To determine whether seropositivity in rheumatoid arthritis patients treated with adalimumab (ADL) is associated with the presence of anti-adalimumab (anti-ADL) antibodies.
Materials and methodsA descriptive observational study that included patients diagnosed with rheumatoid arthritis according to ACR 1987 criteria, and who were on treatment with ADL as the first biological, for at least six months. All patients were evaluated for rheumatoid factor, anti-citrulline antibodies, erythrocyte sedimentation rate, C-reactive protein, clinimetric indices, and level of anti-ADL antibodies.
ResultsA total of 80 patients with a mean age of 56 years were evaluated, of whom 86% were women. The mean duration of the disease was 15 years, and the ADL exposure time was 52 months (median value). The seropositivity for rheumatoid factor tended to be higher in patients who developed anti-ADL antibodies compared to those who did not (90.5% vs. 66.1%). The magnitude of the association between rheumatoid factor and the presence of anti-ADL antibodies was shown to be strong and statistically significant (OR=4.87, 95% CI; 1.03–23.03). Adjusted multivariate regression analyses showed a strong association (OR=9.77, 95% CI; 1.74–54.79) between seropositivity and the presence of anti-ADL antibodies, which, given the low number of patients, lacks precision (95% CI very wide).
ConclusionsSeropositive patients tend to have more anti-ADL antibodies. However, a larger sample size is required to obtain the necessary precision and greater certainty in these findings.
Determinar si la seropositividad en pacientes con artritis reumatoide tratados con adalimumab (ADL), se asocia a la presencia de anticuerpos anti-adalimumab (anti-ADL).
Materiales y métodosEs un estudio observacional descriptivo que incluyó pacientes con diagnóstico de artritis reumatoide según criterios ACR 1987, que estaban en tratamiento con ADL como primer biológico, por al menos 6 meses. Todos los pacientes se evaluaron para factor reumatoide, anticuerpos anticitrulina, velocidad de sedimentación globular, proteína C reactiva, índices clinimétricos y nivel de anticuerpos anti-ADL.
ResultadosSe evaluaron 80 pacientes con edad promedio de 56 años, el 86% fueron mujeres, la duración promedio de la enfermedad fue de 15 años y el tiempo de exposición a ADL de 52 meses (valor mediano). La seropositividad para factor reumatoide tendió a ser mayor en los pacientes que desarrollaron anticuerpos anti-ADL en comparación con los que no (90,5% vs. 66,1%). La magnitud de la asociación entre factor reumatoide y la presencia de anticuerpos anti-ADL tendió a ser fuerte y estadísticamente significativa (OR=4,87; IC 95%: 1,03-23,03). Los análisis ajustados de regresión multivariable mostraron una asociación fuerte (OR=9,77; IC 95%: 1,74-54,79) entre la seropositividad y la presencia de anticuerpos anti-ADL, que dado el bajo número de pacientes carece de precisión (IC 95% muy amplios).
ConclusionesLos pacientes seropositivos tienden a presentar más anticuerpos anti-ADL; sin embargo, se requiere tener un mayor tamaño muestral para obtener la precisión necesaria y tener mayor certeza en estos hallazgos.
Rheumatoid arthritis (RA) is a systemic, chronic and autoimmune disease of unknown etiology. A clearly identified cytokine, responsible for joint damage and destruction, is the tumor necrosis factor alpha (TNF-α).1–4 There are 5 anti-TNF-α agents currently available (etanercept, infliximab, adalimumab [ADL], certolizumab and golimumab).3,5 The use of anti-TNF-α agents has helped many patients with RA; however, some patients fail to respond.5,6 The exact mechanism for the inadequate response to anti-TNF-α has not been fully explored, and any biologic, regardless of its origin – human, chimeric or humanized – may trigger an immune response generating antibodies against the biologic itself.6,7 The development of anti-drug antibodies may be one of the reasons for the loss of response, the development of adverse reactions, and the severity of those adverse reactions.5,8–10 The prevalence of anti-drug antibodies varies considerably among the various anti-TNF-α studies; this observation may be explained based on the differences among the various groups of patients, the use of concomitant immunosuppressants, the moment the sample was drawn, time of drug exposure, and the technique used for measuring.5,7,8,11 The data on ADL immunogenicity suggest that anti-ADL antibodies develop after at least 6 months of exposure, and are associated with sub-therapeutic drug levels, and with a loss of clinical response. Bartelds et al., in 2011, and Balsa in 2018, reported that their prevalence varied between 20% and 25% of patients.11–13
The definition of seropositivity in RA according to the 1987 criteria of the American College of Rheumatology (ACR 1987) is based on the presence of rheumatoid factor (RF),14 while according to the ACR/EULAR 2010 criteria, it is based on the presence of RF or anti-citrulline antibodies (ACCP).15 Being seropositive is associated with more aggressive joint disease, extra-articular manifestations and higher mortality.16,17
The influence of seropositivity in responding to anti-TNF-α is uncertain; however, data from the British registry suggest that the presence of RF or ACCP is associated with a mild DAS28 reduction after 6 months of therapy; likewise, data from the Italian registry suggest that the presence of RF is associated with a lower probability of achieving remission of the disease.3
Approximately 70% of the patients with RA are seropositive.10 There is currently evidence suggesting that seropositive RA is genetically and pathogenically different to seronegative RA, and hence numerous genes have been studied that could enhance the classification. The different loci identified may divide the disease into 4 groups: loci that are often found in seropositive patients, loci frequently found in seronegative individuals, loci often found in both groups, and loci perceived as disease specific.18 Notwithstanding the fact that they seem to be the same entity, being seropositive suggests a more aggressive disease and is associated to the presence of autoantibodies, which would indicate that B lymphocytes are producing antibodies due to an altered regulation. In some patients, the exposure to therapeutic proteins (ADL), could further stimulate the immune system to produce anti-drug antibodies (anti-ADL antibodies) in the presence of non-regulated B lymphocytes, and hence compromise efficacy. The central objective of the study was to assess whether being seropositive in RA is a factor associated with the presence of anti-ADL antibodies in patients receiving ADL as their first biologic. This finding has not been described in previous publications.
Materials and methodsThe patients included had a diagnosis of RA according to the ACR 1987 criteria. The expectation was to identify the serostatus prior to starting ADL therapy; however, were patients some with established RA in whom the ASSP information could not be obtained before the start of ADL. Patients were aged 18 years and over and had been on ADL treatment as their first biologic for at least 6 months; they were part of the Risk of Fracture clinic, CAYRE® IPS and accepted to participate. The collection of samples began on March 16, 2015 and the last sample was drawn on January 20, 2017. The exclusion criteria were as follows: prior use of a different biologic (another anti-TNF-α, abatacept, tocilizumab, rituximab) or having another associated autoimmune rheumatic disease (information obtained from the medical record).
The clinical evaluation was programmed for the day when the drug was to be administered – before the administration (trough) and patients who submitted their consent to participate in the study completed a form that included the demographic and clinical characteristics, and the clinimetric indexes were calculated. In the case of patients where access to the complete medical record was possible, the RF and ACCP information was collected – if available – before starting ADL. After the medical evaluation was completed, a sample of venous blood was drawn (15 cc), which was divided into 2 test tubes: one was processed immediately to determine the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), RF, and ACCP; the second test tube was centrifuged at 3500×g for 10minutes, and stored at −20°C for the determination of anti-ADL antibodies. The anti-ADL antibodies were identified through ELISA (immunoassay) and were reported in units of absorbance per milliliter (UA/ml). It was considered to be positive if the value was >10UA/ml, according to the kit's specifications (Promonitor®. Ref. 5090130000).
Statistical analysisA descriptive analysis of the demographic and clinical variables was initially conducted, using measures of central tendency that included: average, median, percentage, and scatter measures such as standard deviation and interquartile range (IQR). In order to assess the differences among the groups in the continuous variables, the Student “t” test and the Mann–Whitney test were used; and to assess the differences in proportions, Chi-square and Fisher's tests were used for those cases in which the expected frequency was <5 in over 20% of the cells.
Seropositivity (RF) was determined in the participants pursuant to the ACR 1987 criteria. The magnitude of the association between RF and anti-ADL antibodies was determined calculating the indirect relative risk known as raw odds ratio (OR). In order to establish the influence of other variables over the direction and magnitude of the association, bivariate analyses were conducted. Based on these analyses, the variables showing a relationship with the presence of anti-ADL antibodies were identified, and included in the multivariate model. To assess the association between RF and anti-ADL antibodies, controlling for the intervening variables, a logistic multivariate regression model was built. This analysis allowed for the evaluation of the presence of interactions and confusion with the control variables. In order to select the best model, the backward technique was used. Finally, a diagnosis of the model was conducted, using information criteria such as the Akaike Information Criterion and the Bayesian Information Criterion.
The study was adjusted to the national and international human research standards, in accordance with the principles established under the Declaration of Helsinki and under Resolution 8430 of October 4, 1993; the study was considered to be of minimal risk. The research was developed in accordance with the protocol and the good clinical practice guidelines (Resolution 2378 of June 27, 2008). In order to ensure the safety and the integrity of the participants, the study was submitted for consideration of the Ethics Committee of the Risk of Fracture Institution, CAYRE® IPS for evaluation and approval, which was obtained prior to the beginning of the trial.
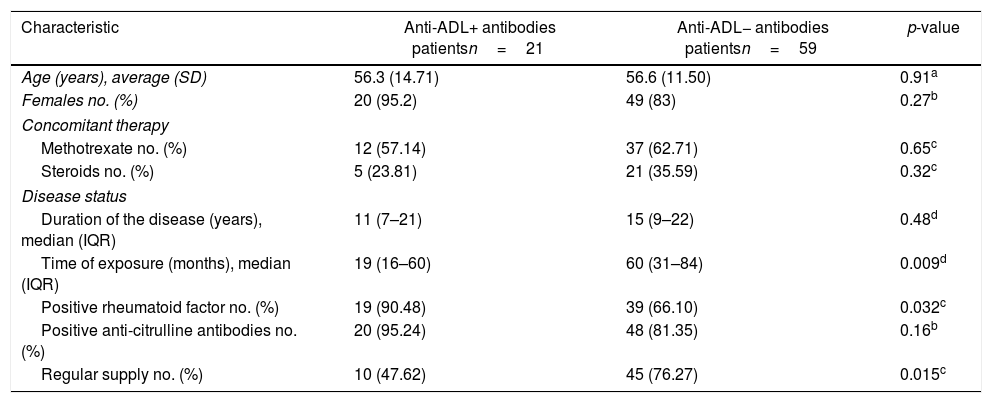
Results80 patients were included, of which 21 were positive for anti-ADL antibodies (26.25%) and 59 were negative (73.75%). The characteristics of each group are shown in Table 1.
Demographic and clinical characteristics of the patients.
| Characteristic | Anti-ADL+ antibodies patientsn=21 | Anti-ADL− antibodies patientsn=59 | p-value |
|---|---|---|---|
| Age (years), average (SD) | 56.3 (14.71) | 56.6 (11.50) | 0.91a |
| Females no. (%) | 20 (95.2) | 49 (83) | 0.27b |
| Concomitant therapy | |||
| Methotrexate no. (%) | 12 (57.14) | 37 (62.71) | 0.65c |
| Steroids no. (%) | 5 (23.81) | 21 (35.59) | 0.32c |
| Disease status | |||
| Duration of the disease (years), median (IQR) | 11 (7–21) | 15 (9–22) | 0.48d |
| Time of exposure (months), median (IQR) | 19 (16–60) | 60 (31–84) | 0.009d |
| Positive rheumatoid factor no. (%) | 19 (90.48) | 39 (66.10) | 0.032c |
| Positive anti-citrulline antibodies no. (%) | 20 (95.24) | 48 (81.35) | 0.16b |
| Regular supply no. (%) | 10 (47.62) | 45 (76.27) | 0.015c |
SD: standard deviation; IQR: interquartile range.
The concomitant use of immunosuppressant medications such as methotrexate was lower in the group with anti-ADL+ antibodies (57.1% vs. 62.7%) than in the group with anti-ADL−, but the difference was not statistically significant (p=0.65). The use of steroids was higher in the group with anti-ADL− antibodies (35.6% vs. 23.8%) than in the group with anti-ADL+ antibodies, but the difference was not significant (p=0.32).
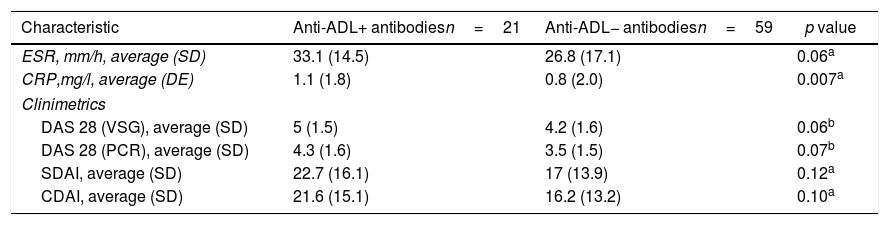
The compounded disease activity indexes (DAS 28 VSG, DAS 28 CRP, SDAI and CDAI) and acute phase reactants (VSG and CRP) tended to be greater in the group with anti-ADL+ antibodies, but only the CRP value showed a statically significant difference (p=0.007), as shown in Table 2.
Acute phase reactants and disease activity index.
| Characteristic | Anti-ADL+ antibodiesn=21 | Anti-ADL− antibodiesn=59 | p value |
|---|---|---|---|
| ESR, mm/h, average (SD) | 33.1 (14.5) | 26.8 (17.1) | 0.06a |
| CRP,mg/l, average (DE) | 1.1 (1.8) | 0.8 (2.0) | 0.007a |
| Clinimetrics | |||
| DAS 28 (VSG), average (SD) | 5 (1.5) | 4.2 (1.6) | 0.06b |
| DAS 28 (PCR), average (SD) | 4.3 (1.6) | 3.5 (1.5) | 0.07b |
| SDAI, average (SD) | 22.7 (16.1) | 17 (13.9) | 0.12a |
| CDAI, average (SD) | 21.6 (15.1) | 16.2 (13.2) | 0.10a |
CDAI: Clinical Disease Activity Index; DAS 28: Disease Activity Score; SD: standard deviation; CRP: C-reactive protein; SDAI: Simplified Disease Activity Index; ESR: erythrocyte sedimentation rate.
Fifty-eight patients (72.5%) were seropositive for RF and for ACCP (double positive), while 10 patients (12.5%) were seropositive for ACCP only and 12 patients were negative for both tests (15% double negative). Of the 58 double positive patients, 19 presented with anti-ADL antibodies (32.7%), and of the 12 double negative patients, one presented with anti-ADL antibodies (8.3%). Of those that were only positive for ACCP, one presented with anti-ADL antibodies (10%).
The average number of suspended ADL doses in those patients that did not have a regular treatment supply was 7 doses (3 and half months) over the last year, with no differences in the number of doses according to the group; however, there was a statistically significant difference with regards to the presence of anti-ADL antibodies, with a regular supply in 47% of the patients with anti-ADL+ antibodies, and in 76% of the anti-ADL− antibodies (p=0.015).
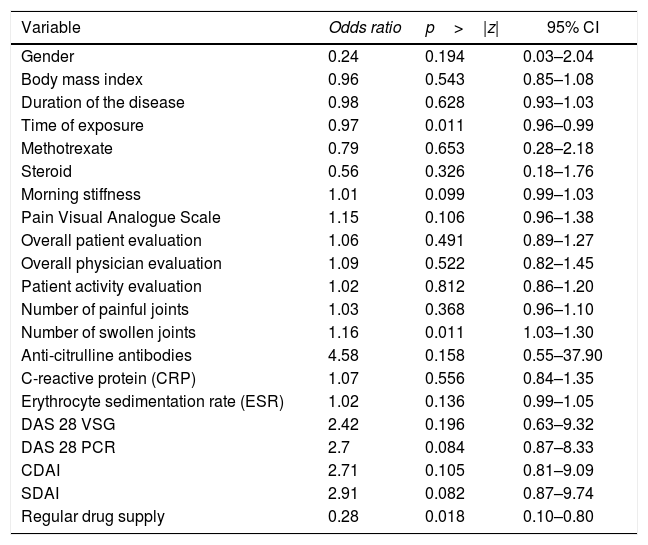
In order to assess the magnitude of the association between RF and anti-ADL antibodies, the raw OR and its 95% confidence interval (CI) was estimated, as shown in Table 3.
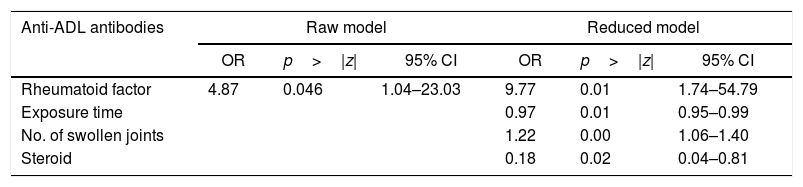
When evaluating whether the association between RF and anti-ADL antibodies is influenced by other variables, a bivariate analysis was conducted, identifying relevant variables that were taken into account to develop the multivariate model (Table 4). Finally, a logistic regression model was developed, to be able to assess the association between RF and the presence of anti-ADL antibodies; A model called “complete”, was designed, in which all variables considered to be relevant were included. This model was evaluated for the presence of interactions (time of exposure, MTX and steroids) using the lrtest test, which turned out to be non-significant for any of the interactions (lrtest=0.1895); therefore, these interactions were excluded from the model. Afterwards, an evaluation for confusion was conducted and the decision was made to keep the following variables in the model: time of exposure, number of swollen joints, and use of steroids. These were the most influential variables on the OR change. Finally, the model that was most suited to the data was selected and it was called “reduced model”; based on this model, a diagnosis was made calculating the information criteria (Akaike Information Criterion and Bayesian Information Criterion) (Table 5).
Bivariate analysis between the presence of anti-ADL antibodies and other variables.
| Variable | Odds ratio | p>|z| | 95% CI |
|---|---|---|---|
| Gender | 0.24 | 0.194 | 0.03–2.04 |
| Body mass index | 0.96 | 0.543 | 0.85–1.08 |
| Duration of the disease | 0.98 | 0.628 | 0.93–1.03 |
| Time of exposure | 0.97 | 0.011 | 0.96–0.99 |
| Methotrexate | 0.79 | 0.653 | 0.28–2.18 |
| Steroid | 0.56 | 0.326 | 0.18–1.76 |
| Morning stiffness | 1.01 | 0.099 | 0.99–1.03 |
| Pain Visual Analogue Scale | 1.15 | 0.106 | 0.96–1.38 |
| Overall patient evaluation | 1.06 | 0.491 | 0.89–1.27 |
| Overall physician evaluation | 1.09 | 0.522 | 0.82–1.45 |
| Patient activity evaluation | 1.02 | 0.812 | 0.86–1.20 |
| Number of painful joints | 1.03 | 0.368 | 0.96–1.10 |
| Number of swollen joints | 1.16 | 0.011 | 1.03–1.30 |
| Anti-citrulline antibodies | 4.58 | 0.158 | 0.55–37.90 |
| C-reactive protein (CRP) | 1.07 | 0.556 | 0.84–1.35 |
| Erythrocyte sedimentation rate (ESR) | 1.02 | 0.136 | 0.99–1.05 |
| DAS 28 VSG | 2.42 | 0.196 | 0.63–9.32 |
| DAS 28 PCR | 2.7 | 0.084 | 0.87–8.33 |
| CDAI | 2.71 | 0.105 | 0.81–9.09 |
| SDAI | 2.91 | 0.082 | 0.87–9.74 |
| Regular drug supply | 0.28 | 0.018 | 0.10–0.80 |
CDAI: Clinical Disease Activity Index; SDAI: Simplified Disease Activity Index.
Raw and reduced logistic regression models to define the association between RF seropositivity and anti-ADL antibodies.
| Anti-ADL antibodies | Raw model | Reduced model | ||||
|---|---|---|---|---|---|---|
| OR | p>|z| | 95% CI | OR | p>|z| | 95% CI | |
| Rheumatoid factor | 4.87 | 0.046 | 1.04–23.03 | 9.77 | 0.01 | 1.74–54.79 |
| Exposure time | 0.97 | 0.01 | 0.95–0.99 | |||
| No. of swollen joints | 1.22 | 0.00 | 1.06–1.40 | |||
| Steroid | 0.18 | 0.02 | 0.04–0.81 | |||
| LR Chi-square | 5.34 | 27.05 |
| Prob>Chi-square | 0.0209 | 0.0000 |
| Log likelihood | −43.384 | −32.5294 |
| McFadden's R2 | 0.058 | 0.2936 |
| Count R2 | 0.738 | 0.800 |
| AIC | 90.7689 | 75.0588 |
| BIC | 95.5329 | 86.969 |
In 66 patients the serostatus (RF) information was obtained before starting the ADL and in 14 of them (21.2%) the RF was negative, while in 52 patients (78.8%) the RF was positive. This data, compared against the RF determination on the day of the visit enabled to show that 9 patients remained RF negative (persistently negative), while 44 patients of the seropositive group remained positive (persistently positive) and 13 patients had changed their serostatus: 8 patients that were initially positive for RF became seronegative (RF−) and 5 patients who were initially negative for RF became seropositive (RF+).
Among the persistently negative group, 2 patients (22%) developed anti-ADL+ antibodies, while among the persistently positive, 13 patients (29.5%) developed anti-ADL+ antibodies; and in 2 patients that changed their serostatus, anti-ADL+ antibodies were found (15.3%) only in the group that became seropositive.
DiscussionThis is the first study evaluating whether being RA seropositive according to the ACR 1987 criteria (RF+ presence) is a factor associated with the development of anti-ADL antibodies in patients receiving ADL as their first biologic. Notwithstanding the fact that the study comprised 80 patients, it contributes with important information for improving our knowledge about immunogenicity to biological products, specifically to ADL in patients in our country.
Keiserman et al. summarized the evidence of the randomized clinical trials studying tumor necrosis factor inhibitors and the incidence on anti-drug antibodies, their relationship with the drug's serum levels and the clinical response in patients with RA. A long term study including 148 patients that were exposed to ADL for 156 weeks found positivity for anti-ADL antibodies in 28% of the patients. Additional studies that included 1559 patients with ADL exposure between 12 and 56 weeks, reported anti-ADL+ antibodies, in a range between 1% and 87% of patients.16,19 The data from this study evidenced anti-ADL+ antibodies in 21 patients (26.25%), which is consistent with the reports.
The proportion of seropositive patients based on RF (90.5%), and on ACCP (95.2%), tended to be higher in patients with anti-ADL positive vs. negative antibodies (66.1% vs. 81.3%) respectively; the difference was statistically significant for RF (p=0.032).
The concomitant use of immunosuppressants (MTX), was lower in the group with anti-ADL+ antibodies, and hence this study further evidences that the co-administration of immunosuppressants may be associated with a reduction in the development of anti-ADL antibodies.4,20
Acute phase reactants and clinimetric indexes tended to be higher in patients with anti-ADL+ antibodies, and this trend is similar to the one reported by Bartelds et al. in 2007, in 2009 and in 2011.11,20,21
An interesting aspect was the finding that the time of ADL exposure in the patients presenting with anti-ADL+ antibodies was shorter than in the patients with anti-ADL- antibodies –(median 19 vs. 60 months) respectively (p=0.009). This is consistent with the reports in the literature by Bartelds et al., who described that seropositivity of anti-ADL antibodies is higher during the first 84 weeks of treatment (21 months), with a tendency to flatten the curve after this time.11 It is important to stress in our study that the inclusion criterion was having received at least 6 months of ADL treatment, which is the minimum exposure time to present anti-ADL antibodies; this reduces the bias of selecting the population with less probabilities of having anti-ADL antibodies.
Evidence shows that the regular supply of the medication induces a state of immunological tolerance, while the irregular use of the medication generates persistent activation of the immune system, every time the patient is exposed to the medication again; this favors the development of anti-ADL antibodies.22,23 This is consistent with the findings in our study, since there was a regular supply of medication in 47% of the patients that were anti-ADL+ antibodies, vs. 76% of patients who were anti-ADL− antibodies; this was a statistically significant difference (p=0.015).
It should be emphasized that the patients who were double positive (RF+ and ACCP+) presented with a higher proportion of anti-ADL+ antibodies (32.7%) in contrast to the double negative patients (RF− and ACCP−), representing 8.33%, showing the trend of presenting anti-ADL+ antibodies in seropositive patients: this finding is consistent in the different analyses conducted so far.
Due to the interference phenomenon that may arise because of the presence of ADL in serum, the blood sample to quantify the anti-ADL antibodies was drawn the day in which the medication was administered to all patients; hence, it is believed that the conditions to do the determination of anti-ADL antibodies were optimal; however, there is a limitation: since ELISA is the technique used for identifying the presence of Anti-ADL antibodies, we are only detecting the free anti-ADL antibodies, and not the ADL-linked antibodies (immune complexes), and therefore the actual presence of anti-ADL antibodies may be undervalued in these results.8,24
One of the limitations of this study was the need to obtain data from historical records, which introduced information biases for the analyses and limited the impact of the results.
Notwithstanding the fact that the values identified suggest a strong association between seropositivity in RA and the presence of anti-ADL antibodies, these results should be carefully interpreted considering the number of patients evaluated, since it is likely that when studying a larger number of patients, the estimated seroposivity effect on the development of anti-ADL antibodies changes direction and magnitude.
FundingColombian Association of Rheumatology, Risk of Fracture, CAYRE, IPS.
Conflict of interestNone.
Colombian Association of Rheumatology, Risk of Fracture, CAYRE, IPS.
Please cite this article as: Domínguez Perilla AM, Vásquez GM, Rojas MX. ¿Es la seropositividad en pacientes con artritis reumatoide tratados con adalimumab un factor para desarrollar anticuerpos anti-adalimumab? Rev Colomb Reumatol. 2019;26:24–30.