To assess the impact of educational level on rheumatoid arthritis through a systematic review of the literature and assessing the age at onset, the educational level as risk factor, and to identify outcomes related to educational level and rheumatoid arthritis.
MethodsA systematic review was performed using PubMed, SciELO and LILACS as data bases in order to identify original articles written in English, Portuguese or Spanish that used accepted classification criteria for rheumatoid arthritis and a comparison was made between educational level and outcomes of rheumatoid arthritis. Final articles were identified by two independent reviewers and three blinded reviewers created a new list and extracted data from selected studies. Each record was classified based on the quality score of the studies.
ResultsThrough the systematic review of the literature, the factors and outcomes related to educational level and rheumatoid arthritis identified were: the risk of developing the disease, radiographic progression, depression and anxiety, work disability, functional disability, quality of life, and mortality.
ConclusionThe information available in the literature about the impact of the educational level in several outcomes related to rheumatoid arthritisis variable. Only work disability is an outcome related to a low education level in all the articles reviewed.
Evaluar el impacto del nivel educativo sobre la artritis reumatoide a través de una revisión sistemática de la literatura analizando la edad de inicio, el nivel educativo como factor de riesgo e identificando los desenlaces relacionados con el nivel educativo y la artritis reumatoide.
MétodosSe realizó una revisión sistemática en PubMed, SciELO y LILACS como bases de datos con el fin de identificar artículos originales en inglés, portugués o español, que utilizaban criterios de clasificación aceptados para artritis reumatoide y comparaban el nivel educativo con diferentes desenlaces de la enfermedad. Los artículos finales fueron identificados por 2 revisores independientes. Tres revisores ciegos crearon una lista y extrajeron los datos de los estudios seleccionados. Cada registro fue clasificado en función de la calidad de los estudios.
ResultadosLos factores y desenlaces identificados relacionados con el nivel educativo y artritis reumatoide fueron el riesgo de desarrollar la enfermedad, la progresión radiográfica, depresión y ansiedad, incapacidad laboral, incapacidad funcional, calidad de vida y mortalidad.
ConclusiónLa información disponible en la literatura sobre el impacto del nivel educativo en la artritis reumatoide en los diferentes desenlaces encontrados es variable. Solo la discapacidad funcional es un desenlace relacionado con bajo nivel educativo en todos los artículos encontrados.
Rheumatoid arthritis (RA) is a systemic, multifactorial autoimmune disease (AD) with an end-stage of chronic inflammation in which there is a response directed toward the diarthrodial joints. Genetic, epigenetic and environmental factors have been implicated, and because of its multifactorial etiology, there is a lot of interest in elucidating factors that could be involved in disease predisposition, onset and outcomes. All of this explains the growing interest in modifiable risk factors.1
During the last few years, several studies have been done to determine the effect of formal educational level on the course of RA. Some studies have found a negative association between a low educational level (LEL) and some outcomes of RA. In a nine-year study of a group of patients with RA, Pincus et al.2 reported that LEL was a significant risk factor for mortality. Leight et al.3 demonstrated that 1 year of schooling is associated with a 0.29% average increase in length of survival.
In other reports, LEL has been associated with a poorer clinical status (e.g., higher erythrocyte sedimentation rate [ESR], higher ratings of pain, greater number of painful joints).4 Wallenius et al.5 showed that LEL was associated with work disability (WD). Rodriguez et al.6 demonstrated more aggressive disease based on findings of increased erosion rate in patients with LEL.
The educational level influences the course of the disease just as it does in other contexts, such as osteoarthritis (OA). Slatkowsky-Christensen et al.7 found that knee OA was more prevalent among people with LEL even after controlling for known risk factors such as age, knee injury, race, obesity and occupation.
A preventive effect of high educational level (HEL) has been reported. In one case–control study done in Sweden, HEL was associated with a decreased risk of RA.8 However, some studies have shown the opposite association, which is that HEL is related to a more severe form of the disease as well as to an earlier onset as shown by Rodriguez et al.6
Consequently, positive and negative effects on the course of RA have been associated with a patient's educational level. However, there is no a paper that synthesizes such evidence. Thus, the aim of this study was to assess the impact of educational level on rheumatoid arthritis (RA) through a systematic review of the literature.
Materials and methodsSystematic literature reviewThe guidelines proposed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were followed.9 A systematic review was done in the following databases: PubMed, SciELO and LILACS, and included articles published up until September, 2013. The review was performed during October 2013. The last date of search was October 25, 2013.
In PubMed the search was done with the following Medical Subject Headings (MeSH) terms: “Education,” “Arthritis, Rheumatoid,” “Educational Status,” “Information Literacy,” “Health Literacy,” “Minority Health,” “Early Intervention (Education).” Also we searched using [Majr] terms, which included “educational level,” “rheumatoid arthritis,” “literacy,” “low educational level,” “education level,” “rheumatoid arthritis erosive,” “education,” “rheumatoid arthritis activity,” and “formal education.”
In addition, MeSH terms were translated into DeCS (Health Sciences Descriptors), the tool that makes it possible to navigate between records and sources of information through controlled concepts and is organized in Portuguese, Spanish and English, in order to search LILACS and SciELO. The terms used were: “Education,” “rheumatoid arthritis,” “Educación,” “artritis reumatoide,” “Educação,” “Artrite Reumatóide,” “Alfabetización.”
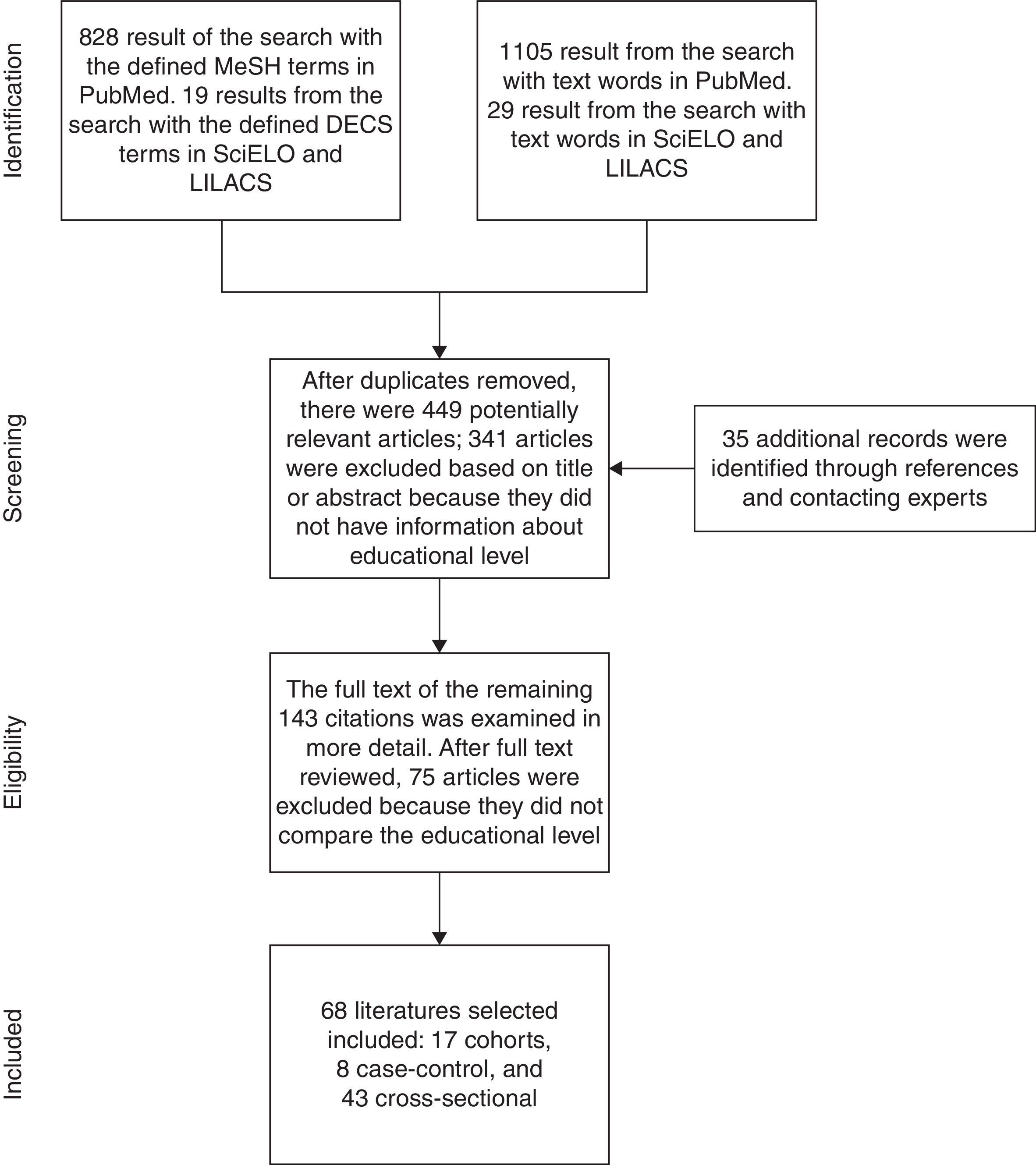
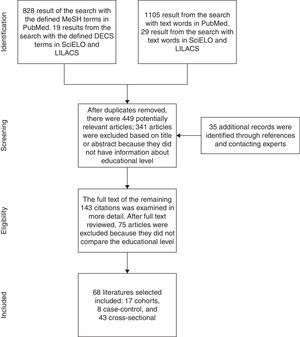
Study selection, data extraction, and quality assessmentFirst, articles were identified through a systematic search done by two independent reviewers (CALC and RCR) applying the same selection criteria. Based on the abstracts and titles, we selected references from the articles that seemed to be relevant for the review. The two resulting lists were compared and disagreements resolved by consensus. After that, of those studies that were eligible, we looked for the full text, and contacted the authors of the articles that were not easily available. In addition, a hand search was done based on the references listed in the full-text articles that we had. Additional studies were identified by contacting clinical experts. Finally, we looked for duplicates and excluded them. The process followed is illustrated in Fig. 1.
Finally, three blinded reviewers (CALC, RCR, and ZDS) created a new list and extracted data from selected studies. Each record was classified based on the quality score of the studies using the levels established by the Oxford Centre for Evidence-Based Medicine 2011.10 The following data were obtained: name of study, author, year of publication, language, country, design of study, level of evidence, number of patients, classification of educational level, prevalence of classification, outcome, and results. When the article had insufficient data or had inadequate statistical power, it was removed.
Inclusion criteria for the systematic review were the following: original articles that used accepted classification criteria for RA and compared educational level with outcomes of RA, and written in English, Portuguese or Spanish. Exclusion criteria were the following: animal models, non-RA patients, juvenile rheumatoid arthritis, review, case report, other topic, and outcomes not related to RA.
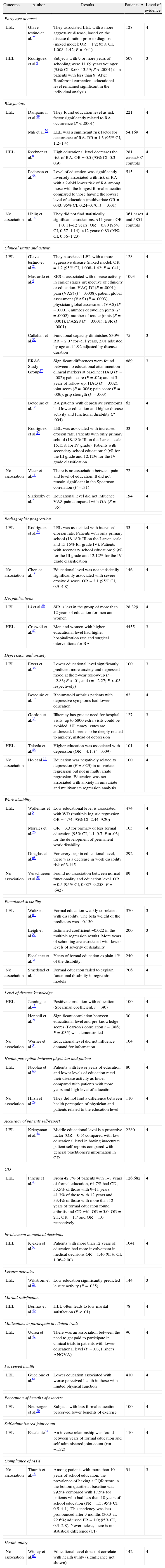
ResultsThe search of PubMed with the defined MeSH terms provided a total of 828 citations and [Majr] terms provided 1105. The search with the defined DECS Terms in SciELO and LILACS provided 19 citations and text words provided 29. After adjusting for duplicates, 449 remained. Of these, 341 studies were discarded after reviewing the titles or abstracts because these papers clearly did not meet the criteria. Finally, 108 papers remained. An additional 35 articles that met the criteria for inclusion were identified by checking the references of located, relevant papers and contacting experts. The full text of the remaining 143 citations was examined in more detail. It appeared that 75 studies did not meet the inclusion criteria as described. Finally, 68 studies2–8,11–69 which did meet the inclusion criteria were included in the systematic review. These included 17 cohorts, 8 case–controls, and 43 cross-sectional (Fig. 1). Table 1 summarizes the selected articles with their respective associations and P-values. The outcomes observed were risk, early age at onset, clinical status, activity and radiographic progression, depression and anxiety, work disability, functional disability, level of disease knowledge, quality of Life (QoL), received treatment and mortality. Details of the search can be found in supplementary material available online.
Outcomes associated to education level.
| Outcome | Author | Results | Patients, n | Level of evidence |
| Early age at onset | ||||
| LEL | Glave-testino et al.25 | They associated LEL with a more aggressive disease, based on the disease duration prior to diagnosis (mixed model: OR=1.2; 95% CI, 1.008–1.42; P=.041) | 128 | 4 |
| HEL | Rodriguez et al.6 | Subjects with 9 or more years of schooling were 11.09 years younger (95% CI, 8.60–13.59; P<.0001) than patients with less than 9. After Bonferroni correction, educational level remained significant in the individual analysis | 507 | 3 |
| Risk factors | ||||
| LEL | Damjanovi et al.49 | They found education level as risk factor significantly related to RA occurrence (P<.0001) | 221 | 4 |
| Mili et al.50 | LEL was a significant risk factor for occurrence of RA. RR=1.3 (95% CI, 1.2–1.4) | 54,169 | 4 | |
| HEL | Reckner et al.8 | High educational level decreases the risk of RA. OR=0.5 (95% CI, 0.3–0.9) | 281 cases/507 controls | 4 |
| Pedersen et al.58 | Level of education was significantly inversely associated with risk of RA with a 2-fold lower risk of RA among those with the longest formal education compared to those having the lowest level of education (multivariate OR=0.43; 95% CI, 0.24–0.76; P=.001) | 515 | 4 | |
| No association | Uhlig et al.18 | They did not find statistically significant associations. <11 years: OR=1.0. 11–12 years: OR=0.80 (95% CI, 0.57–1.14). >12 years: 0.83 (95% CI, 0.56–1.23) | 361 cases and 5851 controls | 4 |
| Clinical status and activity | ||||
| LEL | Glave-testino et al.25 | They associated LEL with a more aggressive disease (mixed model: OR=1.2 (95% CI, 1.008–1.42; P=.041) | 128 | 4 |
| Massardo et al.4 | SES is associated with disease activity in earlier stages irrespective of ethnicity or education. HAQ-DI (P=.0001); pain (VAS) (P=.0008); patient global assessment (VAS) (P=.0003); physician global assessment (VAS) (P=.0001); number of swollen joints (P=.0002); number of tender joints (P=.0001); DAS28 (P=.0001); ESR (P=.0001) | 1093 | 4 | |
| Callahan et al.32 | Functional capacity diminishes ≥30% RR=2.07 for <11 years, 2.01 adjusted by age and 1.92 adjusted by disease duration | 75 | 3 | |
| ERAS Study Group57 | Significant differences were found between no educational attainment on clinical markers at baseline: HAQ (P=.002); pain score (P=.02); and at 3 years of follow up. HAQ (P=.002); joint score (P=.006); pain score (P=.006); grip strength (P=.003) | 689 | 3 | |
| Botequio et al.19 | RA patients with depressive symptoms had lower education and higher disease activity and functional disability (P=.004) | 62 | 4 | |
| Rodriguez et al.24 | LEL was associated with increased erosion rate. Patients with only primary school (18.18% III on the Larsen scale, 15.15% for IV grade). Patients with secondary school education: 9.9% for the III grade and 12.12% for the IV grade classification | 33 | 4 | |
| No association | Vlaar et al.11 | There is no association between pain and level of education. It did not remain significant in the Spearman correlation (P=.31) | 72 | 4 |
| Slatkosky et al.7 | Educational level did not influence VAS pain compared with OA (P=.35) | 194 | 4 | |
| Radiographic progression | ||||
| LEL | Rodriguez et al.24 | LEL was associated with increased erosion rate. Patients with only primary school (18.18% III on the Larsen scale, and 15.15% for grade IV). Patients with secondary school education: 9.9% for the III grade and 12.12% for the IV grade classification | 33 | 4 |
| No association | Chen et al.15 | Educational level was not statistically significantly associated with severe erosive disease. OR=2.1 (95% CI, 0.9–4.8) | 146 | 4 |
| Hospitalizations | ||||
| LEL | Li et al.56 | SIR is less in the group of more than 12 years of education for men and women | 28,329 | 4 |
| HEL | Criswell et al.47 | Men and women with higher educational level had higher hospitalization rate and surgical interventions for RA | 4455 | 3 |
| Depression and anxiety | ||||
| LEL | Evers et al.36 | Lower educational level significantly predicted more anxiety and depressed mood at the 5-year follow-up (t=−2.83; P<.01, and t=−2.27; P<.05, respectively) | 100 | 3 |
| Botequio et al.19 | Rheumatoid arthritis patients with depressive symptoms had lower education | 62 | 4 | |
| Gordon et al.33 | Illiteracy has greater need for hospital visits, up to 6800 extra visits could be avoided if illiteracy issues are addressed. It seems to be deeply related to anxiety, instead of depression | 127 | 3 | |
| HEL | Takeda et al.46 | Higher education was associated with depression (OR=4.1; P=.009) | 101 | 4 |
| No association | Ho et al.14 | Education was negatively related to depression (P=.029) in univariate regression but not in multivariate regression. Education was not associated with anxiety in univariate and multivariate regression analysis. | 100 | 4 |
| Work disability | ||||
| LEL | Wallenius et al.5 | Low educational level is associated with WD (multiple logistic regression, OR=4.74; 95% CI, 2.44–9.20) | 474 | 4 |
| Morales et al.26 | OR=3.3 for primary or less formal education (95% CI, 1.1–9.7; P=.03) for the development of permanent work disability | 105 | 4 | |
| Doeglas et al.68 | For every step in educational level, there was a decrease in work disability risk of 3.145 | 292 | 4 | |
| No association | Verschueren et al.38 | Found no association between normal functionality and education level. OR=0.5 (95% CI, 0.027–9.258; P=.642) | 89 | 4 |
| Functional disability | ||||
| LEL | Waltz et al.64 | Formal education weakly correlated with disability. The beta weight of the predictors was −0.130 | 370 | 3 |
| Leigh et al.55 | Estimated coefficient −0.022 in the multiple regression results. More years of schooling are associated with lower levels of severity of disability | 200 | 3 | |
| Escalante et al.31 | Years of formal education explain 4% of the disability. | 240 | 4 | |
| No association | Smedstad et al.17 | Formal education failed to explain functional disability in regression models | 706 | 4 |
| Level of disease knowledge | ||||
| HEL | Jennings et al.13 | Positive correlation with education (Spearman coefficient, r=.40) | 100 | 4 |
| Hennell et al.21 | Significant correlation between educational level and pre-knowledge scores (Pearson's correlation r=.386; P=.035) was demonstrated | 30 | 4 | |
| No association | Werner et al.34 | Educational level did not influence demand for information | 104 | 4 |
| Health perception between physician and patient | ||||
| LEL | Nicolau et al.60 | Patients with fewer years of education and lower levels of education rated their disease activity as lower compared with patients with more years and high level of education | 80 | 4 |
| No association | Hirsh et al.29 | They did not find a difference between health perception of physician and patients related to the education level | 110 | 4 |
| Accuracy of patients self-report | ||||
| LEL | Kriegsman et al.54 | Middle educational level is a protective factor (OR=0.5) compared with low educational level in having inaccurate patient self-reports compared with general practitioner's information in CD | 2280 | 4 |
| CD | ||||
| LEL | Pincus et al.43 | From 42.7% of patients with 1–8 years of formal education, 64.7% had CD, 53.5% of those with 9–11 years, 41.3% of those with 12 years and 33.4% of those with more than 12 years of formal education found arthritis and CD with OR=5.0, OR=2.1, OR=1.7 and OR=1.0 respectively | 126,682 | 4 |
| Involvement in medical decisions | ||||
| HEL | Kjeken et al.52 | Patients with more than 12 years of education had more involvement in medical decisions OR=1.46 (95% CI, 1.06–2.00) | 1041 | 4 |
| Leisure activities | ||||
| LEL | Wikstrom et al.23 | Low education significantly predicted leisure activity (P=.035) | 144 | 3 |
| Marital satisfaction | ||||
| HEL | Bermas et al.40 | HEL often leads to low marital satisfaction (P<.01) | 78 | 4 |
| Motivations to participate in clinical trials | ||||
| LEL | Udrea et al.42 | There was an association between the need to get paid to participate in clinical trials in patients with lower educational level (P=.03, Fisher's ANOVA) | 96 | 4 |
| Perceived health | ||||
| LEL | Guccione et al.61 | Lower education associated with worse perceived health in those with limited physical function | 410 | 4 |
| Perception of benefits of exercise | ||||
| LEL | Neuberger et al.20 | Subjects with less formal education perceived fewer benefits of exercise | 100 | 4 |
| Self-administered joint count | ||||
| LEL | Escalante67 | An inverse relationship was found between years of formal education and self-administered joint count (r=−1.32) | 110 | 4 |
| Compliance of MTX | ||||
| No association | Thurah et al.16 | Among patients with more than 10 years of school education, the prevalence of having a CQR score in the bottom quartile at baseline was 29.5% compared with 17.5% for patients who had less than 10 years of school education (PR=1.5; 95% CI, 0.5–4.1). This tendency was less pronounced after 9 months (30.3 vs. 22.6%; adjusted PR=1.0; 95% CI, 0.3–2.8). Nevertheless, there is no statistical difference (CI) | 91 | 3 |
| Health utility | ||||
| No association | Witney et al.62 | Educational level does not correlate with health utility (significance not shown) | 142 | 4 |
CD, chronic diseases; CI, confidence interval; CQR, The Compliance Questionnaire-Rheumatology; DAS28, disease activity score; ESR, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire for Rheumatoid Arthritis; HEL, high education level; LEL, low education level; MTX, methotrexate; OR, odds ratio; PR, prevalence ratio; RA, rheumatoid arthritis; RR, relative risk; SIR, standardized incidence ratio; VAS, visual analog scale; WD, work disability.
Several outcomes and their association with educational level in RA were identified through the systematic review. In the following subsections, we will discuss those outcomes that were studied the most or most frequently established.
Respecting LEL prevalence, in the case–control study by Pedersen et al., 58 when we added together the frequencies of the groups with 9 or fewer years, they came to 43.5%. In a work that compared educational level with physical activity and QoL in chronic diseases including RA, 56.3% of the RA patients had 9 or fewer years of schooling.18 De Man et al.12 reported a LEL frequency of 7.2% in Netherlands, Archenholtz et al.37 reported 16% in Sweden, and 19.3% in United States.57 Noteworthy, it is difficult to compare the results of the different studies given the different policy statements of each country regarding the classification of educational level. For example, few articles used 9 or less schooling years to classify the educational level as low.18,25,49,50,58 Indeed, educational level was only reported as a discrete variable in many of the articles reviewed.
SES and household dutiesRecently an article by Massardo et al.4 evaluated the disease activity in early RA patients from many Latin-American countries. In this study, SES was associated with disease activity in the earlier stages irrespective of ethnicity or education. Despite this, assuming formal educational level to be a surrogate of SES, there is a linear relationship to exist between LEL and low SES.
With respect to the relation between LEL and household duties, a report by the Census of the United States showed that a high percentage of housewives are Hispanic, and one of the main reasons is their LEL.70 In fact, in a study of low-income Latinas with arthritis, 38% of them with homemaker status, the mean±standard deviation of education level was 8.4±4.0 years, that is below the present results.71 On the contrary, in a survey of RA patients coming from a developed country (Canada), more hours of unpaid work (including household duties) were associated with having a post-secondary education in addition to more children in the household, greater perceived physical and psychological demand of the work and social support from family.72
Early age at onsetThe information available in the literature about this association is variable. It is well known that early RA is associated with more aggressive disease based on the disease duration prior to diagnosis as observed by Glave-Testino et al.25 These authors suggested that LEL is associated with longer disease duration due to early age at onset, which contrasts with the results of Rodriguez et al.,6 who showed an association between HEL and early onset through a study where subjects with 9 or more years of schooling were 11.09 years younger than patients with less than 9 years.
Perception of disease could explain why people with HEL had an earlier onset of symptoms compared to subjects with LEL, because patients with HEL may be more concerned about the symptoms of the disease and report this aspect more accurately. Correctly reporting the age at onset of symptoms is also subject to the memory of the participants, another possible explanation for why HEL was associated with earliest onset. Better educated individuals might remember when their symptoms started more accurately based on the findings by Farmer et al.73 who found a better cognitive functionality in patients with HEL. However, prospective studies are needed in order to confirm these results.
Another fact that may explain the relationship between HEL and early age at onset is the hardscrabble hypothesis that refers to the increase in the incidence of ADs in connection with higher socioeconomic status.74 Recently, a trend toward an increase in the incidence of AD with a rising SES has been reported.75–78 Chronic tissue damage leads to a phenomenon of toleration in which the immune system has evolved to “expect” a certain level of damage-related antigen exposure. In situations where this damage is reduced, there is under-presentation of self-antigens, reduced toleration, and an accompanying increased risk of AD.74 In the case of the LEL, if we understand it as a surrogate of low SES, there is an ancestral environment that promotes tissue damage. Since those patients with HEL have been removed from that ancestral environment, the development of early-onset RA is now favored.
Educational level as a risk factor to RAEducational level has been reported as a risk factor for RA. Reckner-Osslon et al.8 demonstrated that HEL decreased the risk for RA in a case–control study done in Sweden with 281 cases and 507 controls. A Swedish study found an inverse association between higher education and incident RA, especially RF+ disease (odds ratio [OR]=0.3 [0.2–0.7] for men with schooling beyond compulsory education).17 In a population from Bosnia and Herzegovina with 221 cases and 300 controls, LEL was a significant risk factor for the occurrence of RA.49 In a study done in the United States, with 54,169 subjects, a higher rate of prevalence of RA was found in the LEL group.51 In a Danish study published in 2006, having 10 or more years of schooling was found to be protective against the risk of having RA in comparison to having 7 or less years of schooling.58 In contrast, Uhlig et al.18 did not find an association between formal education and risk of RA.
Clinical status, activity and radiographic progressionArticles from our systematic review reported that LEL is associated with higher disease activity and earlier onset of RA, as reported by Massardo et al.4 and by Glave-Testino et al.25 In the first study, a more severe clinical status was found in RA patients with LEL given by higher ESR, joint count, visual scale analog (VAS), and global self-assessment. The latter author found a more aggressive disease in patients with LEL and argued that it was due to longer disease duration. The ERAS Study Group57 found that HAQ and pain scores at presentation were related to a lower level of education, but the ESR, joint scores and grip strength were not. After 3 years, all these clinical markers, except ESR, showed evidence that their tendency with respect to educational attainment remained the same, e.g., the more highly educated patients had more favorable scores. Callahan et al.32 reported the educational level as a quantitative marker which identifies a surrogate or composite variable associated with increased morbidity in RA. Hirsch et al.35 observed that higher health literacy was associated with lower scores on a multidimensional health assessment questionnaire (MDHAQ). Interestingly, some patients with LEL often classified their disease as less active compared to the physicians’ assessment as pointed out by Nicolau et al.60
LEL was associated with worse perceived health by patients with limited physical function as reported by Guccione et al.61 Even if depression is taken into account, as was done by Botequio-Mella et al.,19 LEL is related to higher disease activity in depressed patients.
LEL has shown a weaker association with pain when this variable is included in the equation as an estimate of disease activity64,65 and its association with disability and emotional support was also weak.64 Other articles found no association between pain and education level.29 When compared to patients with hand osteoarthritis (HOA), RA patients had fewer formal years of education, worse physical functioning and less pain than patients with HOA, but no association was found regarding educational level.7
With respect to the radiologic progression, no statistically significant association was found between educational level and erosions by Chen et al.,15 but are contrary to those reported by Rodriguez et al.,24 where LEL was associated with an increased erosion rate.
Depression and anxietySome articles within the systematic review reported associations of educational level with depression and anxiety. One study found an association between HEL and depression in RA patients.46 In another, education was negatively related to depression in univariate but not in multivariate models and was not associated with anxiety.14 Two other studies found no association of depression with LEL.62,79 However, three studies found a positive association between LEL and depression and/or anxiety. Evers et al.36 found that anxiety and depression were more frequent in patients with LEL and that it was even more frequent in patients who had WD. In a Brazilian study done by Botequio-Mella et al.,19 depressive symptoms had an association with LEL, higher disease activity, and functional disability. Gordon et al.33 showed that illiterate RA patients had more hospital visits that seemed to be related to anxiety.
Work disabilityLEL has been associated with WD in several studies.2,5,26,53,68 One Mexican study by Morales-Romero et al.26 demonstrated that work-disabled women were more likely than working women to have a lower than high school level education or a nonprofessional occupation, compared with the limited association of these variables with work disability in men.22 Doeglas et al.68 showed that with a higher educational level, there was a reduction in work disability risk. Nevertheless, LEL was not a predictor for changing jobs due to disability in a Belgic population.38 Regarding disability pension, those with more than 11.2 years of formal education received less in work disability payments than those with fewer years of education according to the findings of Callahan et al.32
Functional disabilityResults regarding this outcome are not homogenous. In a cross-sectional study of 706 patients from four European countries that evaluated functional disability in early RA, formal education failed to explain functional disability in regression models.17 Other studies have indicated that LEL is associated with more disability as reported by Waltz et al.64 and Leigh et al.55 Escalante et al.31 carried out a study where 4% of disability could be explained by LEL. However, these studies are not comparable since they were done on populations with different RA characteristics and using different classifications of educational level.
Level of disease knowledgeThe study by Jennings et al.13 found a positive association between the level of disease knowledge and education using the Patient Knowledge Questionnaire (PKQ) as Henell et al.21 did, who demonstrated an association between educational level and pre-knowledge scores. In contrast, Werner et al.34 found no association between formal education and disease knowledge using a VAS of disease knowledge.
Quality of lifeIn the review we found that weak or no associations between QoL and educational level were reported. A study by Slatkosky-Christensen et al.7 found no differences in the influence of educational level on QoL when RA was compared to HOA. Salaffi et al.30 compared RA with fibromyalgia and found that patients with RA had more physical limitations which were associated with widespread pain, BMI and educational level. The study by Arne et al.44 found that patients with chronic diseases, including chronic obstructive pulmonary disease (COPD) and RA had a worse QoL and a lower level of psychological well-being. In addition, when comparing these patients to healthy ones, they found that they had a LEL. However, their results should be examined carefully, because they disclosed that there may have been a selection bias in RA patients due to the fact that it was a self-report sent in by mail and that RA prevalence was greater than COPDs. Hurst et al.41 found an association between disability and impairment measures. Within this association formal education was not found to be a significant predictor. Gurdesh et al.51 found no influence of educational level on health-related QoL in Indian patients.
TreatmentIn the systematic review, one article that studied this relationship was found. The study by Criswell et al.47 showed that women with more than 17 years of education had a higher use of hydroxichloroquine and a lower use of the combination of non-steroidal anti-inflammatory drugs (NSAID) plus prednisone. Additionally, men with LEL received less hydroxichloroquine with a higher use of d-penicillamine.47 Treatment adherence was studied by Garcia et al.66 and HEL was associated with better adherence.
MortalityFormal education represents a very important and well described mortality predictor in RA.28,45 In a Cuban study, patients with 8 or 9 years of formal education had a lower mortality rate than patients with less than 8 years of education.48 In an effort to identify possible specific prognostic markers in RA, Pincus et al.2 studied 75 patients over a 9-year period. The results showed higher mortality in RA patients with a lower educational level. Further analysis indicated that patients with LEL showed significantly higher mortality over the period under study. These associations were not explained by age, duration of disease, functional measures, or therapies used. Leight et al.3 reported that 1 more year of schooling was associated with a 0.29% average increase in length of survival by applying a Weibull model. In 1996, Callahan et al.27 hypothesized that formal education may represent a surrogate for cognitive or behavioral variables that may affect health status. Thus, it would reflect associations between poorer psychological, social, and behavioral characteristics and, as a result, increase mortality in many diseases including RA as part of a psychosocial model of disease.
In general, the information available in the literature about the impact of the educational level in several outcomes related to RA is variable. Only work disability is an outcome related to LEL in all papers reported.
It is important to note that the educational status cannot be a risk factor itself, but a surrogate marker of an entire biopsychosocial process, which can also be a consequence and not the cause of the disease and its severity. Hence, in the future the realization of cohort studies and clinical interventions to assess the impact of each of the outcome with the aim of improving the quality of evidence on this topic should be considered.
ConclusionsEducational level significantly can influence the risk and clinical course of RA; moreover it can be assessed with studies of greater statistical power as meta-analysis, which we did not carry out, due to the several outcomes found in this review and the heterogeneity in the study designs which may contain bias and unmeasured confounding factors not assessed in this article. A drawback, for example, is the fact that the definition used to classify HEL or LEL is variable in several papers; even in some cases it is handled as a quantitative variable and as a discrete variable in other cases, making it difficult to perform a more complete analysis of the available data. It is necessary to perform meta-analysis for each outcome found that properly analyzes the impact of education in the course of RA.
FundingThis work was supported by the Universidad del Rosario.
Conflict of interestThe authors declare no conflict of interest.
The authors express their gratitude to their colleagues at the CREA for their fruitful discussions and contributions.