Patients with rheumatoid arthritis have a higher cardiovascular risk due to the prevalence of cardiovascular risk factors and metabolic alterations derived from the inflammatory activity and the treatment of their disease. A systematic evaluation of the effect of statins on lipid fractions and cardiovascular outcomes has not been carried out specifically in patients with rheumatoid arthritis.
MethodsA literature search was conducted in Medline (PubMed), Embase, Lilacs, Scielo, and Cochrane. Randomized and non-randomized clinical trials were selected that evaluated the impact of statins on mortality, cardiovascular events, lipid fractions and disease activity in patients with rheumatoid arthritis. A paired evaluation of risk of bias was carried out, along with a meta-analysis using RevMan 5.3®.
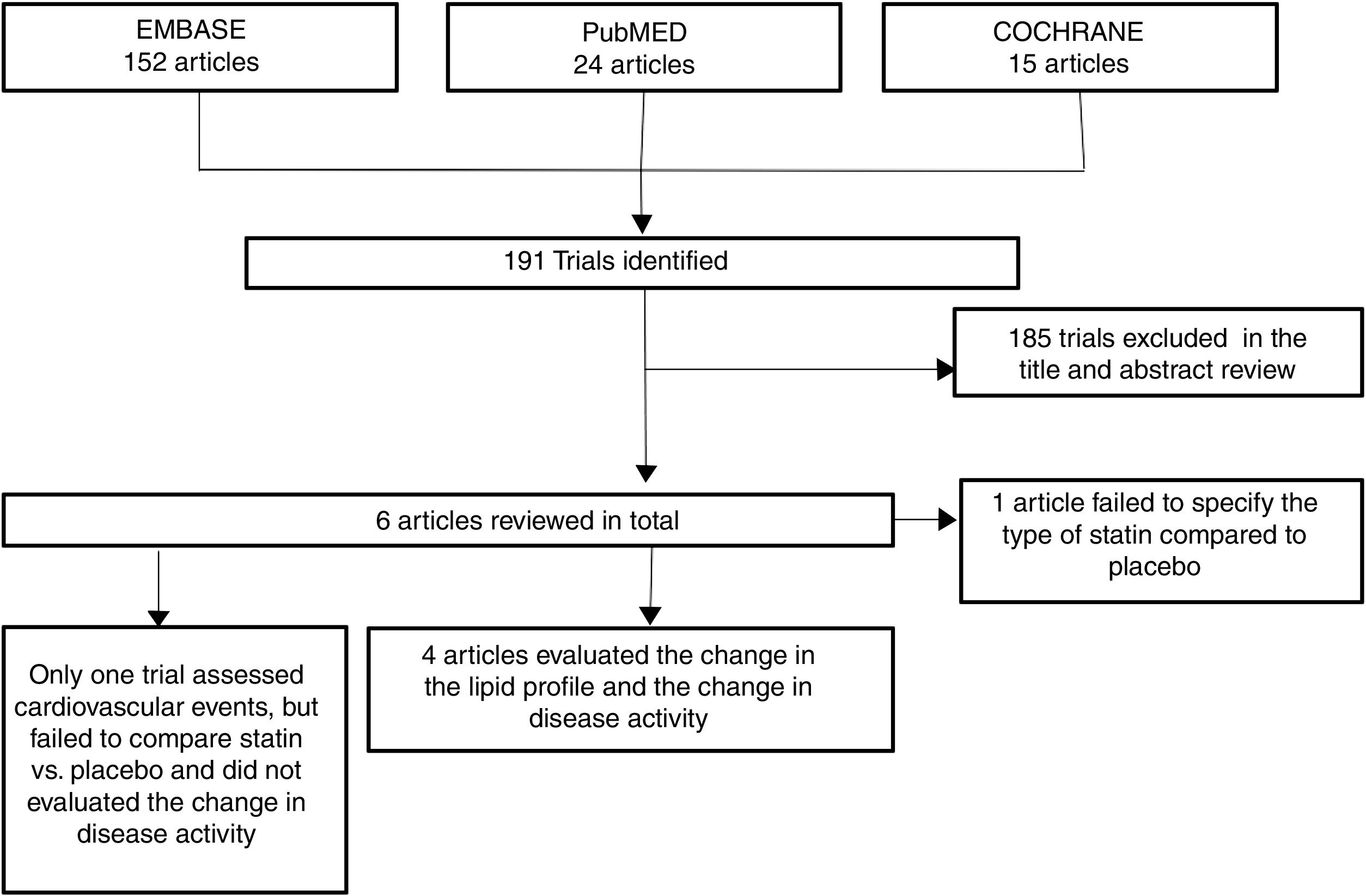
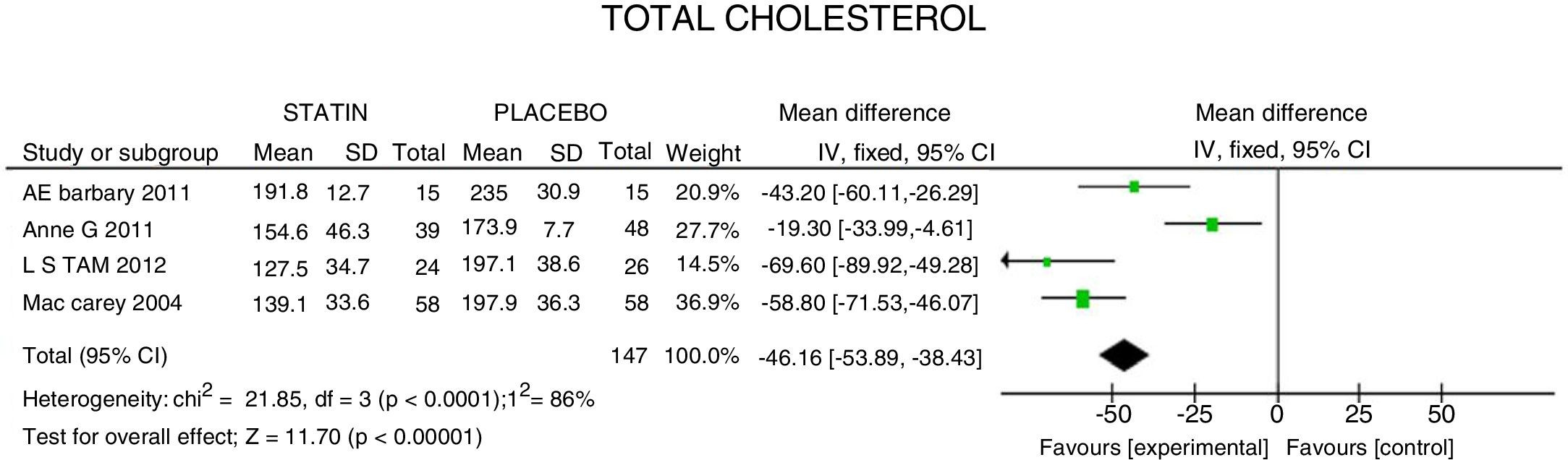
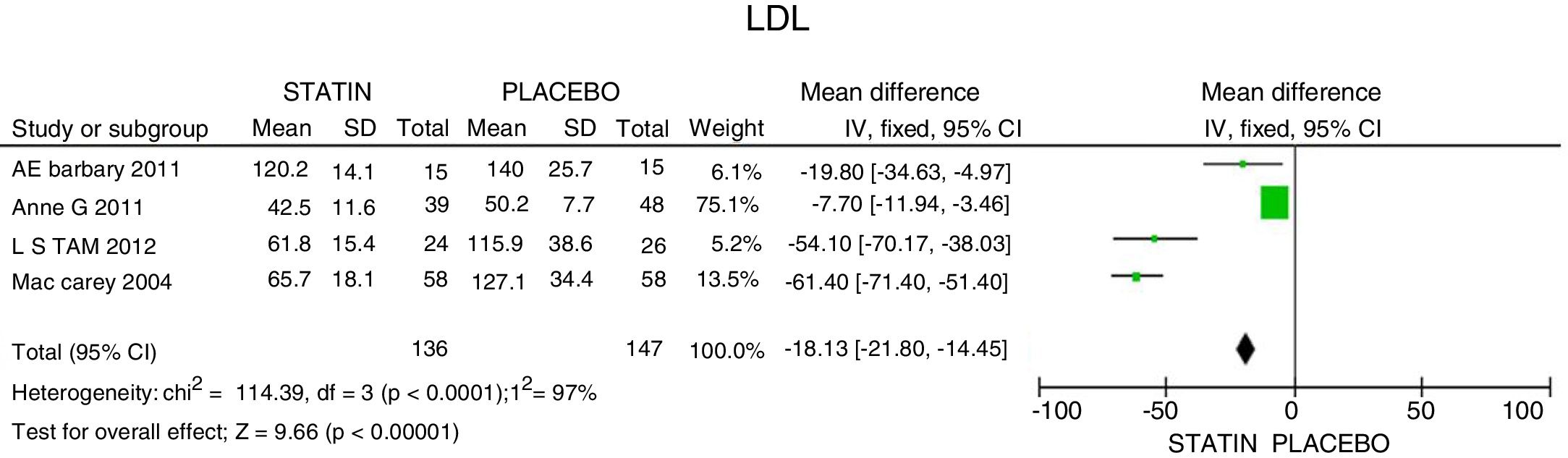
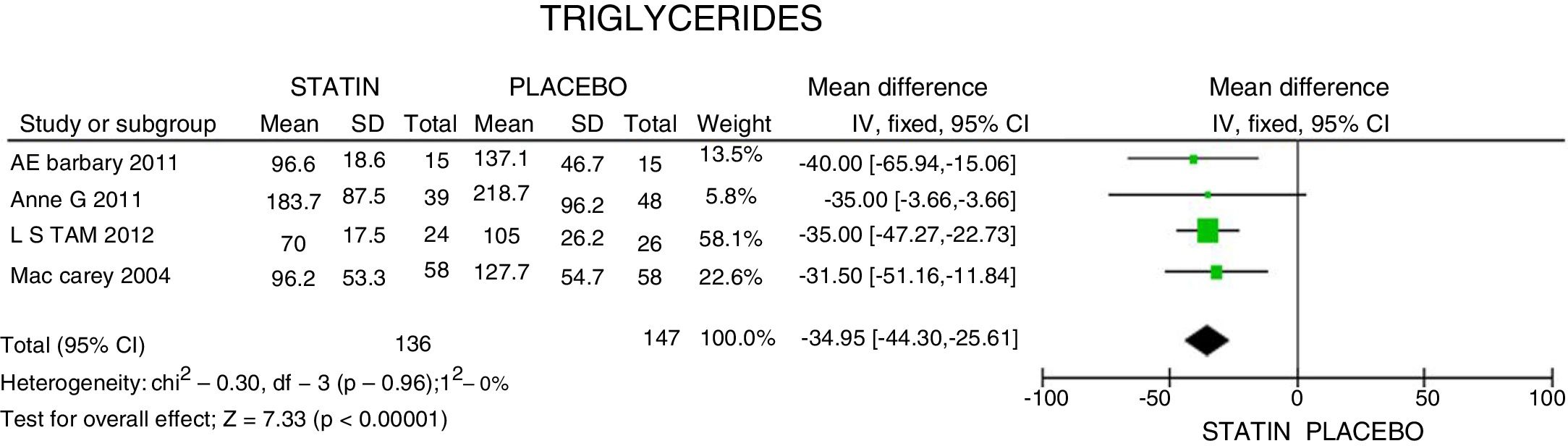
ResultsA total of 5 studies and 383 patients were included in the analysis. The use of statins among patients with rheumatoid arthritis significantly reduced total cholesterol levels: −46.16mg/dL (95% CI: [−53.89, −38.46]; I2: 86%), LDLc: −18.13mg/dL (95% CI: [−21.8, −14.4], I2: 97%) and TG of −34.95mg/dL (95% CI: [−44.3, −25.6], I2: 0%), without significant changes in HDLc. It also reduced the activity of the disease (DAS28) by 0.73 (95% CI: [−0.94, −0.53], I2: 96%). No study was found that reported mortality or cardiovascular outcomes.
ConclusionThe lipid-lowering effect of statins in patients with rheumatoid arthritis is consistent with that found in other populations. There is a slight impact in reducing the activity of the disease, but reducing the risk of cardiovascular events in this specific population has not been studied.
Los pacientes con artritis reumatoide tienen un riesgo cardiovascular elevado por la alta prevalencia de factores de riesgo cardiovascular y de alteraciones metabólicas derivadas de la actividad inflamatoria y del tratamiento de su enfermedad. No se ha realizado una evaluación sistemática del efecto de las estatinas en las fracciones lipídicas y desenlaces cardiovasculares específicamente en pacientes con artritis reumatoide.
MétodosSe realizó una búsqueda de la literatura en Medline (PubMed), Embase, Lilacs, Scielo y Cochrane. Se seleccionaron experimentos clínicos aleatorizados y no aleatorizados (estudios de cohortes) que evaluaran el impacto de las estatinas en mortalidad, eventos cardiovasculares, fracciones lipídicas y actividad de la enfermedad en pacientes con artritis reumatoide. Se realizó la selección, evaluación del riesgo de sesgos de forma pareada y un metaanálisis con RevMan 5.3®.
ResultadosUn total de 5 estudios y 383 pacientes fueron incluidos en el análisis. El uso de estatinas en pacientes con artritis reumatoide reduce significativamente los niveles de colesterol total: −46,16mg/dL (IC 95%: [−53,89; −38,46]; I2: 86%), LDLc: −18,13mg/dL (IC 95%: [−21,8; −14,4]; I2: 97%) y triglicéridos de −34,95mg/dL (IC 95%: [−44,3; −25,6]; I2: 0%), sin cambios significativos del HDLc. Además, reduce la actividad de la enfermedad (DAS28) en 0,73 (IC 95%: [−0,94; −0,53]; I2: 96%). No se encontró ningún estudio que reportara mortalidad ni desenlaces cardiovasculares.
ConclusiónEl efecto hipolipidemiante de las estatinas en pacientes con artritis reumatoide es consistente con lo encontrado en otras poblaciones. Existe un discreto impacto en la reducción de actividad de la enfermedad; sin embargo, la reducción del riesgo de eventos cardiovasculares en esta población específica no ha sido estudiada.
Rheumatoid arthritis (RA) is an independent risk factor for cardiovascular morbidity and mortality.1–3 It has even been said that cardiovascular events are the main cause of death in patients with RA.4,5 RA is a systemic autoimmune disease involving a significant inflammatory process.6 There are multiple pathophysiological mechanisms resulting from inflammation that promote the atherogenic process, increasing the cardiovascular risk, and in particular, the risk of coronary disease, in contrast with the population without RA.7–10
The specific causes of this process in RA are not fully established. It is believed that in addition to the classical cardiovascular risk factors, there is a complex interaction between a genetic component, and the presence of chronic inflammation.11 In patients with RA, particularly those with active disease, there is a significant decline in the levels of high density cholesterol (HDLc) and a constant elevation in the atherogenic index, with loss of the anti-atherogenic and antioxidant function, which then results in a pro-oxidative and pro-atherogenic profile.1,12 Moreover, there is an increase in the inflammatory mediators, which further promotes the atheromatous process and endothelial damage.13
A paradoxical effect has been described in the reduction of total cholesterol (TC) and of low density cholesterol (LDLc) in patients with RA, usually associated with active disease, increased risk of cardiovascular complications, and death.1,10,14 Other studies have documented TC, LDLc and triglyceride (TG) elevations in patients with RA, characteristically during the early phases.13,15,16 Such discrepancies may be related with the population studied, the stage of the disease, or the presence of activity,17 making it increasingly difficult to properly assess the indication and impact of treatment.
Statins are part of the usual management of patients with RA in clinical practice,9 together with DMARDs (disease-modifying anti-rheumatic drugs) treatment, not just because of its potential impact on the reduction of cardiovascular events by modifying and improving the lipid profile,8 but also because these drugs could apparently control the activity of the disease.
In view of the importance of establishing the effect on cardiovascular risk and lipid profile of statins in patients with RA, and bearing in mind the shortage of information regarding their effectiveness in patients with this disease, the decision was made to conduct a systematic review and a meta-analysis to assess the impact of statins and determine to what extent these drugs could be prescribed jointly with anti-rheumatic therapy, as part of the standard management of the disease.
Materials and methodsA literature search was conducted on several databases, including Medline (PubMed), Embase, Lilacs, Scielo and the Cochrane Collaboration database (Cochrane Central Register of Controlled trials), from the beginning and until September 2016. The search terms used were: rheumatoid arthritis, hyperlipidemia, dyslipidemia, hypercholesterolemia, hydroxymethylglutaryl CoA reductase inhibitors, atorvastatin, pravastatin, simvastatin, lovastatin and rosuvastatin, both in free text as in MeSH terms. The search was limited to publications in English and Spanish.
The inclusion criteria were studies that evaluated patients over 18 years old, with a definite diagnosis of RA pursuant to the RA classification criteria published by the American College of Rheumatology (ACR) in 198718 or according to the criteria defined by the ACR and the European League Against Rheumatism in 2010,6 treated with at least one statin, for a minimum of 26 weeks, while the control groups received either placebo or a less potent statin, or no medication at all. The outcomes evaluated were death from any cause, cardiovascular death, and cardiovascular events (acute myocardial infarction [AMI], stroke); the secondary outcomes evaluated were changes in the lipid profile values (TC, LDLc, HDLc, and TG), a decline in the disease activity measured with DAS28 (disease activity score) and changes in C-reactive protein.6 Aware of the shortage of information from randomized clinical trials (RCTa), the decision was made to additionally look for and include non-randomized trials, whether cohort studies, or case control studies.19,20
The studies that failed to provide original data were excluded (letters, comments, perspectives, or editorials), studies including patients with a different type of concomitant inflammatory joint disease in addition to RA, and those that failed to submit data on outcomes of interest or those for which the data were not available despite contacting the authors.
The selection of studies was done in pairs by 2 authors (SC and OR) and any discrepancies were solved by consensus, with the participation of a third reviewer (OM). Data mining was done independently by two investigators, using a standardized format which included the information about the size of the sample, baseline characteristics of the population, the comparative or controlled used, the presence of comorbidities, additional medications used, duration of follow-up and relevant outcomes.4,18 Any discrepancies in the information were settled by a third investigator. The risk of biases in clinical trials was independently assessed by 2 reviewers, using the tool proposed by the Scottish Intercollegiate Guidelines Network21 and the evaluation of the risk of biases of the non-randomized trials used the ROBINS-I22 tool, recommended by the Cochrane Collaboration. Any disagreements were settled through discussion and with the participation of a third reviewer. The input, analysis and processing of the data were done with the Revman 5.3® tool, using a random effect model for the meta-analysis of the clinical experiments. There was an a priori plan for analyzing and independently presenting the information from clinical, no-randomized trials, in an attempt to identify the causes for dissimilar results.19,23,24
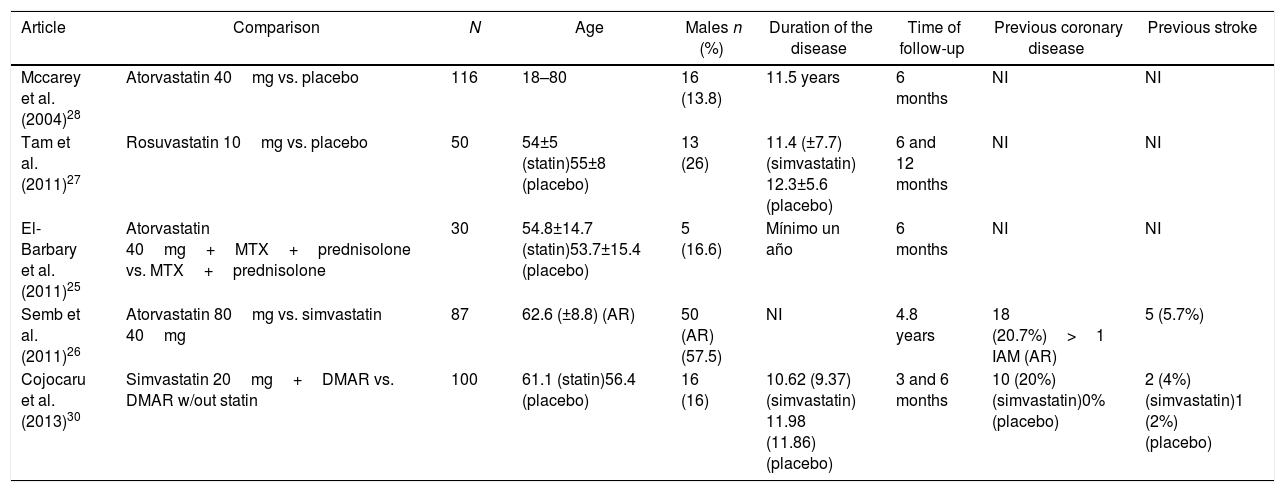
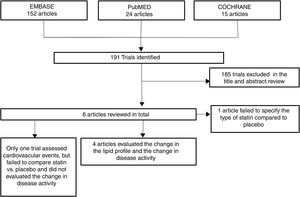
ResultsOut of 191 studies found in the search, 185 were excluded following an evaluation of the titles and abstracts. 6 articles25–30 were selected for full text evaluation. One of them,29 corresponded to a cohort study and was excluded because it failed to indicate the type of statin evaluated. Of the 5 studies finally included, 4 were RCTs25–28 and one was a cohort study30; overall, the studies evaluated included a total population of 383 patients. Fig. 1 shows the results of the search and the selection of the trials. The statin evaluated in 3 trials was atorvastatin25,26,28; in one study, simvastatin30 and in another one, rosuvastatin.27 The characteristics of the patients included in each one of the studies are illustrated in Table 1.
Baseline characteristics of patients included in the trials selected.
| Article | Comparison | N | Age | Males n (%) | Duration of the disease | Time of follow-up | Previous coronary disease | Previous stroke |
|---|---|---|---|---|---|---|---|---|
| Mccarey et al. (2004)28 | Atorvastatin 40mg vs. placebo | 116 | 18–80 | 16 (13.8) | 11.5 years | 6 months | NI | NI |
| Tam et al. (2011)27 | Rosuvastatin 10mg vs. placebo | 50 | 54±5 (statin)55±8 (placebo) | 13 (26) | 11.4 (±7.7) (simvastatin) 12.3±5.6 (placebo) | 6 and 12 months | NI | NI |
| El-Barbary et al. (2011)25 | Atorvastatin 40mg+MTX+prednisolone vs. MTX+prednisolone | 30 | 54.8±14.7 (statin)53.7±15.4 (placebo) | 5 (16.6) | Mínimo un año | 6 months | NI | NI |
| Semb et al. (2011)26 | Atorvastatin 80mg vs. simvastatin 40mg | 87 | 62.6 (±8.8) (AR) | 50 (AR) (57.5) | NI | 4.8 years | 18 (20.7%)>1 IAM (AR) | 5 (5.7%) |
| Cojocaru et al. (2013)30 | Simvastatin 20mg+DMAR vs. DMAR w/out statin | 100 | 61.1 (statin)56.4 (placebo) | 16 (16) | 10.62 (9.37) (simvastatin) 11.98 (11.86) (placebo) | 3 and 6 months | 10 (20%) (simvastatin)0% (placebo) | 2 (4%) (simvastatin)1 (2%) (placebo) |
CVA: cardiovascular accident: RA: rheumatoid arthritis; DMAR: disease modifying anti-rheumatic drugs; AMI: acute myocardial infarction; MTX: methotrexate; NI: no information available.
No study was found that evaluated statins against placebo or no treatment, in terms of the occurrence of cardiovascular events; however, one study26 compared the incidence of cardiovascular events — defined as a compounded final outcome of AMI, stroke, heart failure, and unstable angina, comparing high potency statin against a moderate potency statin — and reported that the rate of cardiovascular events was not significantly different in patients with RA who received atorvastatin (14/39, 35.9%) in contrast with simvastatin (9/48; 18.8%; p=0.24). It was impossible to individualize each of the different events.
Lipid fractions and disease activityTC experienced a mean reduction of 46.16mg/dL (95% CI: [−53.89; −38.46]; I2: 86%). In the meta-analysis of the 4 RCTs (Fig. 2), the heterogeneity of the results may be explained because in one trial26 a high potency statin was assessed against another lower potency statin, with a lower estimated effect (CT: −19.30mg/dL; 95% CI: [−33.9; −4.6]).
The mean LDLc reduction was 18.13mg/dL (95% CI: [−21.8; −14.45]; I2: 97%). The studies by Tam et al.27 and McCarey et al.,28 showed evidence of higher efficacy in LDL reduction (54.10mg/dL; 95% CI: [−70.17; −38.03]) with the use of rosuvastatin; and of 61.4mg/dL (95% CI: [−71.4; −51.4]) with the use of atorvastatin, respectively (Fig. 3).
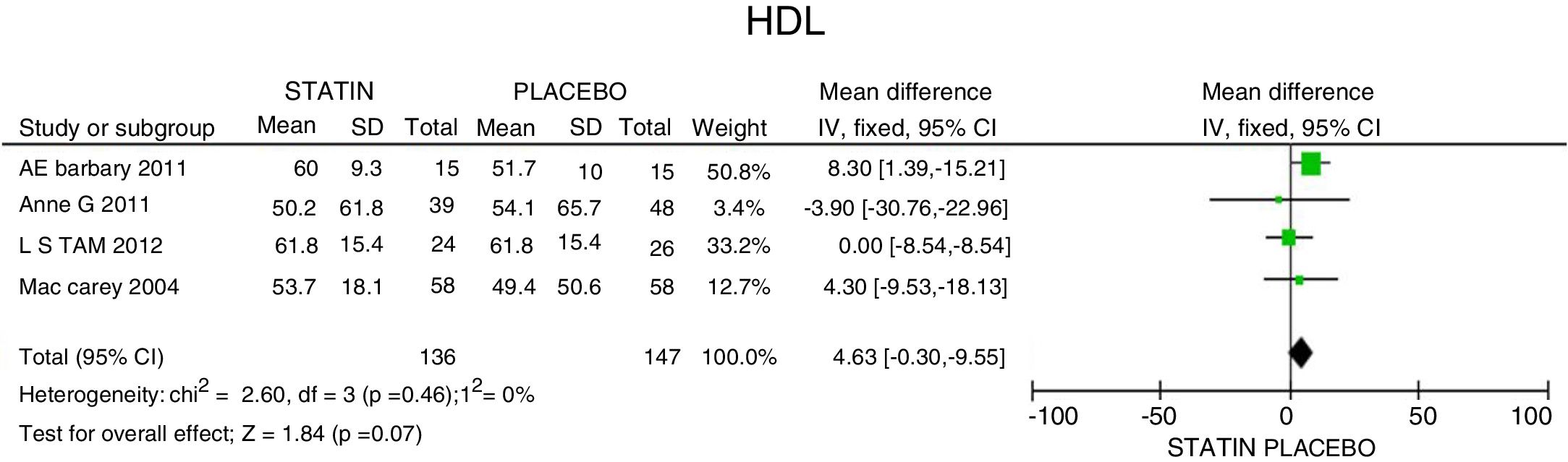
In terms of reducing the level of triglycerides, the results indicate a 34.95mg/dL reduction (95% CI: [−44.3; −25.6]; I2: 0%) after statins treatment (Fig. 4) and for HDLc there was a non-statistically significant elevation of 4.63mg/dL (95% CI: [−0.30; 9.55]; I2: 0) (Fig. 5).
In contrast with the results of the RCTs, the Cojocaru30 cohort study evidenced an apparent increase in the lipid fractions following statins therapy. This was the result of a selection bias, as shall be discussed later. However, the net TC drop corresponded to −27.9mg/dL (95% CI: [19.311; 44.243]; p<0.001), while LDL experienced a reduction of −21.66mg/dL (95% CI: [21.109; 43.972]; p<0.001), with no significant changes in the HDL and TG levels.
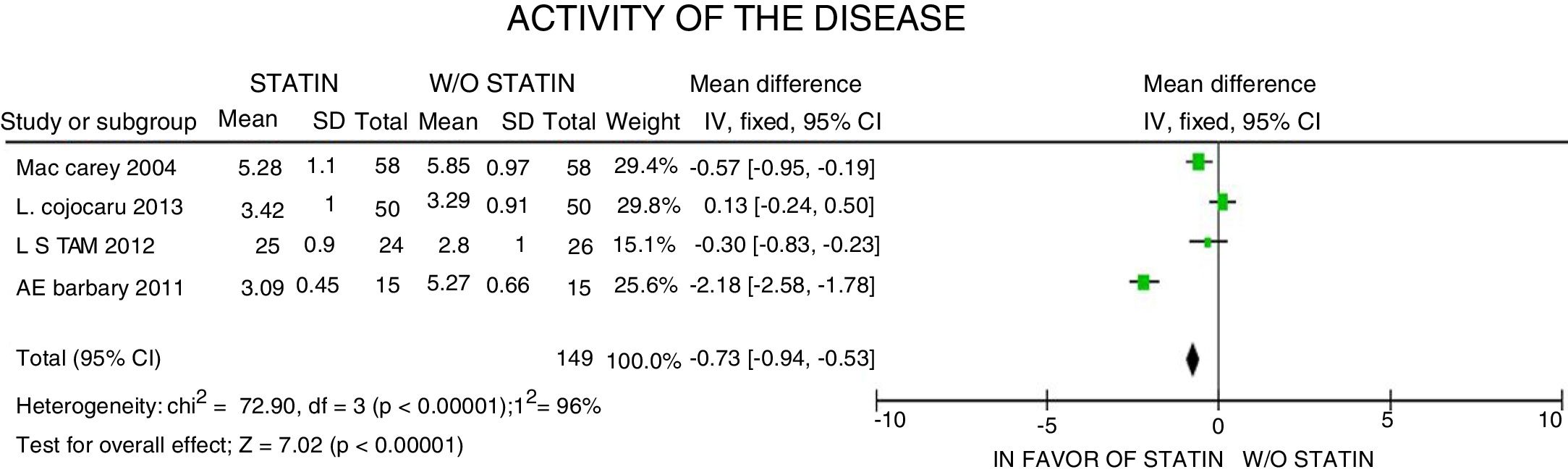
In terms of the disease activity evaluated with the DAS28 scale, a 0.73-point reduction was reported (95% CI: [−0.94; −0.53]; I2: 96%), with considerable heterogeneity of the estimates, and failure to identify the clinical characteristics accounting for such reduction, or to determine the clinical relevance, despite the statistical significance of the data (Fig. 6).
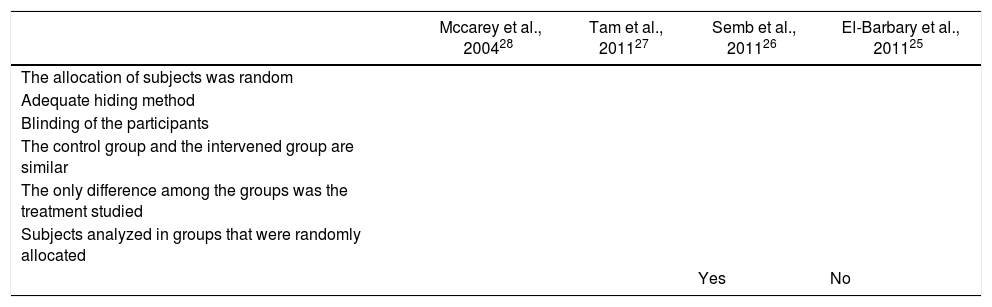
Quality of the trialsThe results of the quality evaluation of the studies included are shown in Table 2. When reviewing the quality of the RCTs to assess the impact of statins and the level of activity of the disease, we found that although in every case the patients were randomized, only 3 trials did a proper blinding.26–28 All of the trials included did a randomized allocation. During the quality evaluation of the cohort study,26 an inadequate management of the confusion factors was identified, with significantly different baseline characteristics among the intervention and control groups, and without a statistical analysis aimed at controlling these differences.
Quality evaluation of the trials selected.
| Mccarey et al., 200428 | Tam et al., 201127 | Semb et al., 201126 | El-Barbary et al., 201125 | |
|---|---|---|---|---|
| The allocation of subjects was random | ||||
| Adequate hiding method | ||||
| Blinding of the participants | ||||
| The control group and the intervened group are similar | ||||
| The only difference among the groups was the treatment studied | ||||
| Subjects analyzed in groups that were randomly allocated | ||||
| Yes | No |
The current systematic review included information from randomized and non-randomized trials, showing the well known lipid lowering effect of statins and a positive impact on reducing the disease activity score (DAS28). The beneficial effect of statins through the reduction of TC, LDLc, and TG levels, with a mild increase in HDLc, is consistent among the studies included and behaves similarly to other populations studied.31,32 Although most of the studies evaluated showed data regarding the change in lipid profile and disease activity, when comparing patients exposed and non-exposed to statins, there were not enough data available to assess the impact on cardiovascular outcomes. The only trial identified with such specification was the IDEAL trial,26 which compared groups of patients with and w/o RA, exposed to high and low potency statins, and showed a significant improvement in reducing the levels of TC, LDLc and TG in favour of the high power statin and HDLc elevation with both statins. However, there were no significant differences in terms of cardiovascular events, and this may be explained by the relatively short follow-up time.
Considering the lack of available evidence to assess the impact of statins on the risk of mortality of patients with RA, it is worth considering a few additional studies published. De Vera et al.33 reported a longitudinal trial based on administrative data from over 16,000 patients, indicating that when statin therapy is discontinued in RA patients, there is an increased incidence of both cardiovascular death and all cause mortality. Likewise, a population study conducted by Sheng et al.29 evaluated the impact of statins to reduce TC, cardiovascular mortality, and all cause mortality; further emphasizing the beneficial effect of statins with regards to these three outcomes in patients with RA. Such studies have significant methodological limitations associated with the risk of poor classification biases because of the limitations inherent to the quality of coding to identify patients with RA based on administrative data and the impossibility to determine exactly which statin they received, as is the case of the above-mentioned Sheng et al. trial. Neither is it possible to evaluate whether patients actually received the drugs or the reasons for their discontinuation. However, there is still the possibility of actually affecting mortality, but this factor was not properly evaluated in the RCTs and cohort studies included in the current review. Further RCTs with a larger sample size and long enough follow-up times to identify this impact shall be conducted in the future.
A strength of this review was the inclusion of non-randomized trials, considering that these trials often have longer follow-up periods and a better assessment of adverse events. Notwithstanding such effort, only one cohort study developed by Cojocaru et al.30 was identified; apparently this study exhibited a trend towards increased lipid fractions with the use of statin, in contrast to what was evidenced in the RCTs included.25–28 Such finding may be explained by a selection bias associated with systematic differences among the patients that received statins versus those who did not, with baseline values for TC, TG, and LDLc considerably higher in the group of patients exposed to statins. The low quality of this trial fails to significantly contribute to the evaluation of the impact of statin treatment in patients with RA.
With regards to the activity of the disease, there was a reduction in the scores of disease activity at 3 months; however, there are no significant differences at 6 months of follow-up. Among the other 3 trials that assessed the activity of the disease, one of them by Tam et al.,27 was not able to achieve statistical significance either, when comparing the intervention with statins against placebo, though surprisingly, one of the inclusion criteria for this trial specified a low level of baseline activity or no activity, which could also explain such phenomenon. Another potential factor for bias is the DMARD therapy used concomitantly with the statin, which could differ in each patient, both in terms of type and doses, and also gives rise to differential baseline activity levels and different control times.
The major limitations of the study include the relatively short follow-up of the trials included, which is suitable to assess the impact on lipid fractions, but is short to evaluate cardiovascular outcomes. Moreover, the patients included in the trial were highly variable and heterogeneous, hindering a correct combination of data and analysis. This systematic review represents a valuable contribution in the light of the shortage of information available and encourages further research in the filed.
ConclusionsThe use of statins as a lipid lowering treatment makes a significant impact on patients with RA because of the reduction of the levels of TC, LDLc and TG, with no evidence of a significant increase in the levels of HDLc. There was a mild impact on the activity of the disease measured with DAS28 in the short term (3 months), but such impact was maintained after 6 months and did not achieve clinical relevance. The information about the impact on reducing cardiovascular outcomes is limited and does not allow for drawing conclusions about its potential benefit or systematic use as part of the antirheumatic therapy in patients that fail to meet the usual indications for statin therapy.
Conflict of interestNone declared.
Please cite this article as: Muñoz ÓM, Reyna Carrasco ÓA, Milena Castelblanco S, García ÁA, Fernández-Avila DG. Impacto terapéutico de las estatinas en el perfil lipídico y riesgo cardiovascular en pacientes con artritis reumatoide: Revisión sistemática de la literatura y metaanálisis. Rev Colomb Reumatol. 2019;26:40–47.