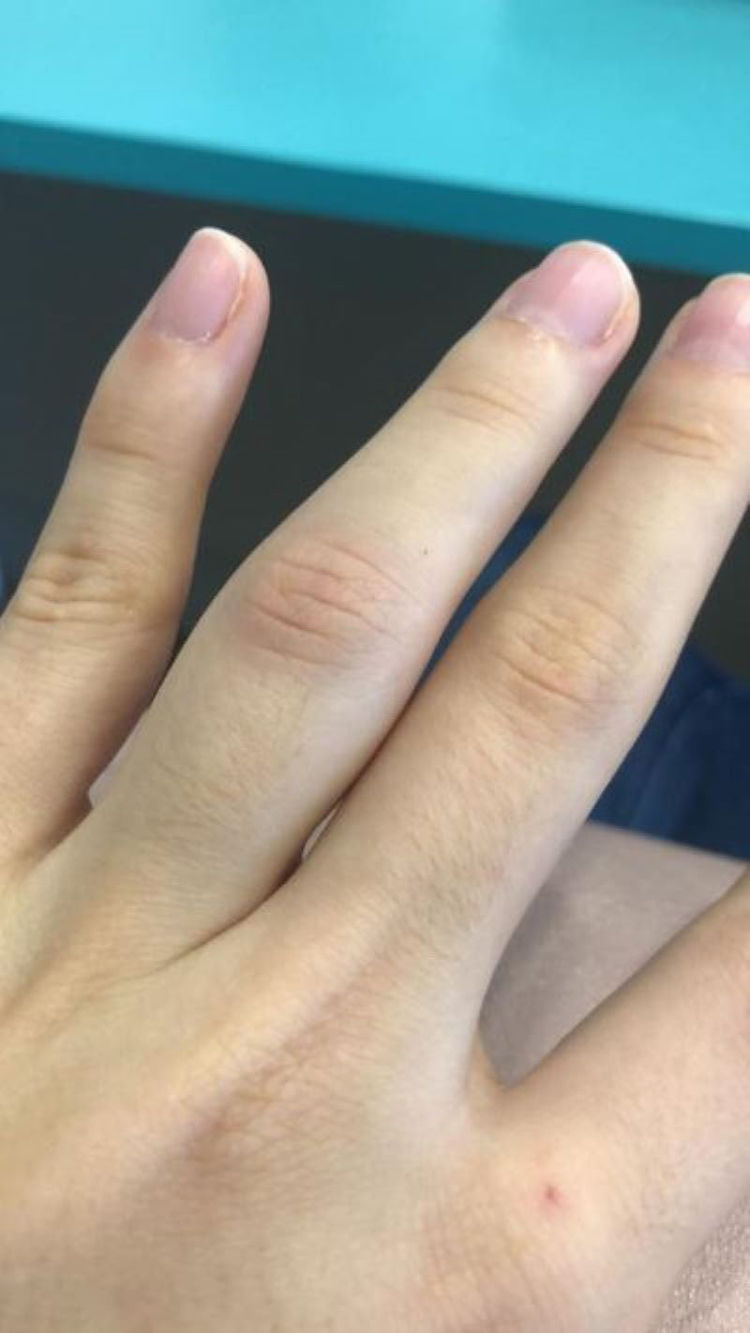
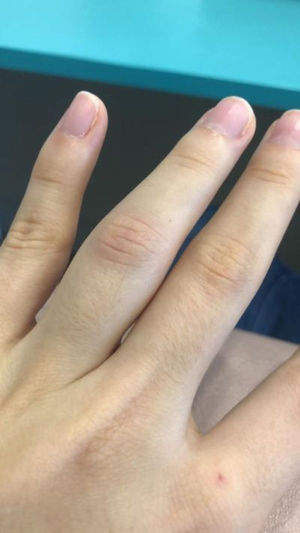
We describe the case of a 25-year-old woman who presented with monoarthritis of the proximal interphalangeal articulation of the fourth left finger 4 days after receiving the second dose of the Oxford-AstraZenecaR SARS-CoV-2 vaccine. She had no abnormalities in her X-ray or blood exams, but she had a cousin diagnosed with juvenile rheumatoid arthritis. The patient had an excellent response to anti-inflammatory medication, the arthritis was transient and left no sequelae. Studies have shown some vaccines may be associated with acute arthritis, in particular the measles–mumps–rubella vaccine. Young women such as our patient seem to be more susceptible to post-vaccination arthritis. Most of the cases reported were transient and left no articular sequelae, thus we did not contraindicate further doses of the Oxford-AstraZenecaR SARS-CoV-2 vaccine (should they be recommended in the future) in this case.
Describimos el caso de una mujer de 25 años que presentó monoartritis de la articulación interfalángica proximal del cuarto quirodáctil izquierdo 4 días después de recibir la segunda dosis de la vacuna Oxford-AstraZenecaR SARS-CoV-2. No presentaba anomalías en sus radiografías ni en los exámenes de sangre, pero tenía una prima diagnosticada con artritis reumatoide juvenil. La paciente tuvo una excelente respuesta a la medicación antiinflamatoria, la artritis fue transitoria y no dejó secuelas. Los estudios han demostrado que algunas vacunas pueden estar asociadas con la artritis aguda, en particular las vacunas contra el sarampión, las paperas y la rubéola. Las mujeres jóvenes como nuestra paciente parecen ser más susceptibles a la artritis posvacunación. La mayoría de los casos notificados fueron transitorios y no dejaron secuelas articulares, por lo que no contraindicamos nuevas dosis de la vacuna Oxford-AstraZenecaR SARS-CoV-2 (en caso de recomendarse en el futuro) en este caso.
The quick spread of the COVID-19 pandemic has launched a race to develop efficient vaccines to contain the virus. Countries are in an extreme hurry to immunize their populations. As more and more people worldwide are vaccinated against SARS-CoV-2, different adverse effects are being reported. Here, we describe a case of monoarthritis following the second dose of the Oxford-AstraZenecaR SARS-CoV-2 vaccine.
We report the case of a 25-year-old female healthcare worker who presented with edema, erythema and pain in the proximal interphalangeal articulation of the fourth left finger (Fig. 1). She also had difficulty in flexing the same finger due to soreness and swelling. Upon examination, no other articulations showed any clinical signs of arthritis. Raynaud's phenomenon was absent. A clinical diagnosis of acute monoarthritis was made.
The patient denied having had fever or any recent infections (including COVID-19). She had a family history of juvenile rheumatoid arthritis (one cousin), but had no personal history of arthritis. She was taking pantoprazole for gastroesophageal reflux disease and oral contraceptive pills. Four days before developing the symptoms, the patient had taken her second dose of the Oxford-AstraZenecaR SARS-CoV-2 vaccine. She reported only local pain in the injection site after the first dose.
The X-ray showed no abnormalities. Total blood count, C-reactive protein, erythrocyte sedimentation rate, creatinine, urea, aspartate transaminase, alanine transaminase, serum protein electrophoresis, total serum proteins, thyroid-stimulating hormone, parathyroid hormone and serum calcium dosage were all within normal range. Antinuclear antibodies, rheumatoid factor, HLA-B27, antibodies for HIV 1 and 2, hepatitis B and C and syphilis were negative.
Faced with a compatible timeline since vaccination and normal laboratory exams, a diagnosis of reactive arthritis secondary to the SARS-CoV-2 vaccine was made. We prescribed 7 days of ibuprofen and 3 days of 20mg prednisone. She showed a good response to treatment and after four days all symptoms were resolved with no articular sequelae.
More than 90% of the patients who receive the Oxford-AstraZenecaR vaccine report some form of adverse effect. The main adverse effects described are local pain, followed by muscle pain, headaches, chills and fever.1 One study performed in South Korea showed that women were more likely to have adverse effects to the vaccine than men and that adverse effects were more prevalent among younger patients, such as our patient.1
There have been reports of arthralgia following vaccination for rubella, hepatitis B, measles-mumps-rubella (MMR), seasonal influenza and tuberculosis (BCG).2,3 A 2019 Systematic Review showed there might be an association between vaccination and acute arthritis, however most papers included in the review studied the seasonal influenza vaccine and the outcome was difficult to analyze due to differences in the outcomes that were evaluated in each study.2 According to the Centers for Disease Control and Prevention (CDC), numbers as high as 10–25% of Women experience arthralgia or arthritis following immunization against rubella.4,5 A Brazilian study that included near 2000 patients who received the MMR vaccine found a prevalence of arthritis of 1.7% among children and adults. Similarly to our patients, the arthritis was transient and none of the subjects involved in the study developed chronic arthritis.6
Regarding the Oxford-AstraZenecaR vaccine against SARS-CoV-2, we have found one Korean study that describes the cases of five patients who developed polyarthralgia and myalgia three to seven days after vaccination. The patients were not previously diagnosed with arthropathy and rheumatologic diseases and the arthralgia occurred in multiple joints, including small and large joints.7 There have also been reports of reactive arthritis following other vaccines against SARS-CoV-2: one report of monoarthritis of the left elbow after the Sputnik-V vaccine8 and three case reports (totalling five cases) of arthritis after the Sinovac vaccine (one case of monoarthritis of the left knee and four cases of polyarthritis).9–11
More studies are required to measure the actual prevalence of arthritis induced by the Oxford-AstraZenecaR vaccine. Younger women seem to be more at risk of developing this condition. Our patient's symptoms were transient and resolved within 4 days with no sequelae, which seems compatible with cases reported after other types of immunizations. Thus, we did not contraindicate the vaccine (should booster doses be recommended in the future) in this case.
We declare that the patient has given her written informed consent for the publication of this case report. This work is in agreement with current bioethical regulations in research. We did not get approval from the Institution's ethics committee, since this is a report of a single case which was written retrospectively and the patient cannot be identified in the photograph. We believe this has not negatively affected the patient's care and has not breached any ethical regulations. The authors declare that his article contains no personal information that could lead to the identification of the patient.
FundingNo funders pertaining to this article.
Conflict of interestAuthors declare to have no conflict of interest.