Unlike other rheumatic diseases, gout is rare in women of childbearing age. Due to the low number of cases available for the study, current information is based mainly on case reports.
ObjectiveTo describe the characteristics and the outcome of the pregnancy of women with gout.
MethodsA systematic literature search was undertaken by the investigators in the PubMed and Embase databases, from the inclusion date to August 2016. Patients were included if they met the definition of gout according to the American Rheumatism Association criteria, and that they had the description of its follow-up during the pregnancy. Data collection and analysis: each pregnancy was treated as a separate observation for analysis. The maternal and fetal-neonatal outcomes data were extracted from the articles finally selected.
ResultsThe search identified 125 potentially relevant articles, but after a full-text review only 12 articles met the inclusion criteria. Of the 23 pregnancies described, there were 16 (69.5%) live births, 5 (21.7%) were aborted, in one (4.3%), the pregnancy was terminated, and in one case report (4.3%) there was no description on the term of pregnancy. No maternal deaths were reported. Two babies died a few hours after birth. Congenital malformations were not described in any case report. The most frequent maternal complications were renal damage, anemia, preeclampsia, and postpartum uremia.
ConclusionsGout during pregnancy is not common, but it is known to occur. While the majority of women with gout delivered healthy infants, they were at increased risk of having maternal complications.
A diferencia de otras enfermedades reumáticas, la gota es una enfermedad rara en mujeres en edad fértil. Debido al escaso número de casos disponibles para el estudio, la información actual se basa, principalmente, en reportes de casos.
ObjetivoDescribir las características y el desarrollo del embarazo en mujeres con gota.
MétodosUna búsqueda sistemática de literatura fue realizada en las bases de datos PubMed, Lilacs, Ebsco y Embase, desde la fecha de inclusión hasta agosto del 2016. Se incluyó a pacientes que cumplieron con la definición de gota según los criterios de la American Rheumatism Association y que tenían la descripción de su seguimiento durante el embarazo. Cada embarazo se trató como una observación independiente para el análisis. A partir de los artículos finalmente seleccionados, se extrajeron los desenlaces materno-fetales.
ResultadosLa búsqueda identificó 125 artículos potencialmente relevantes, después de la revisión de texto completo, 12 artículos cumplieron los criterios de inclusión. Se describen 23 embarazos que resultaron en 16 (69,5%) nacimientos vivos, 5 (21,7%) abortos, una (4,3%) interrupción del embarazo y en un caso (4,3%) no se describió el desenlace. No se reportaron muertes maternas. Dos recién nacidos fallecieron después del parto. No se documentaron malformaciones congénitas. Las complicaciones maternas más frecuentes fueron la insuficiencia renal, la anemia, la preeclampsia y la uremia posparto.
ConclusionesLa gota durante el embarazo no es común, pero se sabe que ocurre. Mientras que la mayoría de las mujeres con gota tuvieron bebés sanos, presentaban un mayor riesgo de tener complicaciones maternas.
Pregnancy in women with rheumatic diseases can be a challenge for the physicians responsible for their care, because the frequency and complications during pregnancy vary between the different rheumatic diseases. In addition, it may be necessary to change the treatment of the patient who plans to become pregnant, with the purpose of using medications that would maintain control of the disease in the mother and which are considered safe for the fetus.
Gout is a disease caused by the formation and precipitation of urate crystals in the joints or in the soft tissues. Epidemiological studies have confirmed that it is an infrequent condition in women (prevalence of 0.04–5%),1,2 and it usually occurs after menopause. It is a rare disease in women of childbearing age, its incidence between 25 and 44 years of age is 1.6 cases per 10,000 patients-years (95% CI 1.1–2.1).3 Studies on the effect of gout in pregnancy are scarce and are mostly case reports. Due to the scarcity of cases for study, many aspects of the interrelationship of this association and the optimal treatment for this disease in pregnancy are unknown. Our objective is to describe the clinical characteristics, the evolution and the treatment of gout during pregnancy and the puerperium, through the presentation of a case of our institution, in addition to 22 published pregnancies.
Patients and methodsThe report of a case of our institution was made, together with a systematic review in the databases: PubMed, Lilacs, Ebsco and Embase, since their origin until August 2016. The search strategy included the keywords: “gout”, “premenopause” “pregnancy”, “hyperuricemia”, “women”, “female”, “gouty arthritis”, “gouty nephropathy”. The search was restricted to articles published in English and Spanish. The publications were considered as the primary information source; however, additional references were sought in the bibliographic references of the selected articles and in the gray literature on this subject.
The selection process was carried out in 2 phases: the first was applied to the titles and abstracts; the second phase was applied to the full texts. The publications which could not be excluded on the basis of the title and the abstract were included for the full text review. The publications in which the patients met the definition of gout according to the criteria of the American Rheumatism Association and that had the description of their follow-up during pregnancy were included.
From each publication were obtained: age, number of pregnancies, presence or absence of acute gout attack during pregnancy, treatment used during pregnancy and the puerperium, comorbidities, and maternal-fetal outcome. The maternal outcomes evaluated were: cesarean delivery, induction of labor, gestational hypertension or preeclampsia, hemorrhage during pregnancy or postpartum and postpartum uremia. The fetal outcomes included: preterm delivery (<37 weeks of gestation [GW]), intrauterine growth retardation, congenital anomalies, and perinatal mortality. Each pregnancy was considered as a unit of analysis. The results obtained are presented in the form of the absolute number of cases and their percentage (n [%]), and the mean±standard deviation (minimum value-maximum value).
ResultsA 30-year-old woman who was known to be healthy until the beginning of her first pregnancy; at 13GW suddenly began to present arthritis of the left ankle and limitation for walking, which was treated with paracetamol 1.5g/day and relented in a period of 2 weeks. At 15GW she suddenly presented arthritis of the right ankle and hyperuricemia (9.1mg/dl), and was treated with paracetamol 1.5g/day and allopurinol 300mg/day.
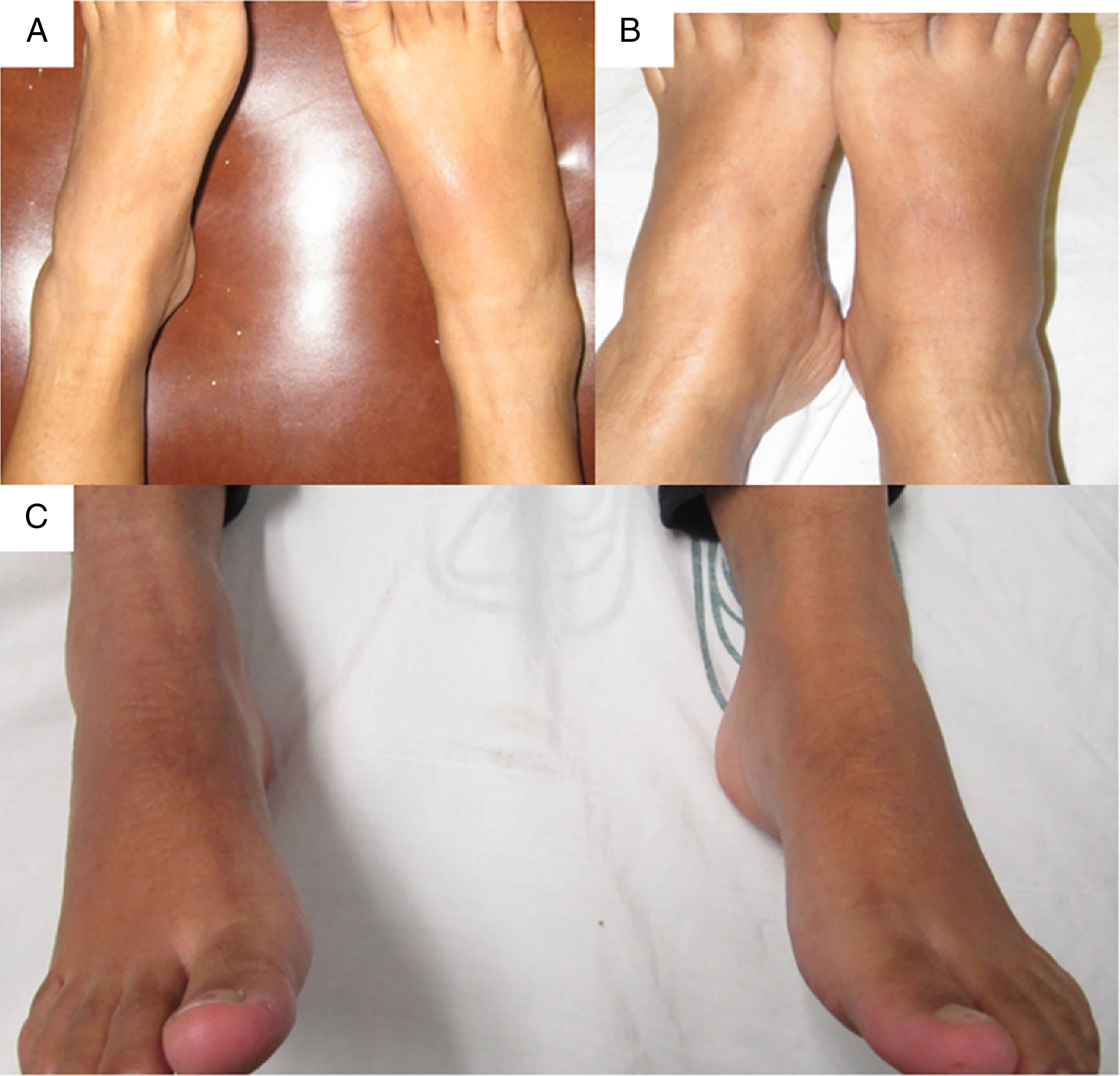
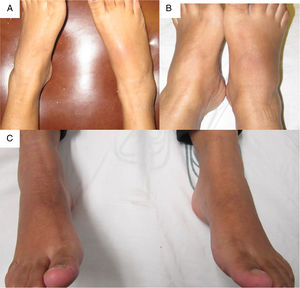
The patient was referred at 17GW to the rheumatology clinic due to persistent ankle arthritis. In her initial assessment, she denied previous clinical pictures of joint pain/arthritis or any knowledge of alterations in renal function; her father suffered from gout. On the physical examination were documented difficulty in walking, arthritis of the right ankle, inflammation and erythema of the right tarsus with intense pain on movement (Fig. 1A); absence of tophi, there was no affection of hands, carpi or elbows, and her body mass index was 22. The laboratory studies (Table 1) showed: mild anemia (hemoglobin 10.4g/dl), mild elevation of creatinine (1.2mg/dl), negative rheumatoid factor and hyperuricemia of 9.1mg/dl. Rest and application of ice in the affected region were prescribed, and treatment was started with prednisone (1mg/kg/day) and paracetamol 2g/day for one week; allopurinol was discontinued because of the risk of teratogenicity. At 20GW, intense pain at the right ankle persisted (Fig. 1B), for which 4mg of intra-articular dexamethasone were infiltrated with resolution of symptoms (Fig. 1C); the renal ultrasound reported kidneys reduced in size and absence of cysts or stones.
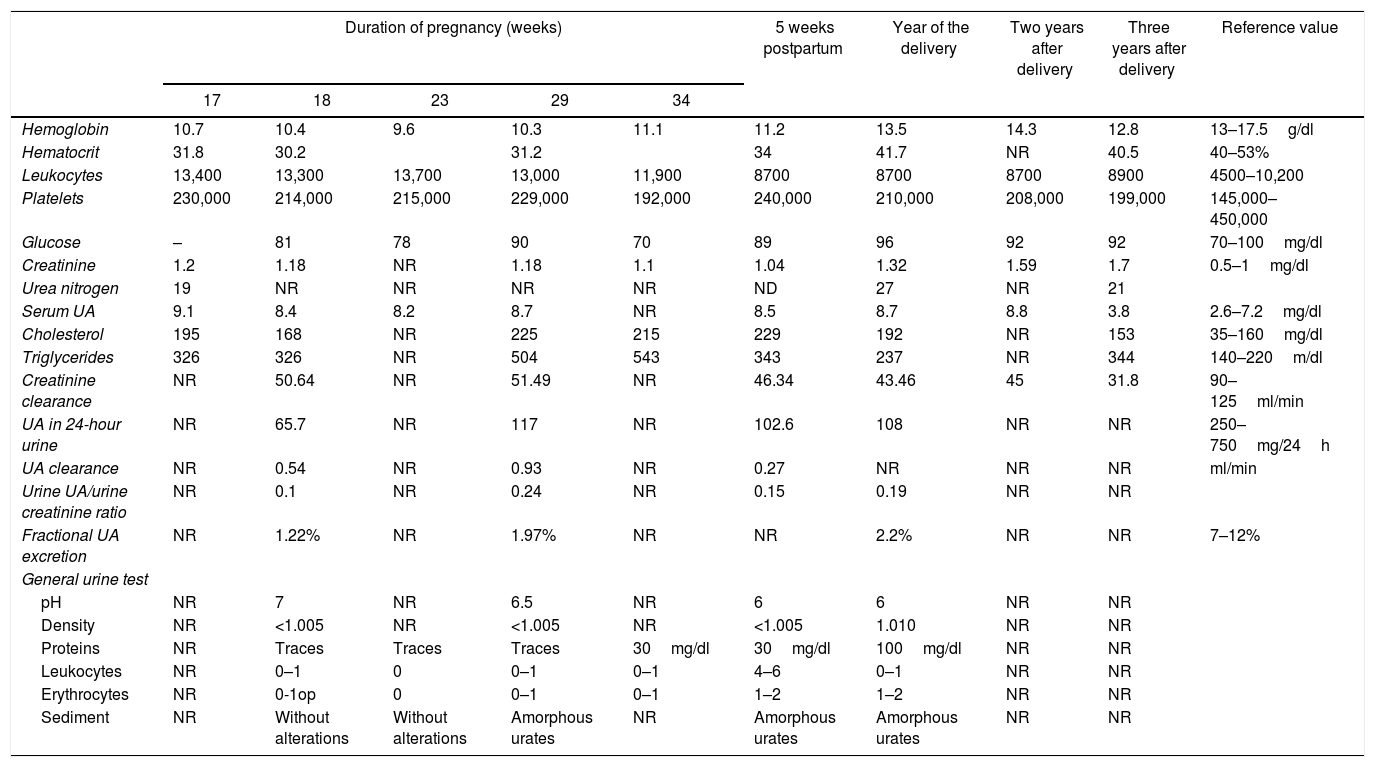
Laboratory results during and after pregnancy of the case presented.
| Duration of pregnancy (weeks) | 5 weeks postpartum | Year of the delivery | Two years after delivery | Three years after delivery | Reference value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 17 | 18 | 23 | 29 | 34 | ||||||
| Hemoglobin | 10.7 | 10.4 | 9.6 | 10.3 | 11.1 | 11.2 | 13.5 | 14.3 | 12.8 | 13–17.5g/dl |
| Hematocrit | 31.8 | 30.2 | 31.2 | 34 | 41.7 | NR | 40.5 | 40–53% | ||
| Leukocytes | 13,400 | 13,300 | 13,700 | 13,000 | 11,900 | 8700 | 8700 | 8700 | 8900 | 4500–10,200 |
| Platelets | 230,000 | 214,000 | 215,000 | 229,000 | 192,000 | 240,000 | 210,000 | 208,000 | 199,000 | 145,000–450,000 |
| Glucose | – | 81 | 78 | 90 | 70 | 89 | 96 | 92 | 92 | 70–100mg/dl |
| Creatinine | 1.2 | 1.18 | NR | 1.18 | 1.1 | 1.04 | 1.32 | 1.59 | 1.7 | 0.5–1mg/dl |
| Urea nitrogen | 19 | NR | NR | NR | NR | ND | 27 | NR | 21 | |
| Serum UA | 9.1 | 8.4 | 8.2 | 8.7 | NR | 8.5 | 8.7 | 8.8 | 3.8 | 2.6–7.2mg/dl |
| Cholesterol | 195 | 168 | NR | 225 | 215 | 229 | 192 | NR | 153 | 35–160mg/dl |
| Triglycerides | 326 | 326 | NR | 504 | 543 | 343 | 237 | NR | 344 | 140–220m/dl |
| Creatinine clearance | NR | 50.64 | NR | 51.49 | NR | 46.34 | 43.46 | 45 | 31.8 | 90–125ml/min |
| UA in 24-hour urine | NR | 65.7 | NR | 117 | NR | 102.6 | 108 | NR | NR | 250–750mg/24h |
| UA clearance | NR | 0.54 | NR | 0.93 | NR | 0.27 | NR | NR | NR | ml/min |
| Urine UA/urine creatinine ratio | NR | 0.1 | NR | 0.24 | NR | 0.15 | 0.19 | NR | NR | |
| Fractional UA excretion | NR | 1.22% | NR | 1.97% | NR | NR | 2.2% | NR | NR | 7–12% |
| General urine test | ||||||||||
| pH | NR | 7 | NR | 6.5 | NR | 6 | 6 | NR | NR | |
| Density | NR | <1.005 | NR | <1.005 | NR | <1.005 | 1.010 | NR | NR | |
| Proteins | NR | Traces | Traces | Traces | 30mg/dl | 30mg/dl | 100mg/dl | NR | NR | |
| Leukocytes | NR | 0–1 | 0 | 0–1 | 0–1 | 4–6 | 0–1 | NR | NR | |
| Erythrocytes | NR | 0-1op | 0 | 0–1 | 0–1 | 1–2 | 1–2 | NR | NR | |
| Sediment | NR | Without alterations | Without alterations | Amorphous urates | NR | Amorphous urates | Amorphous urates | NR | NR | |
UA: uric acid; NR: not reported.
At 23GW she presented arthritis in the left ankle; traces of proteins were documented in the urinalysis; the patient was treated with 80mg of intramuscular methylprednisolone acetate in a single dose and colchicine 1mg/day, ibuprofen 200mg every 6h for necessary reason and prednisone 10mg/day were started, with which remission of the arthritis was achieved. By recommendation of the obstetrician-gynecologist, she suspended the prednisone and colchicine at 34GW. At 37.3GW a male newborn of 2900g, length 48cm, Apgar 9/9, Silverman-Anderson test 0, was obtained by cesarean section. 24h after giving birth, the mother presented arthritis in the right ankle, which was treated with ketorolac 30mg intravenously every 8h, with symptomatic improvement and continued with prednisone 5mg/day. At 3 weeks postpartum she referred improvement in joint symptoms, simple X-rays of the foot without alterations; colchicine 1mg/day and allopurinol 300mg/day were reinitiated with favorable clinical evolution. In the first year after childbirth, she presented 3 acute gout attacks associated with therapeutic non-compliance. The child was found clinically healthy after 3 years of follow-up.
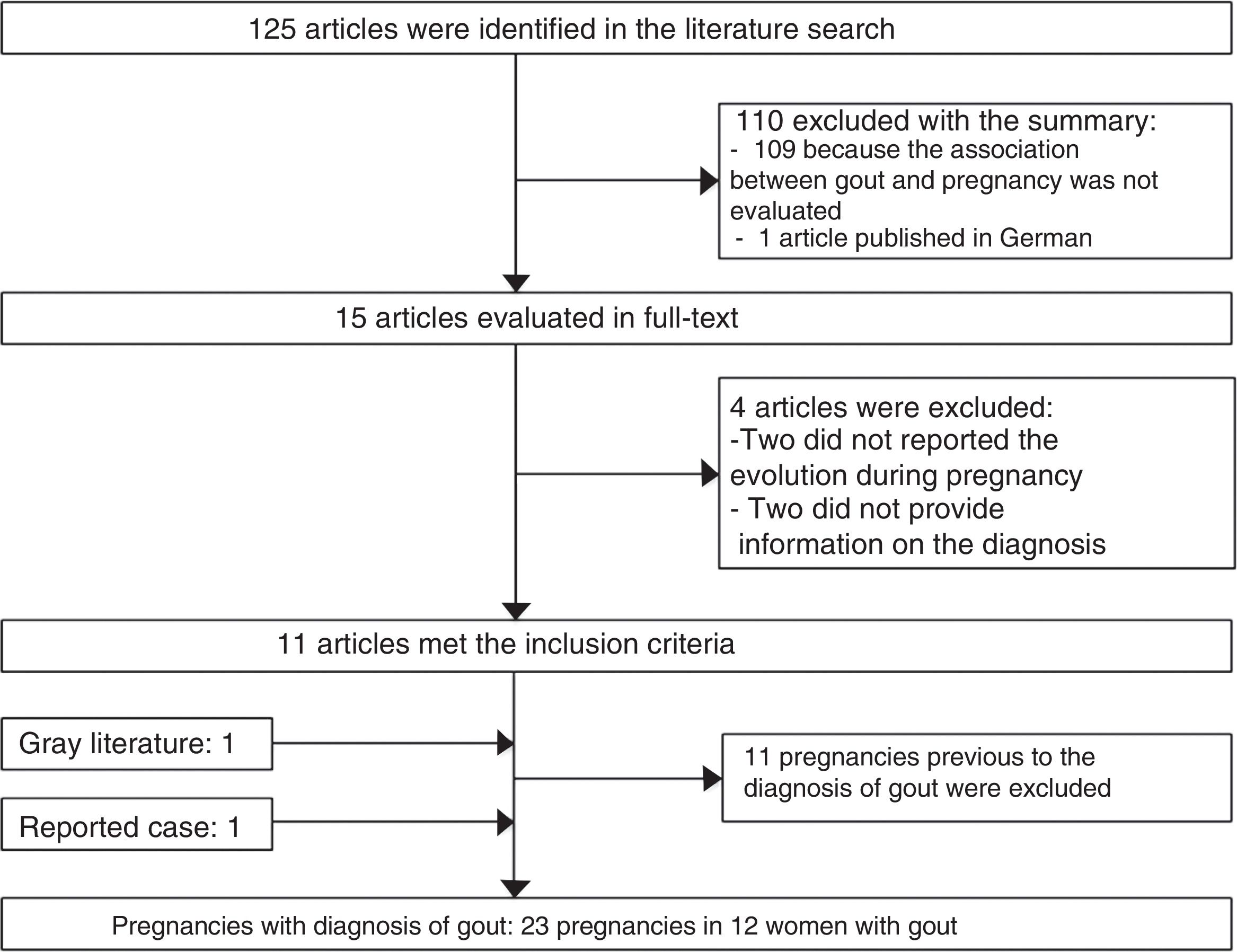
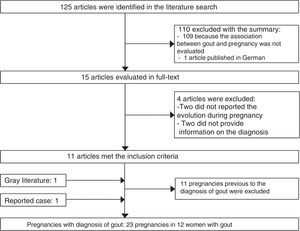
125 potentially relevant publications were identified in the bibliographic review. 110 publications were excluded with the review of the title and abstract, 11 articles were selected with the review of the text, as well as an article identified from the gray literature (Fig. 2).
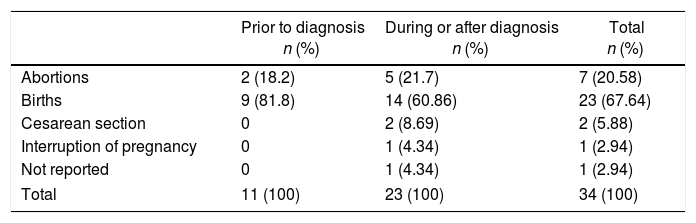
34 published pregnancies were documented in 12 women (Fig. 2), the case of one patient was published by 2 authors, and for this reason both publications are described together (Weingold4 published the evolution of the first 7 pregnancies and Batt et al.5 published the last 3 pregnancies). Six women had only one pregnancy, 3 women had 2 pregnancies, 2 women had 6 and one woman had 10 pregnancies. Eleven pregnancies occurred in 5 patients before the diagnosis of gout and 23 pregnancies occurred in 12 patients with an established diagnosis of gout (Table 2). The fetal survival rate (81.8% vs. 69.5%) was higher prior to the diagnosis of gout (Table 3). Primary gout was diagnosed in 8 women and in 4 cases it was considered a secondary cause (pseudo-Bartter syndrome,6 one case with suspected von Gierke disease,7 one case with familial juvenile hyperuricemic nephropathy8 and in other case an etiology was not concluded).9
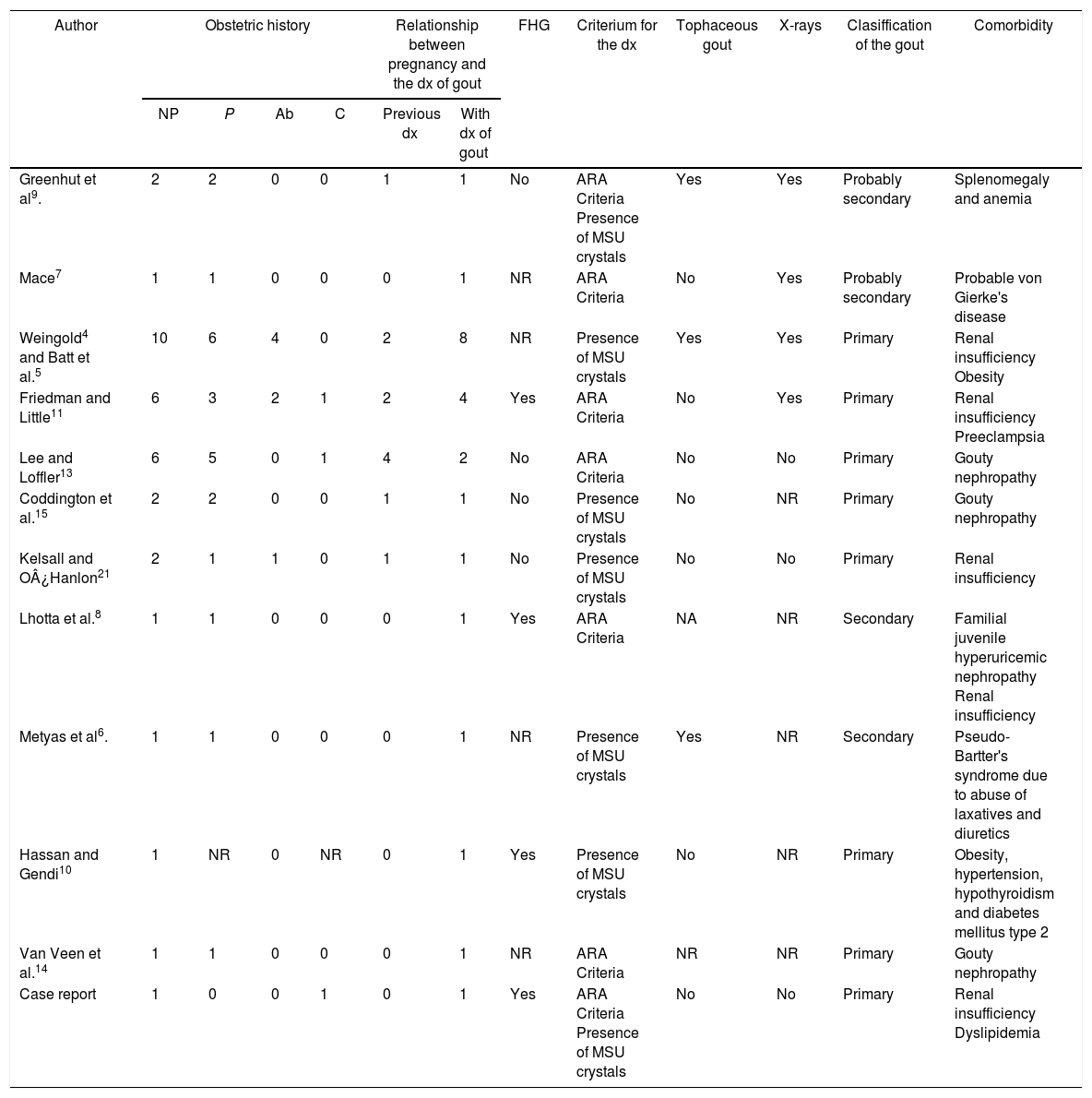
Characteristics and outcomes of pregnancies in women diagnosed with gout.
| Author | Obstetric history | Relationship between pregnancy and the dx of gout | FHG | Criterium for the dx | Tophaceous gout | X-rays | Clasiffication of the gout | Comorbidity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NP | P | Ab | C | Previous dx | With dx of gout | |||||||
| Greenhut et al9. | 2 | 2 | 0 | 0 | 1 | 1 | No | ARA Criteria Presence of MSU crystals | Yes | Yes | Probably secondary | Splenomegaly and anemia |
| Mace7 | 1 | 1 | 0 | 0 | 0 | 1 | NR | ARA Criteria | No | Yes | Probably secondary | Probable von Gierke's disease |
| Weingold4 and Batt et al.5 | 10 | 6 | 4 | 0 | 2 | 8 | NR | Presence of MSU crystals | Yes | Yes | Primary | Renal insufficiency Obesity |
| Friedman and Little11 | 6 | 3 | 2 | 1 | 2 | 4 | Yes | ARA Criteria | No | Yes | Primary | Renal insufficiency Preeclampsia |
| Lee and Loffler13 | 6 | 5 | 0 | 1 | 4 | 2 | No | ARA Criteria | No | No | Primary | Gouty nephropathy |
| Coddington et al.15 | 2 | 2 | 0 | 0 | 1 | 1 | No | Presence of MSU crystals | No | NR | Primary | Gouty nephropathy |
| Kelsall and O¿Hanlon21 | 2 | 1 | 1 | 0 | 1 | 1 | No | Presence of MSU crystals | No | No | Primary | Renal insufficiency |
| Lhotta et al.8 | 1 | 1 | 0 | 0 | 0 | 1 | Yes | ARA Criteria | NA | NR | Secondary | Familial juvenile hyperuricemic nephropathy Renal insufficiency |
| Metyas et al6. | 1 | 1 | 0 | 0 | 0 | 1 | NR | Presence of MSU crystals | Yes | NR | Secondary | Pseudo-Bartter's syndrome due to abuse of laxatives and diuretics |
| Hassan and Gendi10 | 1 | NR | 0 | NR | 0 | 1 | Yes | Presence of MSU crystals | No | NR | Primary | Obesity, hypertension, hypothyroidism and diabetes mellitus type 2 |
| Van Veen et al.14 | 1 | 1 | 0 | 0 | 0 | 1 | NR | ARA Criteria | NR | NR | Primary | Gouty nephropathy |
| Case report | 1 | 0 | 0 | 1 | 0 | 1 | Yes | ARA Criteria Presence of MSU crystals | No | No | Primary | Renal insufficiency Dyslipidemia |
Ab: number of abortions; FHG: family history of gout; ARA: American Rheumatism Association; C: number of cesarean sections; dx: diagnosis; NP: number of pregnancies; NR: not reported; P: number of childbirths; MSU: monosodium urate.
Outcome of the pregnancies according to the relationship with the diagnosis of gout.
| Prior to diagnosis n (%) | During or after diagnosis n (%) | Total n (%) | |
|---|---|---|---|
| Abortions | 2 (18.2) | 5 (21.7) | 7 (20.58) |
| Births | 9 (81.8) | 14 (60.86) | 23 (67.64) |
| Cesarean section | 0 | 2 (8.69) | 2 (5.88) |
| Interruption of pregnancy | 0 | 1 (4.34) | 1 (2.94) |
| Not reported | 0 | 1 (4.34) | 1 (2.94) |
| Total | 11 (100) | 23 (100) | 34 (100) |
Since it is known that the outcomes of pregnancy before and after the diagnosis of rheumatic diseases are associated with different results, pregnancies that occurred prior to the diagnosis of gout were excluded in the analysis, in order to assess the effect of the disease in pregnancy. 23 pregnancies were described, which resulted in 16 (69.5%) live births, 5 (21.7%) abortions, one (4.3%) therapeutic abortion and in one case (4.3%) the outcome was not described (Table 3). The mean age at the time of the pregnancy was 30.6±3.69 years (minimum 23, maximum 37), in 4 cases there was a hereditary familial antecedent of gout. Of the available laboratory results (Appendix A, supplementary material): the mean of uric acid (UA) was 9.18±1.6mg/dl (6.5–12), hemoglobin 10.35±1.6g/dl (9–13.9), creatinine clearance 36.8±15.2ml/min (17–63), blood urea nitrogen 46.48±26.1mg/dl (12–96) and erythrocyte sedimentation rate of 37±16mm/h (24–55).
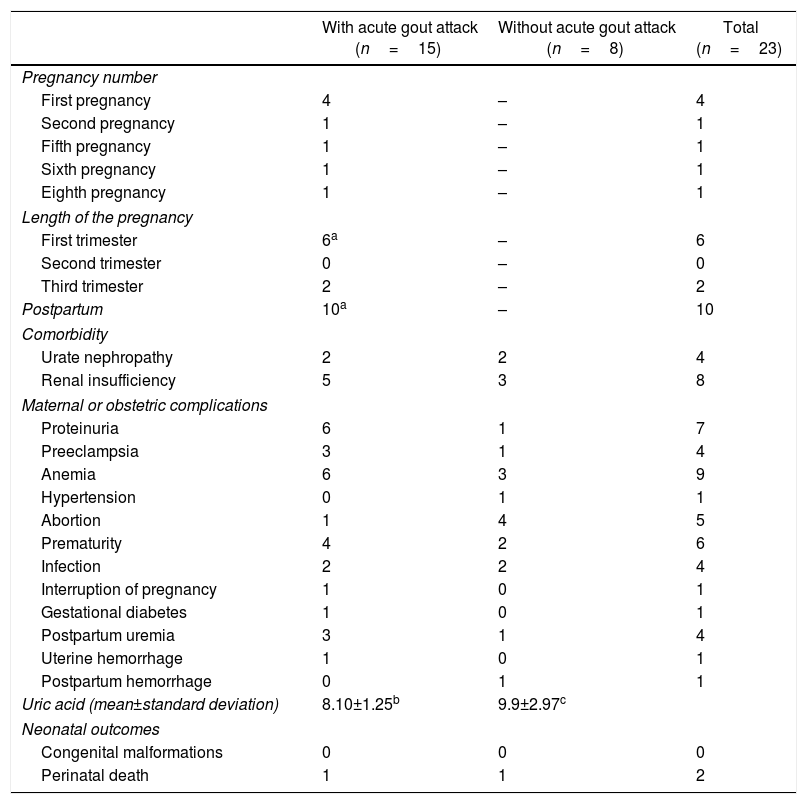
In 2 women, arthritis during pregnancy was the initial manifestation of gout. An acute attack of gout occurred in 8 pregnancies between the 7GW and one week before delivery; in 4 cases the gouty arthritis occurred during the first pregnancy. In 10 pregnancies the exacerbation of the arthritis occurred in the postpartum in a period between 18h and 11 months after delivery. The presence of proteinuria, hypertension and postpartum uremia was more frequent in women who had an acute attack of gout during pregnancy (Table 4). No maternal deaths or fetal malformations were reported. One patient had 2 neonatal deaths4; in her fourth pregnancy, the newborn obtained through pelvic delivery died 15h after due to neonatal hypoxia and subarachnoid hemorrhage; and in the seventh pregnancy, the newborn died 4 days after due to respiratory distress associated with hyaline membrane disease.4
Maternal-fetal outcomes of 23 pregnancies in women with gout classified according to the presence/absence of acute attack of gout during pregnancy or the puerperium.
| With acute gout attack (n=15) | Without acute gout attack (n=8) | Total (n=23) | |
|---|---|---|---|
| Pregnancy number | |||
| First pregnancy | 4 | – | 4 |
| Second pregnancy | 1 | – | 1 |
| Fifth pregnancy | 1 | – | 1 |
| Sixth pregnancy | 1 | – | 1 |
| Eighth pregnancy | 1 | – | 1 |
| Length of the pregnancy | |||
| First trimester | 6a | – | 6 |
| Second trimester | 0 | – | 0 |
| Third trimester | 2 | – | 2 |
| Postpartum | 10a | – | 10 |
| Comorbidity | |||
| Urate nephropathy | 2 | 2 | 4 |
| Renal insufficiency | 5 | 3 | 8 |
| Maternal or obstetric complications | |||
| Proteinuria | 6 | 1 | 7 |
| Preeclampsia | 3 | 1 | 4 |
| Anemia | 6 | 3 | 9 |
| Hypertension | 0 | 1 | 1 |
| Abortion | 1 | 4 | 5 |
| Prematurity | 4 | 2 | 6 |
| Infection | 2 | 2 | 4 |
| Interruption of pregnancy | 1 | 0 | 1 |
| Gestational diabetes | 1 | 0 | 1 |
| Postpartum uremia | 3 | 1 | 4 |
| Uterine hemorrhage | 1 | 0 | 1 |
| Postpartum hemorrhage | 0 | 1 | 1 |
| Uric acid (mean±standard deviation) | 8.10±1.25b | 9.9±2.97c | |
| Neonatal outcomes | |||
| Congenital malformations | 0 | 0 | 0 |
| Perinatal death | 1 | 1 | 2 |
In the pregnancies that ended in miscarriage, there was a remarkable absence of gouty arthritis at the end of pregnancy. Five abortions occurred spontaneously between 2 and 5 months of gestation, and a gout attack during pregnancy occurred only in one case. However, there was no information available on the evolution or the laboratory studies during these pregnancies.
A standard treatment was not followed in the cases described, in some cases even no treatment was used during pregnancy. In the cases in which treatment was prescribed, the most commonly used drugs were colchicine (n=7 [27%]) and probenecid (n=7 [27%]). Allopurinol was used in 3 cases during pregnancy (Appendix A, supplementary material), without inducing fetal malformations.
The association of gout and pregnancy was related to maternal complications: renal insufficiency (n=8 [34.7%]), preeclampsia (n=4 [17.3%]), prematurity (n=6 [26%]), infection (n=4 [17.3%]), anemia (n=9 [39.1%]), obstetric hemorrhage (n=2 [8.6%]) and postpartum uremia (n=4 [17.3%]). A blood transfusion was necessary in 6 pregnancies. Two cases presented acute renal failure; in one case it was considered associated with urate nephropathy complicated by severe preeclampsia and in the other case it was secondary to a salt-losing nephropathy.10 In 6 pregnancies was necessary the induction of labor (26.08%) due to maternal complications. In one case was necessary a cesarean section due to failed induction of labor. One pregnancy was conceived as a result of the failure of contraception and due to the history of potentially fatal complications in the previous pregnancy and to the presence of exacerbation of the arthritis during the first 8GW, therapeutic abortion was indicated at 11GW with performance of hysterectomy. The same patient had gouty arthritis after the interruption of pregnancy.11
DiscussionUnlike in other rheumatic diseases, few works have focused on the evaluation of gout during pregnancy. The case described presented a rare initial manifestation since her first clinical picture of arthritis appeared during her first pregnancy, with multiple relapses during pregnancy but with a response to the combination of glucocorticoids and colchicine. In our patient, a secondary cause of gout was not found, the urine UA/urine creatinine ratio <1 does not support the hyperproduction of UA or that the renal affection is associated with an acute urate nephropathy. Hyperuricemia with a low excreted fraction of UA indicates hyperuricemia associated with renal failure.
Hyperuricemia during pregnancy is a nonspecific alteration which is much more common during pregnancy than gout. The main causes include: preeclampsia, renal failure, severe dehydration, acute fatty liver of pregnancy, toxins and alcoholism. Hyperuricemia is a prerequisite, but in its own is not sufficient for the onset of gouty arthritis. Along with the hyperuricemia, is necessary the presence of other factors, among which stand out: genetic predisposition, obesity, use of diuretics, surgeries and chronic renal insufficiency. Despite inheritance has been strongly implicated in premenopausal patients with gout,12 a hereditary familial history was documented only in 4 women.
The complex adaptive changes that occur during pregnancy can produce a variable clinical picture of gout. Lee and Loeffler reported a beneficial influence of pregnancy on articular symptoms.13 Weingold4 reported the case of a patient who remained free of symptoms without treatment during 2 pregnancies, but with exacerbation of the arthritis in the postpartum period. The hypotheses proposed to explain the higher frequency of arthritis in the postpartum period than during pregnancy were: the high levels of estrogen during pregnancy protect the mother against an acute gout attack;13,14 the increased production of UA related to hematopoiesis after an hemorrhage during childbirth or postpartum; the process of reabsorption of hypertrophied tissues; tissue trauma, and excessive protein catabolism.4,15 These observations generated interest on the influence of pregnancy on the levels of UA. During pregnancy, the UA levels usually decrease, due to the increase in its urinary excretion, making a gout attack less likely. This is due to the physiological adaptations in pregnancy, including: hemodilution, renal hyperperfusion due to increased plasma volume, increase in the glomerular filtration rate from 30% to 50% in the first trimester, which is maintained until week 36. The estimated mean concentration of UA during the first trimester is 2.72±0.62mg/dl, in the second trimester it is 2.60±0.54mg/dl and during the third trimester it reaches the values of a non-pregnant woman (4–6mg/dl).16,17
In addition, it has been reported that serum levels of UA may increase during the first pregnancy18; in this series, most of the described cases of gouty arthritis occurred during the first pregnancy. The maternal risk factors described in hyperuricemia during pregnancy in normotensive women include: being pregnant for the first time, a younger maternal age, an excessive weight gain and the decrease in the glomerular filtration rate.18 The mechanisms by which hyperuricemia occurs in the first pregnancy remain unknown.
In the majority of the cases included, comorbidities could have favored the development of gouty arthritis, especially renal insufficiency. The kidneys play an important role in the regulation of serum levels of UA and the degree of renal insufficiency is an independent risk factor for the development of gout.19 The mechanism most commonly implied in hyperuricemia is an alteration of the renal excretion of UA, due to the reduction of glomerular filtration, the increase of tubular resorption or the decrease of tubular secretion of UA.20 Considering that the majority of cases (7 of the 8 cases) with acute gout attack during pregnancy occurred in women with chronic renal failure and that the exacerbation of arthritis in the postpartum was more frequent in patients with postpartum uremia,5 renal insufficiency was an important factor in the development of arthritis during pregnancy and the puerperium. This may be due to the fact that the decrease in the glomerular filtration rate may favor an increase in the serum levels of UA and that these fluctuations are those which trigger the arthritis. In this sense, Batt et al.5 demonstrated that the maintenance of renal function along with the treatment with colchicine and probenecid prevented the exacerbation in the postpartum.
Preeclampsia per se is associated with hyperuricemia, which may contribute to the increase in UA during pregnancy. Hyperuricemia related to preeclampsia may be due to a reduction in the renal elimination of urates, an increase in their reabsorption in the proximal tubule, renal damage associated with hypertension and hyperactivity of the enzyme xanthine oxidase in the placenta.16,17 Likewise, maternal hyperuricemia is one of the predictive factors for the severity of preeclampsia. The potentially fatal complications in the cases described were due to acute renal failure;5,11 in these 2 patients, the preeclampsia was remarkable, with a recovery of the levels of renal function previous to pregnancy and a rapid clinical improvement in the postpartum, so these complications were associated more with superimposed preeclampsia than with gouty nephropathy.
Women with gout prior to pregnancy are prone to an acute gout attack during pregnancy or the puerperium, unless preventive measures are taken. The form of presentation of gouty arthritis in pregnancy or the puerperium has changed over time, probably due to improvements in medical care. In the publications prior to the 1990s, most cases of arthritis occurred during the postpartum and only in 2 of the 17 pregnancies an acute attack occurred during pregnancy, indicating initially that pregnancy could have a favorable influence by decreasing joint symptoms. On the contrary, in the cases published after the 1990s, the majority of cases occurred during pregnancy and in no case in the postpartum period. In our case, the patient presented arthritis in the postpartum due to the suspension of the treatment established during pregnancy.
The treatment of gout during pregnancy and the puerperium can be a challenge for the physician due to the limited information on the safety of the drugs normally used in the treatment and the presence of comorbidities that can influence the therapeutic decision-making. In general, the treatment of gout during pregnancy should be focused on the monitoring and maintenance of renal function, the evaluation and treatment of comorbidities, the prevention of acute attack and the treatment of gout exacerbations if they occur during pregnancy or the puerperium. Since it is advisable to avoid the use of medications, it is recommendable to use non-pharmacological measures: rest, applying ice on the affected joint, healthy diet and maintaining adequate hydration. In none of the published cases there was evidence of the recommendation of non-pharmacological measures.
If a pharmacological treatment is necessary, the medication should be evaluated according to the indications and comorbidities of the patient, as well as to the risk/benefit ratio. Unintentional exposure to drugs can easily occur in cases of unplanned pregnancies and the patients and their doctors will require information about the use of medications during pregnancy. The majority of the drugs used in the treatment of gout are classified in category C of the Food and Drugs Administration (FDA) for their use in pregnancy. In the cases described there was no evidence of maternal-fetal toxic effects of colchicine, probenecid, allopurinol and glucocorticoids.
The pharmacological treatment of gout is divided into 2 main categories: drugs for symptomatic relief during an acute gout attack and medications to decrease UA levels. The main therapeutic agents for the treatment of acute attacks are: non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids and colchicine.
Despite NSAIDs are considered as first-line treatment for acute gout attack, they were used only in 3 cases (aspirin,13 indomethacin21 and ibuprofen in the current report). Their use during pregnancy can induce maternal-fetal adverse events. There are contradictory data on whether NSAIDs increase the risk of miscarriage, indicating that they should be used with caution in the first 2 trimesters and should not be prescribed after 30GW because of the risk of premature closure of the ductus arteriosus.22,23 Of the NSAIDs, although they are not exempt of risks, ibuprofen and naproxen do not appear to be teratogenic during the first trimester (category B of the FDA).24 Some authors have indicated that ibuprofen is the drug of choice in cases of gout attacks during pregnancy.25,26
Colchicine is considered a drug of first choice in the prophylaxis of gouty arthritis, but of second choice in the treatment of acute gout attack. Colchicine was used both for the treatment of acute gout attack and for its prophylaxis in the cases studied. The greatest experience of its use during pregnancy comes from women treated for familial Mediterranean fever and Behçet's disease, where the results indicate that its administration before or during pregnancy is not associated with adverse results.26–28 In the context of pregnant women, the indication of colchicine may be advisable in doses of 1.2–1.5mg/day one week before delivery to prevent exacerbation in the postpartum period and it is recommended to maintain it during the puerperium.5,6 In women with chronic renal insufficiency, it should be used in lower doses, because of the risk of presenting neuromuscular adverse events. In general, in subjects with a glomerular filtration rate ≤30ml/min, it is recommended to start with a dose of 0.3mg/day, and dose adjustment is not required in cases with a glomerular filtration rate ≥30ml/min.29
In patients with renal insufficiency, the drugs of choice for the treatment of acute attack of gout are glucocorticoids, especially in patients with contraindications for NSAIDs and colchicine. Prednisone, prednisolone, and methylprednisolone do not cross the placental barrier, and no serious effects have been described when doses ≤30mg of prednisone or its equivalent during pregnancy are used.30 Intra-articular injections of glucocorticoids are recommended in cases of arthritis of 1 or 2 joints.29 In our case, and in one published case,6 remission of the arthritis was achieved with the intra-articular infiltration.
For the treatment of hyperuricemia, probenecid 0.5g 3 times a day is the drug of choice for the reduction of UA; the available data do not indicate any evidence of teratogenic effects when it is used during pregnancy (category B of the FDA).25,31 Probenecid is metabolized by the liver and is excreted primarily in the urine. In subjects with mild renal insufficiency (glomerular filtration≥50ml/min) the dose adjustment is not necessary, but in patients with a glomerular filtration≤30ml/min is not effective and should be avoided.29,31 Because allopurinol is structurally similar to xanthines, there is a theoretical possibility that it will be incorporated into the nucleic acids of the embryo. In the cases described, allopurinol was used during pregnancy in 2 cases14,15 and during the first trimester in other 2, without reporting adverse events. However, the possible teratogenicity of allopurinol is known32 and it is recommended to avoid its use, especially in the first trimester. The administration of allopurinol does not justify the termination of pregnancy, but treatment should be changed to probenecid and a detailed fetal echographic evaluation is recommended.25,26 There is limited information on the use of febuxostat during pregnancy in humans and, currently, its use is considered contraindicated.
The limitations inherent to the quality of the data that can be derived from retrospective studies are the main limitation of this study. Gout in pregnancy is an uncommon condition and the publications are based on case reports with limited data for their comparison, differences in the evaluation time in the publications make generalization difficult (level of evidence: iii).33 Regarding the outcome of the pregnancies, it should be mentioned that 6 of the 12 publications included were published between the years 1950 and 1960, a period with higher obstetric morbidity than at present. In the same way, a publication bias cannot be ruled out, since recently published articles include only patients with acute gout attack during pregnancy. There are no evidence-based recommendations for the treatment of gout in pregnancy and the puerperium. The recommendations of this study are based on the information available (grade of recommendation: C).33
To our best knowledge, this is the first systematic review that evaluates the association between gout and pregnancy. A definitive statement about the potential interaction between gout and pregnancy cannot be made on the basis of the cited cases. More studies are needed to confirm the effect of gout in pregnancy. On the basis of what has been described above, the treatment of choice for acute gout attacks during pregnancy would be ibuprofen or naproxen, and glucocorticoids in patients with chronic renal insufficiency, probenecid for the treatment of hyperuricemia, and colchicine to prevent acute gout attacks during pregnancy or after delivery.
Finally, we can conclude that gout is a rare disease in women of reproductive age. However, it should constitute a differential diagnosis of arthritis in pregnant women. A conclusive relationship between gout and complications during pregnancy cannot be established due to the limited number of available cases; the limited data on the cases described and the differences in the evaluation time in the publications which make it difficult to generalize. While the majority of women with gout have healthy newborns, mothers are at higher risk of complications, which indicates that pregnancy should be monitored closely.
Ethical aspectsThe written consent of the patient was obtained for the publication of this case report. The manuscript does not contain clinical studies or data that could identify the patient.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Horta-Baas G, Hernández-Cabrera MF, Vergara-Sánchez I, Romero-Figueroa MD. Gota y embarazo: revisión sistemática, incluyendo el reporte de un nuevo caso. Rev Colomb Reumatol. 2017;24:219–229.