Sociodemographic, clinical, and paraclinical features of patients with LN.
| Variable | n (%) |
|---|---|
| Female | 110 (83) |
| Age at diagnosis of SLE (years) | 21.5 (16–31)* |
| Time elapsed between SLE diagnosis and LN (months) | 4.5 (0–66)* |
| Arterial hypertension | 26 (20) |
| Nephritic syndrome | 61 (46) |
| Nephrotic syndrome | 50 (38) |
| Acute renal failure | 22 (17) |
| Chronic renal failure | 15 (11) |
| Hematuria | 85 (64) |
| Leukocyturia | 53 (40) |
| Proteinuria | 105 (80) |
| Cylindruria | 27 (20) |
| Telescopic urine sediment | 20 (15) |
| Serum creatinine (mg/dL) | 0.9 (0.7–1.3)* |
| 24-hour proteinuria (mg) | 2450 (813–4995)* |
| Positive anti-DNA antibodies | 96 (73) |
| C3 levels (mg/dL) | 78 (54–97)* |
| C4 levels (mg/dL) | 12 (9–21)* |
Abbreviations: LN, lupus nephritis; SLE, systemic lupus erythematosus.
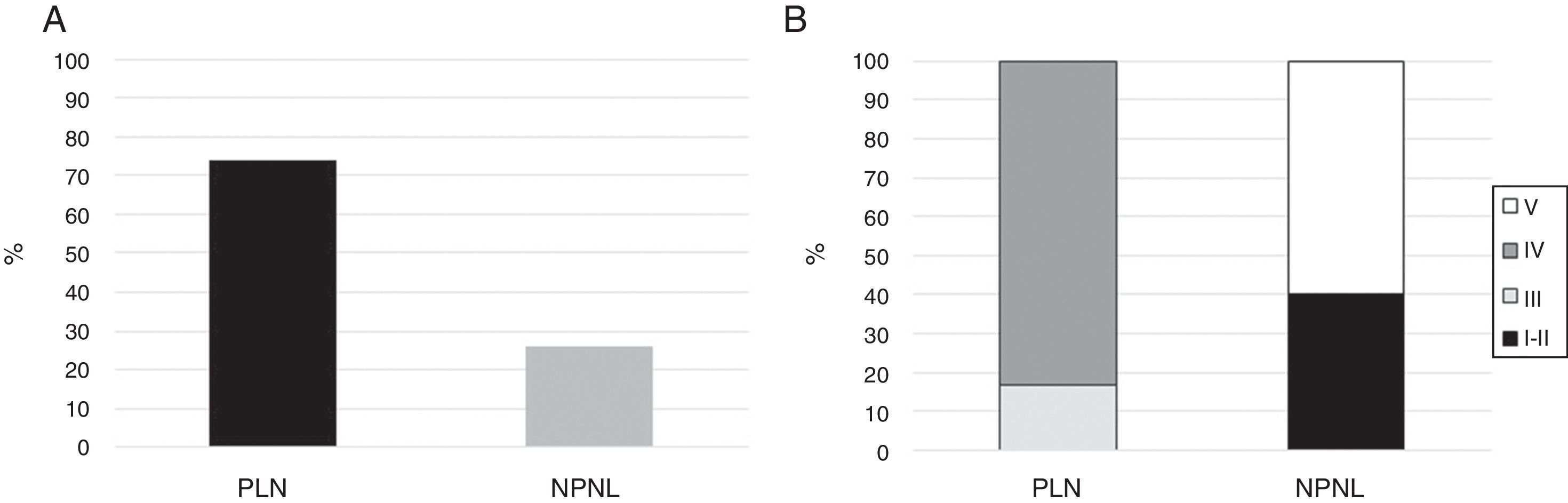
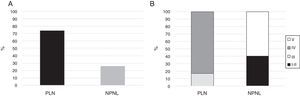
Fifteen (11.4%) patients had mixed histological types (III–V: n=7; IV–V: n=8) and were not included in the final analysis. Of the remaining 116 patients, 86 (74%) had PLN, where type IV was the most common as it was observed in 71 (82.6%) of the subjects (Fig. 1). None of the patients had type VI nephritis.
The median of activity and chronicity indices were 5 (IQR: 1.3–9) and 1 (IQR: 0–3), respectively. When activity and chronicity indices were compared, PLN had a higher activity index, with a median of 7.5 (IQR: 4–11.3), compared to NPLN (median: 0; IQR: 0–1.25; p<0.0001). The chronicity index was also higher in PLN (median 1; IQR 0–3) compared to NPLN (median 0; IQR 0–1.25; p=0.015).
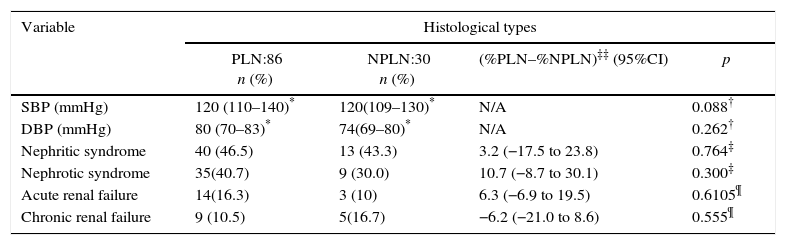
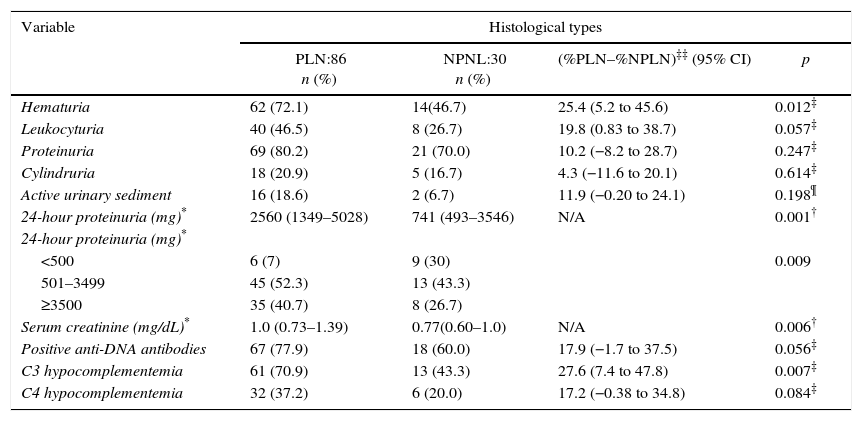
Clinicopathological relationshipNo association was found between arterial hypertension or any clinical syndrome (nephritic, nephrotic, acute, or chronic renal failure) with the presence of either PLN or NPLN (Table 2). Nonetheless, in patients with PLN, there was a higher presence of hematuria (72.1 vs. 46.7%; p=0.012), C3 hypocomplementemia (70.9 vs. 43.3%; p=0.007), 24-h proteinuria (2560 vs 741mg; p=0.001), and serum creatinine (1 vs. 0.77mg/dL; p=0.006), when compared with NPLN (Table 3).
Clinicopathological relationship of patients with lupus nephritis.
| Variable | Histological types | |||
|---|---|---|---|---|
| PLN:86 n (%) | NPLN:30 n (%) | (%PLN–%NPLN)‡‡ (95%CI) | p | |
| SBP (mmHg) | 120 (110–140)* | 120(109–130)* | N/A | 0.088† |
| DBP (mmHg) | 80 (70–83)* | 74(69–80)* | N/A | 0.262† |
| Nephritic syndrome | 40 (46.5) | 13 (43.3) | 3.2 (−17.5 to 23.8) | 0.764‡ |
| Nephrotic syndrome | 35(40.7) | 9 (30.0) | 10.7 (−8.7 to 30.1) | 0.300‡ |
| Acute renal failure | 14(16.3) | 3 (10) | 6.3 (−6.9 to 19.5) | 0.6105¶ |
| Chronic renal failure | 9 (10.5) | 5(16.7) | −6.2 (−21.0 to 8.6) | 0.555¶ |
Laboratory features of patients with lupus nephritis according to histological type.
| Variable | Histological types | |||
|---|---|---|---|---|
| PLN:86 n (%) | NPNL:30 n (%) | (%PLN–%NPLN)‡‡ (95% CI) | p | |
| Hematuria | 62 (72.1) | 14(46.7) | 25.4 (5.2 to 45.6) | 0.012‡ |
| Leukocyturia | 40 (46.5) | 8 (26.7) | 19.8 (0.83 to 38.7) | 0.057‡ |
| Proteinuria | 69 (80.2) | 21 (70.0) | 10.2 (−8.2 to 28.7) | 0.247‡ |
| Cylindruria | 18 (20.9) | 5 (16.7) | 4.3 (−11.6 to 20.1) | 0.614‡ |
| Active urinary sediment | 16 (18.6) | 2 (6.7) | 11.9 (−0.20 to 24.1) | 0.198¶ |
| 24-hour proteinuria (mg)* | 2560 (1349–5028) | 741 (493–3546) | N/A | 0.001† |
| 24-hour proteinuria (mg)* | ||||
| <500 | 6 (7) | 9 (30) | 0.009 | |
| 501–3499 | 45 (52.3) | 13 (43.3) | ||
| ≥3500 | 35 (40.7) | 8 (26.7) | ||
| Serum creatinine (mg/dL)* | 1.0 (0.73–1.39) | 0.77(0.60–1.0) | N/A | 0.006† |
| Positive anti-DNA antibodies | 67 (77.9) | 18 (60.0) | 17.9 (−1.7 to 37.5) | 0.056‡ |
| C3 hypocomplementemia | 61 (70.9) | 13 (43.3) | 27.6 (7.4 to 47.8) | 0.007‡ |
| C4 hypocomplementemia | 32 (37.2) | 6 (20.0) | 17.2 (−0.38 to 34.8) | 0.084‡ |
When assessing the relationship between clinical variables with activity and chronicity indices, positive correlations were found between activity index with serum creatinine values (Spearman's Rho: 0.40; 95% CI: 0.25–0.55; p<0.0001) and 24-h proteinuria (Spearman's Rho: 0.37; 95% CI: 0.18–0.55; p<0.0001), whereas a negative correlation was described amongst activity index and serum complement levels (C3 Spearman's Rho: 0.31; 95% CI: −0.47 to −0.13; p=0.001; C4 Spearman's Rho: −0.24; 95% CI: −0.41 to −0054; p=0.01); no correlations were detected for chronicity index. Additionally, the activity index values were higher in patients with positive anti-DNA antibodies, compared to subjects without them (median: 6; IQR: 3–11 vs. 3; IQR: 0–5.5; p=0.001).
DiscussionIn the present study, we found a clinicopathological relationship between PLN and hematuria, 24-h proteinuria, elevated serum creatinine, and C3 hypocomplementemia; nonetheless, no association was found with any clinical syndrome or other variables. This relationship is logical since proliferative forms are related to increased deposition of immune complexes in the glomeruli, with subsequent complement consumption and alterations in kidney function.32 These results are similar to those reported by Okpechi et al.,22 where they found that PLN was linked to hematuria on dipstick and C3 hypocomplementemia in a South African lupus population. Conversely, Wen, in Taiwan, found no clinicopathological relationship, except for anti-DNA antibodies and C4 hypocomplementemia with PLN. One possible explanation for this difference when compared with our study is the inclusion of mixed LN forms in the study of Wen et al., which could obscure this association.
When analyzing specific results, the correlation of hematuria with PLN is expected since the endothelial damage caused by immune complexes can lead to extravasation of red blood cells into Bowman's space, with its subsequent urinary excretion.4 Even though leukocyturia would be expected to behave in the same way, we did not find statistical association, albeit there was a clinically significant difference (46.5 vs. 26.7% in PLN and NPLN, respectively) with a borderline statistically significant p value (0.057), which probably reflects lack of power of our study to detect such difference and could also be explained by the many causes other than glomerular damage, which could cause leukocyturia. Since the sample in this study was a convenience one with the total of the detected subjects, there were no other interventions that could be implemented to mitigate this problem. It would be important to consider the inclusion of additional rheumatology centers in the future to increase the sample size and clarify this borderline finding.
Concerning the lack of correlation between the clinical syndromes and histological types, renal biopsy was performed in a systematic manner in patients with 24-h proteinuria greater than 500mg or persistent alterations in urinary sediment, instead of waiting until renal function was impaired, which could explain the lack of correlation (at least partially), since prompt treatment was initiated. This was evidenced through nephritic and nephrotic syndrome rates in our study (46 and 38%, respectively).
When compared with other authors, the frequency of nephrotic and nephritic syndromes is variable in non-proliferative and proliferative forms.22,33 For instance, in the study of Shariati-Sarabi et al.,34 the overall prevalence of nephrotic syndrome was only 8.5%, compared to 38% in our study, yet they did not analyze PLN versus NPLN, and criteria to perform renal biopsy were also different; thus, the comparison of both studies is not possible. It is important to remember that the definition of these syndromes are composite measures of different clinical variables (dyslipidemia, edema, hypertension, among others), susceptible to variation between observers, which constitutes a confounding factor when comparing with other studies, in addition to the inclusion, in some studies, of mixed forms of lupus nephritis.33
One of the striking features of the results of this investigation is the greater range of 24-h proteinuria in proliferative forms, as opposed to that described in other lupus nephritis cohorts.18,35 One of the possible explanations for this finding is that, in our cohort, 40% of non-proliferative forms corresponded to histological types I and II, in which proteinuria is minimal and generally would not be included in normal clinical practice studies, which require a minimum threshold of proteinuria and urinalysis abnormalities to perform RB. Additionally, one could then argue that class V was shadowed by classes I and II when grouped in the NPLN for analysis; nonetheless, we analyzed each class separately and class V had a lower 24-h proteinuria compared with class III and IV and analysis was unchanged whether we analyzed PLN versus NPLN or each class separately (data not shown). We did not find a satisfactory explanation for these phenomena other than the inherent variation of RB, which analyses a limited number of glomeruli and may not reflect the entire renal histopathology.
The other possible reason is that, in literature, there are descriptions of important podocytopathy in PLN, which shows, in some series, that proteinuria is higher in these histological subtypes. Rsende et al. found, in a four-year follow-up, a tendency toward lower mean levels of proteinuria in patients with NPLN, in relation to a preserved synaptopodin, Wilms tumor protein 1, glomerular epithelial protein 1 and nephrin, suggesting structural podocyte damage and higher proteinuria in proliferative types.36
One of the difficulties in trying to compare the findings of this study with previous reports is that the 1977 WHO histological classification was used in these surveys and does not include activity and chronicity indices evaluated in this research.37,38 Due to the type of population and the adoption of the current classification of lupus nephritis, the present study is similar to Polanco et al.19; correspondingly, the most common clinical syndrome was nephritic, while proliferative forms occurred with a similar frequency (77 vs. 79%), although the nephritic syndrome was higher in Polanco's study (46 vs. 63%), probably attributed to a longer history of nephritis (13.1±28.1 vs. 4.5 months in this study, although it must be clarified that times were calculated as means and medians, respectively). Unfortunately, Polanco et al. did not refer to anti-DNA titers or activity and chronicity indices. The clinical resemblance of both studies might indirectly suggest a comparable behavior of PLN in Latin American patients. Such findings could be explained by the early aggressive treatment, as well as the prompt diagnosis of lupus nephritis, and the rapid onset of immunosuppressive therapy in these patients.
We explored the relationship between activity and chronicity scores of renal biopsies with PLN and NPLN. Like the results of Mavragani et al.,18 an elevated serum creatinine was associated with a high activity index; an identical finding was reported in the study of Shariati-Sarabi34); additionally, they found a higher frequency of anti-DNA antibodies in PLN. It should be noted that, in the work of these authors, the presence of patients with type VI nephritis constituted 7.1% of its population and that, presumably, it had clearly higher creatinine levels than other types of nephritis.
Certain specific limitations were recognized in this study, such as the high frequency of proliferative forms; therefore, the external validity of the results can only be inferred to these specific types. Furthermore, as a cross-sectional study design, there is no data concerning monitoring or treatment response, which are relevant aspects from a clinical perspective. We understand that this design overrepresents chronic and less severe cases, and excludes both extremely ill patients and those who had a fatal outcome. Moreover, despite having included the entire population that met eligibility criteria for a period of eight years in two high-complexity institutions, only 132 patients were admitted, which could affect the power of the study to detect any clinical differences between them; this aspect also limits the conclusions of this paper. However, with the available information, relationships were explored using appropriate statistical analyses.
We recognize the possibility of reference bias; it is nonetheless unavoidable, since renal biopsy can only be performed in reference centers and the international criteria by which this procedure was performed already generates a selection bias per se.39,40 This limitation could also be one of the strengths of our study since this survey was carried out under real-life conditions; we believe that our results can be transferred into clinical care, due to the inclusion of a significant number of individuals using the current histological classification. To our knowledge, this is the first study which adopts this classification at the local level.
Finally, even though our results could guide the clinicians in order to define immunosuppression in situ, renal biopsy continues to be of paramount importance in order to correctly classify patients as well as rule out other causes of renal involvement, such as antiphospholipid syndrome nephropathy or thrombotic microangiopathy.
ConclusionIn a population of Colombian patients with lupus nephritis, there is a clinicopathological relationship between proliferative types and certain laboratory features, such as hematuria, elevated 24-h protein excretion, serum creatinine level, and C3 hypocomplementemia; nonetheless, no association was found with any other clinical syndrome nor other variables. Renal biopsy continues to be the gold standard in the classification and correct evaluation of lupus nephritis.
Conflicts of interestThe authors declare no conflicts of interest of finantial disclosures.
The authors would like to thank to Clinica Universitaria Bolivariana and Hospital Pablo Tobón Uribe for their support in the development of this study.