Psoriasis is a chronic disease associated with multiple comorbidities including inflammatory intestinal disease. There are no previous studies in Colombia regarding the relationship of gastrointestinal symptoms in patients with psoriasis.
ObjectiveTo determine the frequency of symptoms and autoantibodies suggestive of gastrointestinal disease in patients with psoriasis, and to compare the results with healthy controls, as well as their relationship with disease activity.
Materials and methodsCross-sectional study with an analytical component, including a questionnaire on symptoms and serum analysis of antibodies suggestive of gastrointestinal disease in cases and controls.
ResultsThe analysis included 84 individuals, separated into groups of 44 cases and 40 controls. Mild psoriasis was observed in 64% of cases, and the remaining 36% had moderate to severe psoriasis. Topical treatments were given to 71% of cases, with another 16% using oral treatments, and 14% received biological treatment. The main symptoms of gastrointestinal disease were, abdominal distention (45%), constipation (39%), and fatigue (34%). A comparison showed a higher prevalence of gastrointestinal symptoms in cases vs. controls, and fatigue was statistically significant (p=0.04). Only the antinuclear autoantibodies in the serum analysis were statistically significant (0 vs. 43%; p=0.01).
ConclusionsThe prevalence of symptoms and autoantibodies suggestive of gastrointestinal disease in patients with psoriasis is considered important, even although there are no significant differences when compared with healthy controls. Some patients with gastrointestinal symptoms had positive autoantibodies. This suggests that an adequate clinical follow-up should be carried out. Dermatologists have a decisive role in the integral management of patients.
La psoriasis es una enfermedad crónica asociada con múltiples comorbilidades, incluida la enfermedad inflamatoria intestinal. Sin embargo, no existen estudios previos en Colombia sobre la relación de los síntomas gastrointestinales en pacientes con psoriasis.
ObjetivosSe determinó la frecuencia de síntomas sugestivos de enfermedad gastrointestinal y autoanticuerpos asociados en pacientes con psoriasis, y su relación con la actividad de la misma. Además, se comparó con controles sanos.
Materiales y métodosEstudio transversal con componente analítico que incluyó una encuesta de síntomas y el análisis sérico de autoanticuerpos sugestivos de enfermedad gastrointestinal en casos y controles.
ResultadosOchenta y cuatro individuos analizados, distribuidos en 44 casos y 40 controles. El 64% de los casos tenían psoriasis leve y el 36% presentó psoriasis moderada a severa. El 71, el 16 y el 14% fueron tratados con tratamientos tópicos, orales y biológicos, respectivamente. Los principales síntomas gastrointestinales fueron distensión abdominal (45%), estreñimiento (39%) y fatiga (34%). La comparación arrojó una mayor prevalencia de síntomas gastrointestinales en los casos, y la variable fatiga fue estadísticamente significativa (p: 0,04). Solo el perfil de autoanticuerpos antinucleares fue estadísticamente significativo (0 vs. 43%; p: 0,001). No se encontró asociación con actividad de la enfermedad.
ConclusionesLa prevalencia de síntomas y autoanticuerpos sugestivos de enfermedad gastrointestinal en pacientes se considera importante, a pesar de no mostrar diferencias significativas con los controles. Algunos pacientes con síntomas gastrointestinales mostraron autoanticuerpos positivos. Esto sugiere la necesidad de un seguimiento clínico adecuado. El dermatólogo tiene un papel decisivo en el manejo integral de los pacientes.
Psoriasis is a multifactorial, inflammatory, chronic disease, characterized by the over-proliferation of keratinocytes. Such over-proliferation is secondary to the activation of the immune system mediated by T lymphocytes.1,2 It is considered a systemic inflammatory disease with significant impact on the patients’ quality of life, and has been associated with concomitant diseases such as psoriatic arthritis, cardiovascular disease, non-alcoholic fatty liver, inflammatory bowel disease, lymphoma, skin cancer, anxiety, and depression.3
Some of the gastrointestinal comorbidities studied that have been associated with psoriasis are inflammatory bowel disease (Crohn's disease and ulcerative colitis), and celiac disease.3
Crohn's disease is a chronic disorder associated with an inadequate immune response. It is a granulomatous and scarring inflammatory disorder.4 It may affect any aspect along the GI tract, from the mouth to the anus. Its involvement usually presents in a segmented fashion, although its most frequent localization of the ileum. The main symptoms of Crohn's disease are diarrhea and abdominal pain. Its diagnosis is based on the combination of clinical suspicion and laboratory tests such as acute phase reactants and fecal markers, endoscopy, biopsy, and radiological findings. Over the course of the disease, there are frequent periods of activity that alternate with periods of remission.4
Different serological markers have been studied in association with Crohn's disease, such as antinuclear antibodies (ANA), anti-Saccharomyces cerevisiae antibodies (ASCA), and the anti-neutrophil cytoplasmic antibody pattern (pANCA). A higher prevalence of ASCA has been found in patients with Crohn's disease, while pANCA is more prevalent in ulcerative colitis.5
Ulcerative colitis is a chronic disease, characterized by ulceration of the superficial mucosae and presents with rectal bleeding, diarrhea, and abdominal pain. In contrast to Crohn's disease, ulcerative colitis is restricted to the mucosal layer and continuously affects the colon. Among the extra-intestinal manifestations, primary sclerosing cholangitis and inflammatory arthropathies are the most relevant. Its immune pathogenesis is not fully understood yet, but the believe is that there are some immune-mediated deregulated mechanisms in response to antigens in genetically predisposed individuals. Environmental factors have been said to play an important role, including the intestinal microbiota and the defense mechanisms of the intestinal barrier.6 Autoantibodies that have shown a stronger relationship to ulcerative colitis are ANCA, with pANCA, and atypical ANCA found in 50–70% of patients.6
The association between psoriasis and gastrointestinal disease has been studied, and the inflammatory and genetic pathways that are common for psoriasis and inflammatory bowel disease have been described.7–16 However, the epidemiology of the relationship between psoriasis and inflammatory bowel disease is poorly defined. Several studies have shown an increased prevalence and incidence, with varying degrees of association and sometimes no association whatsoever.11,15
Einarsdottir et al., in 2009, described the association of variants of single nucleotides (SNP) in the IL23R in patients with inflammatory bowel disease with Crohn's disease and ulcerative colitis in patients with psoriasis. This association has been found to be significant.7
Cohen et al. reported that the association of psoriasis and Crohn's disease is stronger than the association with ulcerative colitis.16 Similarly, a cohort study among women in the USA found an increased risk of Crohn's disease among patients with psoriasis (RR: 3.86 [95% CI: 2.23–6.67]), whilst the risk of ulcerative colitis was attenuated and showed no statistical significance (RR: 1.17 [95% CI: 0.41–3.36]).11
Li et al., in 2013, evaluated the association between psoriasis, psoriatic arthritis, ulcerative colitis, and Crohn's disease, in a population of 174,476 women.11 The association was confirmed via medical records that ratified the diagnosis of psoriasis or psoriatic arthritis, and through questionnaires about inflammatory bowel disease, and previous medical records. 188 incidental cases of Crohn's disease and 240 cases of ulcerative colitis were documented. Psoriasis was associated with Crohn's disease. There was no significant risk increase in the association between ulcerative colitis and psoriasis.11
In the above-mentioned trial, a joint analysis of both cohorts showed that women with psoriasis experienced a significant increase in the risk of Crohn's disease, but not in the risk of ulcerative colitis. The risk of Crohn's disease was particularly evident in patients with psoriasis and concomitant psoriatic arthritis (RR: 6.43 [95% CI: 2.04–20.32]). Therefore, there is an increased risk of Crohn's disease incidence associated with psoriasis and concomitant psoriatic arthritis.11
Moreover, the association between psoriasis and celiac disease has shown different results, with mostly a low grade of association.17 Zamani et al., in a population of 328 patients with psoriasis, reported a 0.3% prevalence of celiac disease but did not find a significant association between these 2 conditions.18 In contrast, De Bastiani et al., in a multicenter primary care study showed a high prevalence of celiac disease in patients with psoriasis, with an improvement in skin lesions after 6 months of gluten-free diet.19
Therefore, it should be relevant to ask patients with psoriasis about the presence of gastrointestinal symptoms, including diarrhea, bloody stools, painful bowel movements, urgency, rectal tenesmus, and abdominal pain.3 Whenever there are one or more symptoms, the patient should be referred to gastroenterology. If the patient presents with inflammatory bowel disease and psoriasis, multidisciplinary management is required. Likewise, inflammatory bowel disease may be underdiagnosed in patients with psoriasis because the gastrointestinal symptoms are usually attributed to the side effects of medications.
This first study in Colombia was intended to establish the frequency of symptoms suggestive of gastrointestinal disease and autoantibodies such as ANA, ASCA, pANCA, anti-transglutaminase (tGT), and anti-gliadin peptide (DGP) in a group of patients with psoriasis, by association with their activity and comparing against healthy individuals.
Materials and methodsA cross-sectional study and analysis were conducted at the Hospital Militar Central in Bogotá, Colombia, including 44 outpatients of the psoriasis clinic and 40 unpaired controls with similar housing, lifestyle, and labor characteristics. No sample sized calculations were made; the patients recruited were patients receiving care at the dermatology service of the institution in one year, who voluntarily accepted to participated in the trial. Non-probability convenience sampling was used, excluding around 30%.20
The inclusion criteria were as follows: individuals over 18 years old, with a diagnosis of psoriasis, selected by dermatologists with more than 5 years of experience at the Hospital Militar Central.1 The controls were randomly selected males and females over 18 years old, without psoriasis, who signed the informed consent. The exclusion criteria for both cases and controls were: presence of any psychiatric or neurological condition that interfered with the decision to participate in the trial. Also, individuals with autoinflammatory neoplasms, autoimmune diseases and antibiotic therapy during the past 3 months; patients with a diagnosis of irritable bowel syndrome, inflammatory bowel disease or celiac disease.
During the visit to the doctor, the cases and controls were asked about their symptoms during the past 6 months, including: abdominal pain, diarrhea (considered as more than 3 bowel movements per day and the duration in weeks),21 fever, weight loss, bloody stools, mucus in the stools, nocturnal incontinence, constipation, painful bowel movements, nausea or vomiting, loss of appetite, abdominal distension, number of bowel movements per day, stools consistency, food intolerance and what type, current systemic treatment, whether the gastrointestinal symptoms appeared after initiation of the drug, or if there was any previous gastrointestinal diagnosis.
All cases were administered the Psoriasis Area Severity Index (PASI), and additionally, the psoriasis was classified in terms of severity: mild psoriasis, patient undergoing topical therapy with a PASI score of less than 10; moderate to severe psoriasis, patient with a PASI score over 10 or undergoing phototherapy or traditional or biologic systemic immunomodulation.22
Serum markers were analyzed at the immunology laboratory of the Hospital Militar Central and at the Quimiolab Ltda. Laboratory. Immunoenzyme tests were used to determine the presence of anti-tGT IgG/IgA autoantibodies, anti-DGP IgG/IgA autoantibodies, and ASCA IgG/IgA. All tests were done using ELISA.
The determination of each antibody was done independently to determine each specific isotype and additionally, the total IgA was measured using kinetic nephelometry, since some patients with autoimmune diseases may present with a congenital deficit of this immunoglobulin, which means that the interpretation of ASCA and tGT should be done based on the IgG quantification data. Finally, ANCA and ANA were determined through indirect immunofluorescence (IIF).
Determination of ASCA (AESKULISA®, Ref 3508 and 3507, Aesku Diagnostics, Wendelsheim, Germany)The determination of ASCA IgG/IgA antibodies was made using an ELISA-based technique for the quantitative detection in human serum, using a positive reference value of >18IU/ml. The positive determinations were independently done (in order to establish which specific isotype was positive), in addition to the total quantification of IgA.
Anti-tTG and anti-DGP determination (AESKULISA®, Ref 3510, 3511, 3512, 3515, 3513 and 3514, Aesku Diagnostics, Wendelsheim, Germany)A solid phase immunoassay method was used to quantitatively identify IgG and IgA antibodies in serum against tissue transglutaminase neoepitopes and antibodies against -synthetic deamidated gliadin peptides with positive reference values >24UI/ml.
ANCA and ANA determination through indirect immunofluorescence (AESKULISA®, Ref 54.200, 54.201 and 55.100, Aesku Diagnostics, Wendelsheim, Germany)The neutrophil measurements were done on ethanol-and-formalin fixed slides using the IIF test for the semi-quantitative determination of anti-neutrophil cytoplasmic antibodies in human serum. The positive reference values for ANCA were >1:20 and for ANA >1:80. All the positive tests for ANCA by IIF (AESKULISA®, Ref 3301, Aesku Diagnostics, Wendelsheim, Germany) (elastase, lactoferrin, myeloperoxidase, proteinase 3, cathepsin G, lysozyme and protein that increases the bactericidal permeability) were confirmed with ELISA.
The measurements of the variables obtained based on the questionnaire on gastrointestinal symptoms, the type of severity of the psoriasis, the PASI and the analysis of autoantibodies, enabled the development of a database using the Microsoft Office Excel® v. 8.0 spreadsheet that was then imported into the statistical software SPSS® 17 to process the information based on the distribution of frequencies, percentages, central tendency measurements of dispersion and association, in accordance with the quantitative and qualitative nature of the socio-demographic variables.
Central tendency measures were analyzed for the quantitative variables, in accordance with their distribution; i.e., median and dispersion measures as interquartile range. Relative frequencies and proportions were calculated with the qualitative variables. The proportion of positive cases was inferred, obtaining 95% confidence intervals. Pearson's or Fisher's Chi-square test was used for the statistical association in categorical variables, in accordance with the number of observations collected, with a statistical significance of p<0.05.
The study was approved by the Research and Ethics Committee of Hospital Militar Central (2014-057).
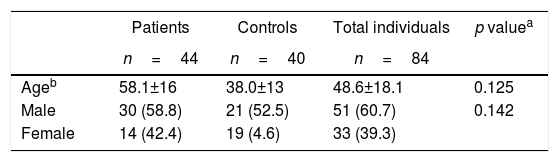
Results64 individuals with psoriasis and 55 controls were considered and 84 individuals were selected (n=84), 44 patients with a diagnosis of psoriasis and 40 controls; in total, there were 60% males and 40% females. The average age of the sample was 48.6±18.1 years. Among the cases, 30 (68%) were males and 14 (32%) were females, with an average age of 58.1±16 years. With regard to recruited controls (n=40), 52% were males and 47% females, with an average age of 38±13 years (Table 1).
Among the cases, the average absolute PASI was 4.4±5.4, with a PASI > 10 in 11% of the patients. Twenty-eight (63%) of the patients were classified as mild grade psoriasis and 16 (36.4%) severe. With regard to treatment, 71% received topical therapy, 16% oral systemic therapy, and 14% biological therapy. Of the total number of patients that received systemic therapy, 13 (30%), 4 (9.1%) received methotrexate, 3 (7.0%) adalimumab, 3 (7.0%) etanercept, one (2.3%) infliximab, and 2 (4.5%) ustekinumab.
In terms of the frequency of abdominal pain, it was higher among patients, but the result showed no statistically significant difference (53 vs. 47%; p: 0.95); weight loss (80 vs. 20%; p: 0.21); bloody stools (56 vs. 44%; p: 0.56), constipation (53 vs. 47%; p: 0.91), loss of appetite (60 vs. 40%; p: 0.51), nausea/vomiting (57 vs. 43%; p: 0.55), abdominal distension (64 vs. 35%; p: 0.08) in patients with psoriasis vs. the controls; none of the above results were statistically significant. Diarrhea was more prevalent among the controls (30%), as was fever (15%); a symptom non-associated with gastrointestinal disorders was fatigue with 71% vs. 29% in patients and controls respectively, p: 0.04.
Moreover, 20% of the patients with psoriasis were receiving oral treatment; 2 of them reported bloody stools and were receiving Methotrexate.
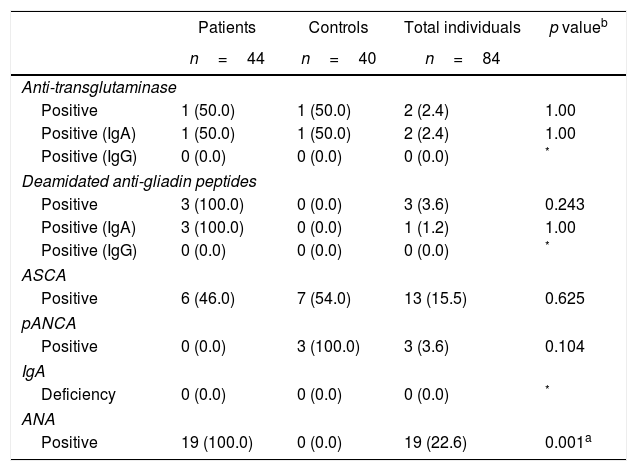
The study on serological antibodies in the total sample (cases and controls) reported that 2 (2%) of the patients were positive for anti-tTG IgA/IgG (both for anti-tTG IgA), 3 (4%) for anti-DGP IgA/IgG (one positive for anti-DGP IgA and none for anti-DGP IgG), 13 (16%) had ASCA positive, 3 (4%) pANCA positive, 19 (23%) ANA positive and none had an IgA deficit.
When comparing the results of the autoantibodies between cases and controls, there were statistically significant differences, with the exception of ANAs which were negative in all controls and positive for 43% of the cases (p: 0.001) (Table 2).
Autoantibodies associated with gastrointestinal disorders in patients with psoriasis and healthy controls.
| Patients | Controls | Total individuals | p valueb | |
|---|---|---|---|---|
| n=44 | n=40 | n=84 | ||
| Anti-transglutaminase | ||||
| Positive | 1 (50.0) | 1 (50.0) | 2 (2.4) | 1.00 |
| Positive (IgA) | 1 (50.0) | 1 (50.0) | 2 (2.4) | 1.00 |
| Positive (IgG) | 0 (0.0) | 0 (0.0) | 0 (0.0) | * |
| Deamidated anti-gliadin peptides | ||||
| Positive | 3 (100.0) | 0 (0.0) | 3 (3.6) | 0.243 |
| Positive (IgA) | 3 (100.0) | 0 (0.0) | 1 (1.2) | 1.00 |
| Positive (IgG) | 0 (0.0) | 0 (0.0) | 0 (0.0) | * |
| ASCA | ||||
| Positive | 6 (46.0) | 7 (54.0) | 13 (15.5) | 0.625 |
| pANCA | ||||
| Positive | 0 (0.0) | 3 (100.0) | 3 (3.6) | 0.104 |
| IgA | ||||
| Deficiency | 0 (0.0) | 0 (0.0) | 0 (0.0) | * |
| ANA | ||||
| Positive | 19 (100.0) | 0 (0.0) | 19 (22.6) | 0.001a |
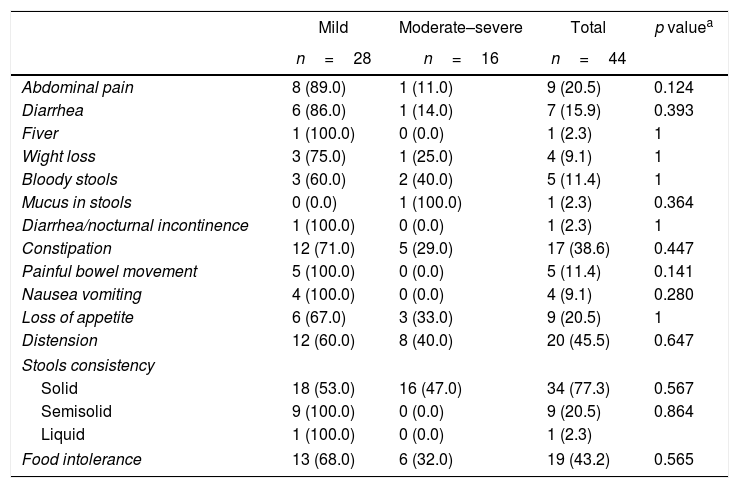
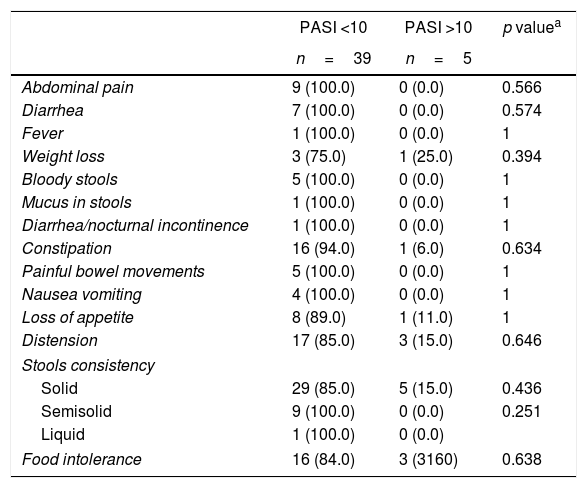
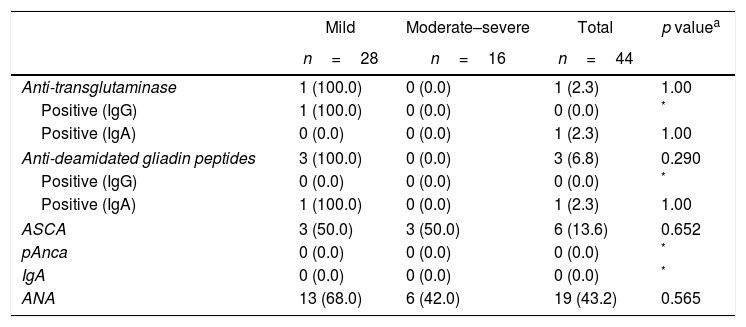
The symptoms of the gastrointestinal disease and antoantibodies were contrasted against severity, PASI and patient gender. In terms of severity, patients with mild psoriasis (PASI <10) presented more gastrointestinal symptoms as compared to patients with moderate-to-severe psoriasis (PASI >10), with no statistically significant findings (Tables 3 and 4).
Association between severity of the psoriasis and gastrointestinal disease-related symptoms.
| Mild | Moderate–severe | Total | p valuea | |
|---|---|---|---|---|
| n=28 | n=16 | n=44 | ||
| Abdominal pain | 8 (89.0) | 1 (11.0) | 9 (20.5) | 0.124 |
| Diarrhea | 6 (86.0) | 1 (14.0) | 7 (15.9) | 0.393 |
| Fiver | 1 (100.0) | 0 (0.0) | 1 (2.3) | 1 |
| Wight loss | 3 (75.0) | 1 (25.0) | 4 (9.1) | 1 |
| Bloody stools | 3 (60.0) | 2 (40.0) | 5 (11.4) | 1 |
| Mucus in stools | 0 (0.0) | 1 (100.0) | 1 (2.3) | 0.364 |
| Diarrhea/nocturnal incontinence | 1 (100.0) | 0 (0.0) | 1 (2.3) | 1 |
| Constipation | 12 (71.0) | 5 (29.0) | 17 (38.6) | 0.447 |
| Painful bowel movement | 5 (100.0) | 0 (0.0) | 5 (11.4) | 0.141 |
| Nausea vomiting | 4 (100.0) | 0 (0.0) | 4 (9.1) | 0.280 |
| Loss of appetite | 6 (67.0) | 3 (33.0) | 9 (20.5) | 1 |
| Distension | 12 (60.0) | 8 (40.0) | 20 (45.5) | 0.647 |
| Stools consistency | ||||
| Solid | 18 (53.0) | 16 (47.0) | 34 (77.3) | 0.567 |
| Semisolid | 9 (100.0) | 0 (0.0) | 9 (20.5) | 0.864 |
| Liquid | 1 (100.0) | 0 (0.0) | 1 (2.3) | |
| Food intolerance | 13 (68.0) | 6 (32.0) | 19 (43.2) | 0.565 |
Association between PASI and gastrointestinal disease-related symptoms.
| PASI <10 | PASI >10 | p valuea | |
|---|---|---|---|
| n=39 | n=5 | ||
| Abdominal pain | 9 (100.0) | 0 (0.0) | 0.566 |
| Diarrhea | 7 (100.0) | 0 (0.0) | 0.574 |
| Fever | 1 (100.0) | 0 (0.0) | 1 |
| Weight loss | 3 (75.0) | 1 (25.0) | 0.394 |
| Bloody stools | 5 (100.0) | 0 (0.0) | 1 |
| Mucus in stools | 1 (100.0) | 0 (0.0) | 1 |
| Diarrhea/nocturnal incontinence | 1 (100.0) | 0 (0.0) | 1 |
| Constipation | 16 (94.0) | 1 (6.0) | 0.634 |
| Painful bowel movements | 5 (100.0) | 0 (0.0) | 1 |
| Nausea vomiting | 4 (100.0) | 0 (0.0) | 1 |
| Loss of appetite | 8 (89.0) | 1 (11.0) | 1 |
| Distension | 17 (85.0) | 3 (15.0) | 0.646 |
| Stools consistency | |||
| Solid | 29 (85.0) | 5 (15.0) | 0.436 |
| Semisolid | 9 (100.0) | 0 (0.0) | 0.251 |
| Liquid | 1 (100.0) | 0 (0.0) | |
| Food intolerance | 16 (84.0) | 3 (3160) | 0.638 |
Additionally, 68% of the patients with ANA positive, 50% of the patients with ANCA positive, and all of the patients with anti-tTG and anti-DGP positive had mild grade psoriasis (Table 5). Similarly, 100% of the patients with anti-tTG, anti-DGP positive, 83% with ASCA positive, and 84% with ANA positive had PASI <10. These differences were not statistically significant.
Association between severity of the psoriasis and gastrointestinal disease-related autoantibodies.
| Mild | Moderate–severe | Total | p valuea | |
|---|---|---|---|---|
| n=28 | n=16 | n=44 | ||
| Anti-transglutaminase | 1 (100.0) | 0 (0.0) | 1 (2.3) | 1.00 |
| Positive (IgG) | 1 (100.0) | 0 (0.0) | 0 (0.0) | * |
| Positive (IgA) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 1.00 |
| Anti-deamidated gliadin peptides | 3 (100.0) | 0 (0.0) | 3 (6.8) | 0.290 |
| Positive (IgG) | 0 (0.0) | 0 (0.0) | 0 (0.0) | * |
| Positive (IgA) | 1 (100.0) | 0 (0.0) | 1 (2.3) | 1.00 |
| ASCA | 3 (50.0) | 3 (50.0) | 6 (13.6) | 0.652 |
| pAnca | 0 (0.0) | 0 (0.0) | 0 (0.0) | * |
| IgA | 0 (0.0) | 0 (0.0) | 0 (0.0) | * |
| ANA | 13 (68.0) | 6 (42.0) | 19 (43.2) | 0.565 |
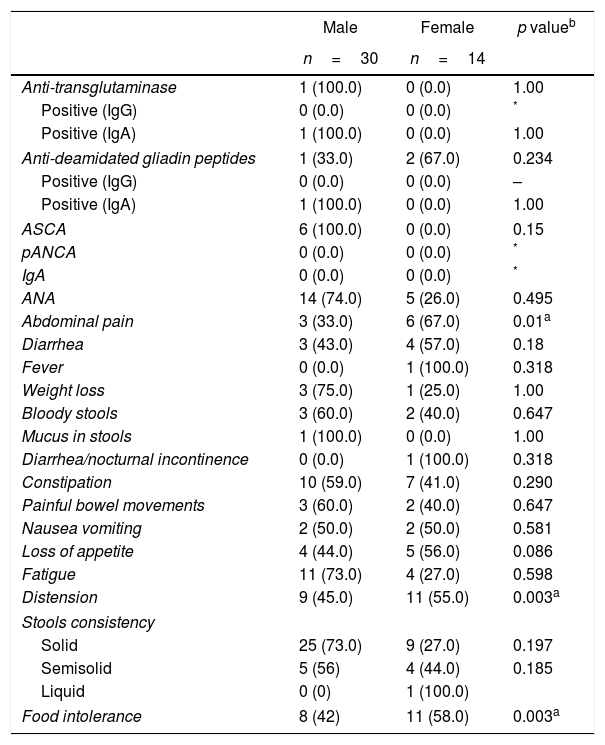
When reviewing the variables by gender among the cases, it was striking to find that females reported more fever (100%), nocturnal fecal incontinence (100%), loss of appetite (56%), distension (55%), abdominal pain (67%) and food intolerance (58%) as compared to males; the last 3 are the most statistically significant symptoms (p: 0.003, p: 0.001, p: 0.003, respectively). The comparison of the autoantibodies profile by gender did not show any statistically significant differences (Table 6).
Association between autoantibodies and gastrointestinal disease-related symptoms in accordance to gender.
| Male | Female | p valueb | |
|---|---|---|---|
| n=30 | n=14 | ||
| Anti-transglutaminase | 1 (100.0) | 0 (0.0) | 1.00 |
| Positive (IgG) | 0 (0.0) | 0 (0.0) | * |
| Positive (IgA) | 1 (100.0) | 0 (0.0) | 1.00 |
| Anti-deamidated gliadin peptides | 1 (33.0) | 2 (67.0) | 0.234 |
| Positive (IgG) | 0 (0.0) | 0 (0.0) | – |
| Positive (IgA) | 1 (100.0) | 0 (0.0) | 1.00 |
| ASCA | 6 (100.0) | 0 (0.0) | 0.15 |
| pANCA | 0 (0.0) | 0 (0.0) | * |
| IgA | 0 (0.0) | 0 (0.0) | * |
| ANA | 14 (74.0) | 5 (26.0) | 0.495 |
| Abdominal pain | 3 (33.0) | 6 (67.0) | 0.01a |
| Diarrhea | 3 (43.0) | 4 (57.0) | 0.18 |
| Fever | 0 (0.0) | 1 (100.0) | 0.318 |
| Weight loss | 3 (75.0) | 1 (25.0) | 1.00 |
| Bloody stools | 3 (60.0) | 2 (40.0) | 0.647 |
| Mucus in stools | 1 (100.0) | 0 (0.0) | 1.00 |
| Diarrhea/nocturnal incontinence | 0 (0.0) | 1 (100.0) | 0.318 |
| Constipation | 10 (59.0) | 7 (41.0) | 0.290 |
| Painful bowel movements | 3 (60.0) | 2 (40.0) | 0.647 |
| Nausea vomiting | 2 (50.0) | 2 (50.0) | 0.581 |
| Loss of appetite | 4 (44.0) | 5 (56.0) | 0.086 |
| Fatigue | 11 (73.0) | 4 (27.0) | 0.598 |
| Distension | 9 (45.0) | 11 (55.0) | 0.003a |
| Stools consistency | |||
| Solid | 25 (73.0) | 9 (27.0) | 0.197 |
| Semisolid | 5 (56) | 4 (44.0) | 0.185 |
| Liquid | 0 (0) | 1 (100.0) | |
| Food intolerance | 8 (42) | 11 (58.0) | 0.003a |
Augustin et al. reported in Germany in 2010, the results of 2 cross-sectional studies; the first was conducted in adults, and the second one in pediatric population; an association between Crohn's disease and psoriasis was identified, with a prevalence of 0.92% PR=2.06 (95% CI: 1.84–2.31) and of 0.51% PR=3.69 (95% CI: 2.15–6.35), respectively.23 Makredes et al., in 2009, found an increased risk of Crohn's disease associated with psoriasis and psoriatic arthritis and the risk is compounded when the latter two conditions are concomitant, with a RR of 1.6 (95% CI: 1.4–2.0) for psoriasis and a RR of 2.1 (95% CI: 1.3–3.3) for psoriatic arthritis.18
Hence the interest to determine the frequency of symptoms suggestive of gastrointestinal disease and associated autoantibodies, in a group of Colombian patients with psoriasis, and to associated it with the clinical activity, in addition to establishing comparisons against healthy controls.
A higher frequency of gastrointestinal symptoms was identified in patients with psoriasis: abdominal pain, weight loss, bloody stools, constipation, loss of appetite, nausea/vomiting, with no statistically significant differences and a significant tendency toward abdominal distension; all of these factors are consistent with several publications previously discussed, associating psoriasis to the presence of gastrointestinal disease symptoms.7–9 It should be mentioned however, that the general population presented symptoms associated with gastrointestinal disorders, which has been reported in several trials. The group of symptoms identified include: constipation, flatulence, abdominal pressure, abdominal bloating, acid reflux, diarrhea, intestinal heaviness, intestinal pain, burning pain and gastric pain, inter alia. Notwithstanding the fact that these problems are minor, they do have a considerable impact on vitality and self-image, as well as on emotional, social and physical wellbeing. The interviewees thought that lifestyle, food, and lack of discipline, are the main culprits.24,25
Interestingly enough, patients with mild psoriasis (PASI <10) showed a tendency to present increased gastrointestinal symptoms, as compared with patients with moderate–severe psoriasis (PASI >10). However, no associations were found between the autoantibodies profile and the severity of the disease. Probably the underlying reason is the sample size analyzed.
As expected, some patients with gastrointestinal symptoms had positive autoantibodies. The ASCA are more frequent in Crohn's disease, while the pANCA are more common in ulcerative colitis, with a sensitivity of 45–60% and a specificity of 85–95%.5 However, these autoantibodies are not part of the diagnostic algorithm for Crohn's disease.5 Among our cases, 8 had positive ASCA in the absence of pANCA, with this being the most prevalent autoantibody among the cases; nevertheless, none presented symptoms associated with Crohn's disease, but this serological study may be useful to promote the search for inflammatory disease in general, with a view to making a clinical diagnosis in this group of patients.6 There is actually a lot of controversy regarding the prevalence of the previous studies vs. gastrointestinal symptoms; some describe associations between psoriasis and inflammatory bowel disease, reporting up to 4-fold the level in the general population,26,27 2.5 times more for Crohn's disease and 1.6 for ulcerative colitis,28,29 but there is very little reported on the levels of associated autoantibodies in this type of patients.
The ANA were negative for all the controls and positive in 43% of the cases with psoriasis. Among the patients with plaque psoriasis, the ANA were present in 25% of the subjects, and in 80% of the patients with psoriatic arthritis. After treatment, there was an increase in the title or occurrence of antibodies in 66.7% in the infliximab group; 18.2% in the etanercept group, and 54.7% in the adalimumab group. In the group of patients studied, 7 received anti-TNF therapy, 4 of them with positive titles in the ANA group.30
Moreover, we found 2 patients with mild psoriasis with serology suggestive of celiac disease, because of positive anti-tTG IgA and anti-DPG antibodies, but no specific symptoms of the disease were still present. This is a rare occurrence and is consistent with the controversial findings of the association between psoriasis and celiac disease described in the literature.18,19. De Bastiani et al., in 2015, found that 9/218 (4.1%) patients with psoriasis had tTG IgA antibodies, in contrast with just one control individual (0.4%, p<0.05; OR: 2.03; 95% CI: 1.42–90.11). The celiac disease diagnosis was histologically confirmed in the 10 individuals at 6 months, associated with considerable improvement of the skin lesions in 7 of 8 patients with psoriasis.19 The patients that presented with symptoms and serology suggestive of gastrointestinal disease were referred to gastroenterology for complementary evaluation.
Since 20% of the patients with psoriasis were receiving oral treatment with methotrexate, and some reported bloody stools, it must be kept in mind that systemic medications frequently used in the management of psoriasis (for example, infliximab, adalimumab and methotrexate), ustekinumab and cyclosporine, may result in gastrointestinal symptoms secondary to the use of these medications.13
So there is a need to be aware of the gastrointestinal side effects when initiating systemic therapy in patients with psoriasis that previously presented gastrointestinal symptoms. Some of the side effects of the systemic medications used in the management of psoriasis include: infliximab: abdominal pain, diarrhea, nausea and dyspepsia; adalimumab: abdominal pain, nausea and vomiting; ustekinumab: diarrhea; methotrexate: loss of appetite, nausea, vomiting, abdominal pain, ulcers of the oral mucosa, stomatitis and dyspepsia; cyclosporine: nausea, vomiting, abdominal pain and diarrhea.13
The frequency of fatigue should be highlighted in this group of patients. Just as in other inflammatory diseases, fatigue represents a relevant clinical finding in psoriasis. Multiple factors are involved in this process, among them potential complex interactions of the inflammation in psoriatic disease, both directly via inflammatory cytokines, and indirectly via psychological and physiological factors.31 Otherwise, unspecific gastrointestinal symptoms if not found within a clinical and serological context of intestinal or celiac inflammatory disease, could be found in diseases that have a higher prevalence in the population, such as irritable colon (however, this was an exclusion criterion), or in diseases that may coexist more often with chronic inflammatory diseases such as fibromyalgia, that may be separated into subgroups that warrant additional studies.32,33
In conclusion, it is important to stress the relevance of the dermatologist when adopting a comprehensive approach to patients with psoriasis; a simple comprehensive interview may result in an adequate study and proper management of the existing comorbidities. In this case, in terms of gastrointestinal disease, this is the first study – to the extent of our knowledge – conducted in Colombia, on the frequency of symptoms and autoantibodies suggestive of gastrointestinal disease, in a cohort of patients with psoriasis. Further studies, with a larger number of patients should be conducted in Colombia, in order to keep a record of this very interesting association and to be able to continue searching for the adequate management and improved quality of life of the patients with psoriasis.
FundingThis paper was sponsored by Aesku Diagnostics, Quimiolab Ltda and the Hospital Militar Central.
Conflict of interestThe authors declare not having any conflict of interest to disclose.
To the Immunology Laboratory of the Hospital Militar Central and the Universidad El Bosque, particularly to Lorena Chila for her technical support in our work with autoantibodies and immunoglobulins. To Mr. Alejandro Ramos for his collaboration in drafting the manuscript.
Please cite this article as: Restrepo Samper S, Camilo Prieto A, Castro LA, Romero-Sánchez C. Frecuencia de autoanticuerpos y síntomas sugestivos de enfermedad gastrointestinal en un grupo de pacientes con psoriasis. Rev Colomb Reumatol. 2019;26:31–39.