To produce recommendations for patients with rheumatological diseases receiving immunomodulatory and immunosuppressive therapies (conventional drugs, biologicals, and small molecules) during the COVID-19 pandemic.
Materials and methodsThe recommendations were determined using the Delphi method as an agreement tool. A panel of experts was formed, with academic backgrounds and research experience in rheumatology. A literature search was conducted and 42 questions were generated. The level of agreement was made with 80% of approval by the participants.
ResultsA group of eleven rheumatologists from 7 cities in the country participated. The response rate was 100% for the three consultation rounds. In the first round, agreement was reached on 35 questions, on 37 in the second round, and on 42 questions in the third round.
ConclusionThe recommendation for the majority of the pharmacological treatments used in rheumatology is to continue with immunomodulatory or immunosuppressive therapies in patients who do not have the infection, and to suspend it in patients with a diagnosis of SARS-CoV-2/COVID-19.
Generar las recomendaciones para la atención de pacientes con enfermedades reumáticas que reciben terapias inmunomoduladoras e inmunosupresoras (fármacos convencionales, biológicos y moléculas pequeñas) durante la pandemia por COVID-19.
Materiales y métodosLas recomendaciones se realizaron utilizando el método Delphi como herramienta de acuerdo. Se conformó un panel de expertos con trayectoria académica y experiencia en investigación en reumatología. Se realizó la búsqueda de la literatura y se generó el cuestionario del ejercicio Delphi conformado por 42 preguntas. El grado de acuerdo se logró con el 80% de aprobación de los participantes.
ResultadosSe conformó un grupo de 11 reumatólogos de 7 ciudades del país. La tasa de respuesta fue del 100% para las 3 rondas de consulta. En la primera ronda se logró acuerdo en 35 preguntas, en la segunda ronda 37 y en la tercera ronda se logró el acuerdo de las 42 preguntas.
ConclusiónLa recomendación para la mayoría de los tratamientos inmunomoduladores utilizados en reumatología es continuar con las terapias en pacientes que no tengan la infección y suspenderlas en aquellos con diagnóstico de SARS-CoV-2/COVID-19.
The coronavirus 19 (COVID-19) disease is the name given to the pathology caused by the severe acute respiratory syndrome coronavirus-2 infection (SARS-CoV-2).1 This new condition emerged in December 2019 in Wuhan city, Hubei province, China, and became a worldwide public health emergency threatening mankind since the World Health Organization (WHO) declared COVID-19 a pandemic on March 11, 2020.2 The first case reported in Latin America was in Brazil, on February 25. In Colombia, the first case was reported in March 6.3 This pandemic has put the different healthcare systems to a test, particularly in developing countries where the logistic and economic limitations may be a significant challenge.
Due to the concern following the announcement of the infection, in addition to the statement by the Ministry of Health through circular letter 0018 on March 2020,4 there was a need to generate recommendations addressed to healthcare professionals for the care of patients with rheumatological conditions and receiving immunomodulatory and immunosuppressive therapies (conventional drugs, biologics, and small molecules) in the current epidemiological setting. This document of the Colombian Association of rheumatology is intended to give some guidance in these difficult times, considering that patients with autoimmune and inflammatory diseases represent part of the population at risk, and therefore should keep mandatory social distancing, in accordance with the government guidelines. The evidence available is limited and hinders our understanding about the risk-benefit ratio of using immunomodulatory and immunosuppressive therapies at this time. Consequently, the following recommendations are subject to changes and updates as the scientific evidence becomes stronger, through new trials including larger numbers of patients, to be able to provide stronger recommendations.
Materials and methodsThe Delphi method is an iterative process designed to combine the opinions of a group of experts into a consensus. It is a structured methodology to systematically collect the opinions of specialists on a particular problem, process the information, and finally build a general group agreement.5 This paper was developed using this methodology as a consensus tool to design the recommendations. Following is a description of the steps taken in this project, with strict adherence to the methodological guidelines applicable to this type of exercise.6
Panel composition: through an open and ad honorem invitation to the members of the Colombian Association of Rheumatology, 11 rheumatologists, with at least 5 years of healthcare experience, were selected, including one pediatric rheumatologist.
The principal coordinator of the recommendations circulated the objectives, the scope and the proposal for the initial questions. The 11 participating rheumatologists selected the questions during an on-line meeting and then 2 of them were appointed to control the flow of information among the participants, during the iterative consultation process, providing the corresponding feedback and preparing the subsequent questionnaires. One of the group members who controlled the information, had no voting power.
Upon completion of the three rounds to reach an agreement on the questions, another on-line session was held to discuss certain aspects such as authorship and collaboration to draft the manuscript. The manuscript was submitted to the group of participating rheumatologists for review and publication by the principal author.
Literature review: A search was conducted in Medline, Embase, Clinical Key, Web of Science, Google Scholar, and Scielo-Bireme, in order to identify the available literature on possible options to consider in patients with or without COVID-19, in terms of continuity of treatment, dose adjustment, or duration of the various medications used to treat rheumatic diseases. The following search terms were used: “Coronavirus infections”; “SARS”; “SARS-CoV-2”; “MERS”; “Influenza”; “Pandemics”; “Anti-inflammatory agents, Non-steroidal”; “Glucocorticoids”; “Antimalarials”; “Methotrexate”; “Leflunomide”; “Sulfasalazine”; “Cyclophosphamide”; “Azathioprine”; “Cyclosporine”; “Mycophenolic Acid”; “Colchicine”; “Immunoglobulins”; “Tumor Necrosis Factor Inhibitors”; “Abatacept”; “Rituximab”; “Tocilizumab”; “Belimumab”; “Interleukin 1 Receptor Antagonist Protein”; “Ustekinumab”; “Interleukin-17”; “Janus Kinase Inhibitors”; “Antibodies, Monoclonal, Humanized”. The results of the search were restricted to articles in English and Spanish, with no limitation as to the date of publication. Based on this search, information was identified about the prescription of the different drugs used in rheumatology, in the context of the SARS-CoV-2 pandemics, within the framework of an infectious process of similar etiology (SARS, MERS, seasonal influenza), based on the assumption that there would be little information available about a new infection. These publications were the basis for the development of the first questionnaire using the Delphi methodology. No results were found with regards to the pediatric population.
Design of the Questionnaire: Using Go ToMeeting® with license of the Colombian Association of Rheumatology, a virtual meeting was held on March 26, 2020 where the group of participants defined the questions to be part of the questionnaire for this document, based on the information collected from the literature search. The questionnaire comprised 42 multiple choice, single answer questions; to complement their answers, each rheumatologist could make his/her own relevant comments.
Consultations with participants and definition of agreement: 3 consultation rounds were held. To define agreement, a question was considered “accepted” for inclusion in the document when at least 80% of the participants chose that question to be part of the questionnaire. The questions where no agreement was reached (selected by 70% or less of the participants) were carried forward for a second round of consultation. The questionnaire for the second round comprised the list of questions for which agreement was not reached, the answers submitted by the participants – without identifying who answered each question -, the statistical analysis of the group answers of the previous round, and the observations made by the experts, also presented anonymously. From this point on, the participants were asked to reconsider their views, taking into account the opinion of the group, so that each participant could keep or change the answer he/she gave in the previous round as considered appropriate, in light of the new information received.
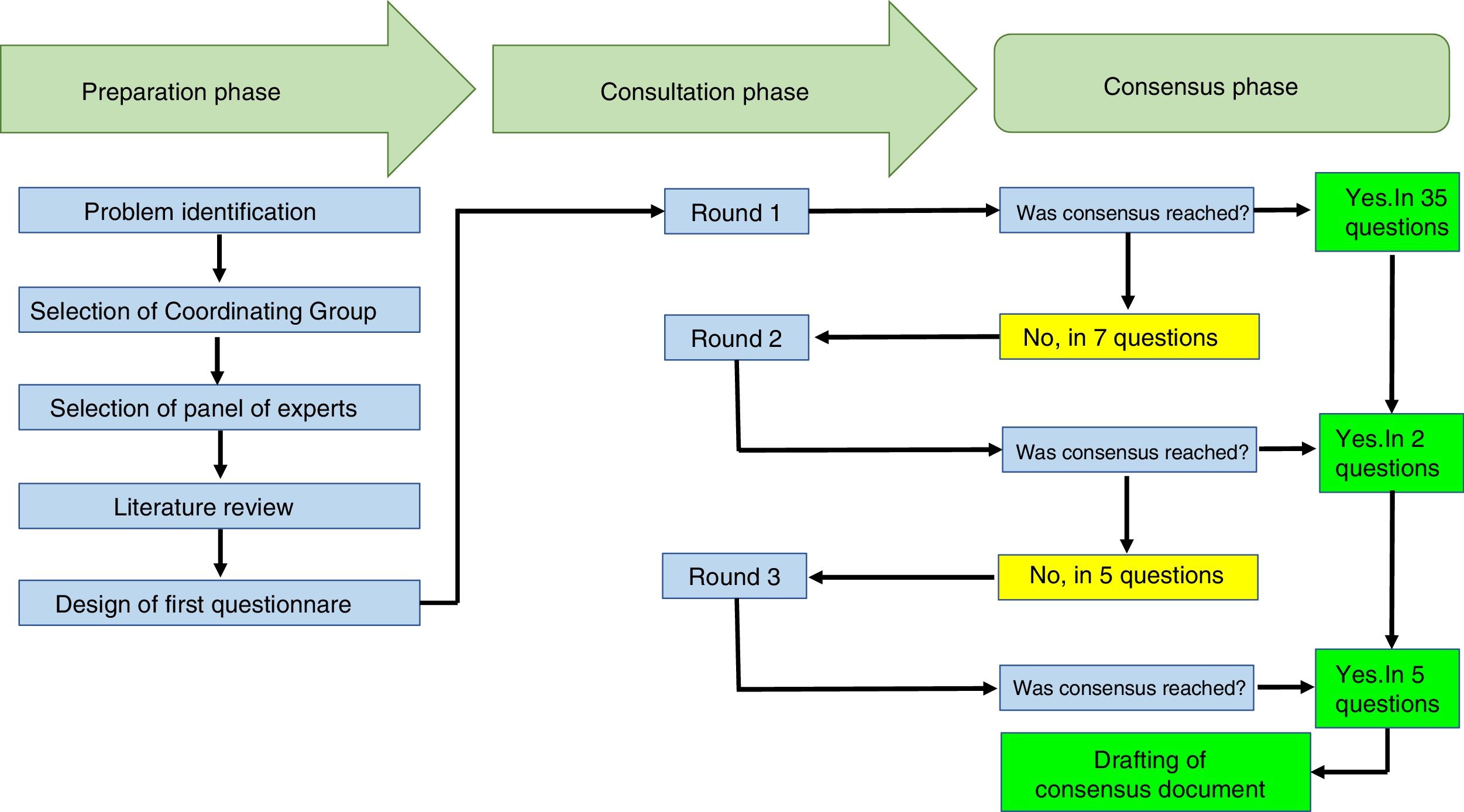
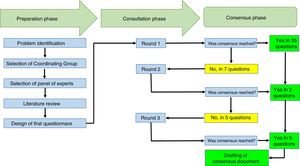
ResultsThe Delphi exercise included the participation of 10 rheumatologists from 7 cities in Colombia (Armenia, Bogotá, Cali, Manizales, Medellín, Neiva and Pereira), with an average experience as practicing rheumatologists of 11.6 years (±6.6). Eight of them worked in hospitals that provide care to rheumatology patients, and 5 of them were university professors. The response rate to the Delphi exercise was 100% in the 3 rounds of consultation completed. The duration of the total exercise was 3 weeks.
The participants received 42 questions. In the first round, agreement was reached on 35 questions and in 7 questions no agreement was reached, so these questions were carried forward to the second round. In the second round, 7 questions were submitted and agreement was reached on 2 of them. In the third round, 5 questions were submitted and agreement was reached on all of them. The text with the questions is attached herein as Appendix A.
The mean time to receive answers from the participants was 2.6 days (±1.1). Table 1 shows the recommendations to the participants and Fig. 1 summarizes the processes completed during the Delphi exercise, in the 3 phases comprised in this consensus technique.
Panel recommendation about the continuity or discontinuation of treatment of rheumatic diseases in the context of the SARS-CoV-2/COVID-19 infection pandemic.
| Recommendation | Votes |
|---|---|
| NSAIDs and/or Cox 2 inhibitors should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 9 |
| NSAIDs and/or Cox 2 inhibitors should be continued at the same dose in patients with rheumatic diseases with COVID-19 | 8 |
| Glucocorticoids should be continued at the same dose in patients with rheumatic diseases without COVID-19 | 8 |
| Glucocorticoids should be continued at a lower dose in patients with rheumatic diseases and COVID-19 | 9 |
| Antimalaria agents should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 10 |
| Antimalaria agents should be continued at the same dose in patients with rheumatic diseases, and COVID-19 | 10 |
| Methotrexate should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 9 |
| Methotrexate should be discontinued in patients with rheumatic diseases, with COVID-19 | 10 |
| Leflunomide should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 9 |
| Leflunomide should be discontinued in patients with rheumatic diseases, with COVID-19 | 10 |
| Cyclophosphamide should be continued in patients with rheumatic diseases, without COVID-19 | 9 |
| Cyclophosphamide should be discontinued in patients with rheumatic diseases, with COVID-19 | 10 |
| Azathioprine should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 9 |
| Azathioprine should be continued at the same dose in patients with rheumatic diseases, with COVID-19 | 10 |
| Ciclosporin should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 8 |
| Ciclosporin should be discontinued in patients with rheumatic diseases, with COVID-19 | 10 |
| Mycophenolate should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 9 |
| Sulfasalazine should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 8 |
| Sulfasalazine should be discontinued in patients with rheumatic diseases, with COVID-19 | 10 |
| Colchicine should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 10 |
| Colchicine should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 8 |
| Immunoglobulin should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 10 |
| Immunoglobulin should be continued at the same dose in patients with rheumatic diseases, with COVID-19 | 8 |
| TNF-inhibitors should be continued at the same dose in patients with rheumatic diseases without COVID-19 | 10 |
| TNF-inhibitors should be continued at the same dose in patients with rheumatic diseases, with COVID-19 | 10 |
| Abatacept should be continued at the same dose in patients with rheumatic diseases, without COVID-19 | 9 |
| Abatacept should be discontinued in patients with rheumatic diseases with COVID-19 | 9 |
| Rituximab should be continued at the same dose in patients with rheumatic diseases without COVID-19 | 8 |
| Rituximab should be discontinued in patients with rheumatic diseases with COVID-19 | 10 |
| Tocilizumab should be continued at the same dose in patients with rheumatic diseases without COVID-19 | 10 |
| Tocilizumab should be discontinued in patients with rheumatic diseases with COVID-19 | 8 |
| Belimumab should be continued at the same dose in patients with rheumatic diseases without COVID-19 | 10 |
| Belimumab should be discontinued in patients with rheumatic diseases with COVID-19 | 8 |
| IL-1 inhibitors should be continued at the same dose in patients with rheumatic diseases without COVID-19 | 9 |
| IL-1 inhibitors should be discontinued in patients with rheumatic diseases with COVID-19 | 9 |
| Ustekinumab should be continued at the same dose in patients with rheumatic diseases without COVID-19 | 9 |
| Ustekinumab should be discontinued in patients with rheumatic diseases with COVID-19 | 10 |
| IL-17 inhibitors should be continued at the same dose in patients with rheumatic diseases without COVID-19 | 9 |
| IL-17 inhibitors should be discontinued in patients with rheumatic diseases with COVID-19 | 10 |
| Janus kinase inhibitors should be continued at the same dose in patients with rheumatic diseases without COVID-19 | 9 |
| Janus kinase inhibitors should be discontinued in patients with rheumatic diseases with COVID-19 | 8 |
This chart summarizes the processes conducted during the Delphi exercise to establish the recommendations of the Colombian Association of Rheumatology regarding the management of patients with rheumatic diseases, receiving immunomodulation/immunosuppressive therapies (conventional, biologics and small molecules), in the context of the SARS-CoV-2/COVID-19 pandemic.
The panel recommends that patients with rheumatic diseases and no COVID-19 should continue their NSAIDs treatment, except when in the course of therapy for their underlying rheumatic disease, the typical adverse effects of this type of medications develop. With regards to the patients diagnosed with COVID-19 and use of NSAIDs, there is information supporting their use but there is also information against; however, both are low quality data in terms of the level of evidence. Ibuprofen has been associated with an increased expression of the angiotensin converting enzyme-2, when used in diabetic rats,7 and this effect could theoretically worsen the susceptibility and the clinical evolution of patients with COVID-19.8 A note published in the British Medical Journal9 reports the note issued by the French Ministry of Health with regards to the use of NSAIDs, claiming that they could worsen the symptoms and the evolution of the disease in patients with COVID-19. Therefore, they suggest using acetaminophen. The European Medicines Agency (EMA) reports that there is “no scientific evidence establishing a relationship between ibuprofen and further decline of COVID-19; EMA is closely monitoring this situation and shall review any new information available on the pandemic”.10 In a letter addressed to the editor of Science magazine, Fitzgerald argues that asymptomatic patients with COVID-19, or with mild forms of the infection, will not develop severe presentations because of the use of NSAIDs.11 In a communication on March 19, 2020, the FDA stated that “no scientific evidence has been reported saying that NSAIDs, particularly ibuprofen, worsen COVID-19 symptoms”.12 Notwithstanding the limited evidence currently available, with contradictory arguments about the use of NSAIDs in COVID-19 patients, the panel considers that in the context of COVID-19, patients with rheumatic diseases could continue using NSAIDs, in accordance with the particular situation for each case.
GlucocorticoidsThe panel recommends that patients with rheumatic diseases and no COVID-19 should continue using glucocorticoids at the minimum doses required to control their rheumatic condition. In patients with rheumatic disease and diagnosed with COVID 19, glucocorticoids should be continued, reducing the dose to the minimum required to control their rheumatic disease. After reviewing the current literature about the use of oral or intravenous glucocorticoids in patients with rheumatic diseases, it was impossible to find any support in favor or against their use in these patients. Most of the articles published on the use of glucocorticoids in the current pandemic, focus on addressing the question about the use of this type of drugs in patients with severe COVID-19-assocaited pneumonia.13 In terms of the specific group of patients with rheumatic conditions, there are very few articles directly referring to the topic. There are general recommendations with regards to the high risk for rheumatic patients to acquire infections, both because of the nature of the autoimmune disease, and because of the need to administer immunosuppressive therapy. Regarding the use of glucocorticoids in this patients, the recommendations are as follows: glucocorticoid therapy should not be initiated unless there is a clear indication and should not be discontinued abruptly if chronically received.
These initial recommendations are part of official documents on the treatment of rheumatic patients during the SARS-CoV-2 pandemic, of the Australian Association of Rheumatology14 and the National Health Service of the United Kingdom.15 Very little information can be found in the current literature regarding the use of glucocorticoids in each rheumatic disease. Some recommendations have been made, particularly in patients with rheumatoid arthritis,16 stating that glucocorticoids in patients with viral infections may lower the clearance of the viral burden, but may also reduce the host inflammatory response, which is one of the causes of adult respiratory distress syndrome (ARDS). However, their role in the management of pulmonary complications is yet to be determined. In conclusion, the current literature is scanty and unclear with regards to the use of glucocorticoids in patients with rheumatic disease, in the context of the SARS-CoV-2 pandemic. Some of the aspects found in the literature that are worth highlighting, include: do not start glucocorticoids unless there is a clear indication in this current situation; maintain the minimum dose required to control the underlying autoimmune disease, avoiding the use of high doses (>10mg/day); do not discontinue glucocorticoid therapy abruptly, but taper down slowly, based on the level of disease control and the concomitant rheumatological therapy.
Antimalaria agents (hydroxychloroquine and chloroquine)The panel recommends that patients with rheumatic diseases and without COVID-19 should continue using antimalaria agents. There is no strong scientific evidence to answer this question; however, there are some recommendations for patients – made by EULAR (European League Against Rheumatism)17 experts – whereby immunomodulatory drugs help to prevent a decline of the underlying rheumatic disease, and if they are discontinued, this may result in a relapse of the disease. So this argument suggests that treatment with these medicines shall not be discontinued.
With regards to the effect of these drugs in a probable case of COVID-19, the National Health Service of the United Kingdom says that patients may continue to use hydroxychloroquine when affected by SARS-CoV-2.15 Antimalaria agents are considered safe medications in the context of infections. In a case control trial that was part of a cohort, including 23,733 patients with rheumatoid arthritis, the risk of severe infections associated with the use of the conventional DMARDs was assessed. The study showed that the relative risk (RR) of infections requiring hospital admission in patients using anti-malaria agents was 1.06 (95% CI: 0.94–1,19), and the RR for pneumonia was 1.06 (95% CI: 0.92–1.22). Hence, the conclusion was that hydroxychloroquine and chloroquine did not show a statistical association with a higher risk of infection.18 A retrospective cohort trial with 24,530 patients with rheumatoid arthritis concluded that these patients had a higher risk of hospital-acquired infection, and the risk varied according to the treatment. The trial found that the rate of a first hospital infection was higher in the cohort of patients with rheumatoid arthritis, but the use of hydroxychloroquine was associated with a risk reduction, with an adjusted RR of 0.74 (95% CI: 0.62–0.89).19
Since 1969 there is scientific evidence showing the in vitro efficacy of chloroquine as potential broad spectrum antiviral agent.20 The antimalaria agents (chloroquine and hydroxychloroquine) block the viral infection, increasing the endosomal pH required for the virus-cell fusion, and it also interferes with the glycation process of the SARS-CoV-2 cell receptors, and hence have been used in different countries to treat patients with COVID-19. Their efficiency in combination with azithromycin21 and antiviral medications22 has been assessed, with favorable results in some trials,22,23 including a recent systematic review24; however these trials had a limitation because they were conducted in small groups of patients. Therefore, the evidence does not allow for making conclusions about the actual effectiveness of antimalaria agents in the treatment of patients with COVID-19; however, in view of the shortage of therapeutic options to treat these patients, antimalaria agents have been included in the treatment protocols in different countries, including Colombia; such is the case of the Colombian Consensus of Care, Diagnosis and Management of the SARS-CoV-2 Infection, recently published in the Journal of the Colombian Association of Infectious Diseases.25 Considering that antimalaria agents are part of the treatment regimen for COVID-19, this panel recommends that patients with rheumatic diseases with a diagnosis of COVID-19 should continue using the antimalaria agents at the usual dose, after receiving specific treatment for the infection
Conventional Anti-rheumatic agents (methotrexate, leflunomide, azathioprine and sulfasalazine) and other immunosuppressors (mycophenolate, cyclophosphamide and ciclosporin)The panel recommends that patients with rheumatic diseases without COVID-19 should continue with the conventional antirheumatic drugs and immunosuppressive medications. Methotrexate is a drug with immunomodulator effect rather than immunosuppressor, at the dose prescribed for the treatment of rheumatic diseases, and has not been associated with opportunistic infections,26 even when used together with other immunomodulators, glucocorticoids or biologics. Leflunomide has a safety profile similar to methotrexate,27 even when these two drugs are used in combination.28 Azathioprine has also shown an adequate safety profile for infections,29 just as sulfasalazine.30 Mycophenolate has shown and increased risk of infection for some viruses such as herpes zoster31 and cytomegalovirus32; however, this has been the case in the context of patients with solid organ transplant receiving additional immunosuppressants. The safety information on mycophenolate, with regards to infection in the treatment of rheumatic diseases such as systemic lupus erythematous, has shown a similar profile to other antirheumatic agents.33 A similar situation to that of mycophenolate presents with cyclosporine, a drug that has shown increased risk of infections in some post-transplant patients,34 but with a lower profile in the context of rheumatic diseases, because other potent immunosuppressors are used concomitantly. In some studies, cyclophosphamide has been associated with bacterial, viral, and opportunistic germ infections,35 with low risk of infection; nevertheless, clinical follow-up is warranted in patients receiving this cytostatic agent for the treatment of rheumatological diseases. In general, the likelihood of developing a severe infection is not significantly increased in patients with rheumatic diseases receiving conventional anti-rheumatic drugs and immunosuppressors. The preliminary data from the international registry of patients with rheumatic diseases has not shown that the use of this type of drugs is associated with a higher susceptibility to severe infections, such as COVID-19.36 However, in the context of active SARS-CoV-2 infection, similar to the recommendations in other types of infections that may develop in a rheumatic patient, the panel recommends that patients with rheumatic conditions, diagnosed with COVID-19, should discontinue the conventional anti-rheumatic drugs and immunosuppressors.
The continued use of these drugs in the presence of viral, bacterial or fungal infection, is associated with worse medical outcomes as compared to their discontinuation.37–40 In terms of the current COVID-19 challenge, the impact of continuing with the conventional anti-rheumatic agents and immunosuppressors is still unknown. Therefore, the information available from other infections, particularly respiratory viruses, is extrapolated, and the recommendation is to discontinue the use of these drugs until complete resolution of the acute infectious condition.
ColchicineThe panel recommends that patients with rheumatological conditions without COVID-19 should continue with colchicine. There is yet no information about the effect of colchicine use in a potential COVID-19 case. Neither is there any evidence of this drug causing the development of a severe illness, or on the contrary, causing a beneficial impact if the patient becomes infected. What is indeed known is that its toxicity increases with some antibiotics and antiviral agents that cause cytochrome P450 inhibition.41 Therefore, precautions should be taken for the combined use of colchicine and the medicines used for COVID-19 treatment. The panel recommends that patients with rheumatic diseases and a COVID-19 diagnosis should continue colchicine treatment.
There are a few randomized, multicenter, double blind, placebo-controlled trials currently underway, to assess that efficacy and safety of colchicine in adult patients diagnosed with COVID-19, and who meet at least one high-risk criteria. These are the GREECO-19 (NCT04322790) trial from the university of Athens42 and the COLCORONA (NCT04322682) trial from the University of New York and the Heart Institute of Montreal.43
ImmunoglobulinThe panel recommends that patients with rheumatic diseases without COVID-19 should continue the use of immunoglobulin. Intravenous immunoglobulin was originally developed in the late 70s, and is probably one of the safest immunomodulators for long-term use in all age groups43; however, it may cause adverse reactions, usually dose-dependent.44 During the SARS outbreak in 2003, immunoglobulin was widely used in Singapore, at a daily dose of 0.4g/kg for 3 days, and in one third of the critical patients, venous thromboembolism was documented as an adverse effect.45 This may be due to the fact that immunoglobulin increases the viscosity in hypercoagulation conditions of SARS patients. However, immunoglobulin has been used in some COVID-19 patients, with variable results, so this agent should be considered an option, particularly in severe ICU patients.46 The panel recommends that patients with rheumatic diseases diagnosed with COVID-19, should continue immunoglobulin therapy.
A randomized controlled trial is currently underway to assess the efficacy of IV immunoglobulin in patients with severe COVID-19 (NCT04261426).47
Anti-TNFThe panel recommends that patients with rheumatic diseases without COVID-19 should continue with anti-TNF (adalimumab, certolizumab, etanercept, golimumab and infliximab) biologic therapy. Most records of patients with anti-TNF therapy focus on bacterial and opportunistic infections,48 but very few assess the association with viral infections. Just as with the recommendation about the conventional antirheumatic agents and immunosuppressors, the panel considered it was not wise to continue administering anti-TNF biologic therapies during active infection, including COVID-19; the recommendation is that patients with rheumatic diseases and a diagnosis of COVID-19 should discontinue the anti-TNF (adalimumab, certolizumab, etanercept, golimumab and infliximab) biologic therapy. COVID-19 patients present with an overregulation of proinflammatory cytokines, including IL-1, anti-TNF and gamma interferon. Feldmann et al. consider that there is enough evidence to support clinical anti-TNF trials in patients with COVID-19.49 A clinical randomized trial is currently being registered in China, using adalimumab in patients with COVID-19 and severe pneumonia.50
AbataceptThe panel recommends that patients with rheumatic diseases, without COVID-19, should continue biologic therapy with abatacept. According to the clinical trials, the rates of infections with abatacept are similar to those of patients with methotrexate and are lower than with other biologics.51 In a network analysis of clinical trials using biologics, the risk of severe infection with abatacept was numerically inferior to the rest, and significantly lower when compared against certolizumab, infliximab and tocilizumab.52 In the current SARS-CoV-2 setting, no evidence has yet been published showing that the use of abatacept leads to the development of a severe illness, if the rheumatic patient receiving this biologic agent presents with COVID-19. The panel considered that it is not prudent to continue biologic therapy with abatacept during active infectious processes, including COVID-19; therefore, the recommendation is that patients with rheumatic diseases, diagnosed with COVID-19, should discontinue abatacept treatment.
RituximabThe panel recommends that patients with rheumatic diseases, without COVID-19 should continue biologic therapy with rituximab. No increase in the incidence of opportunistic infections has been yet documented in the clinical trials with rituximab for rheumatoid arthritis,53 although there have been some reports of infections by Pneumocystis jirovecci,54 cryptococci meningitis55 and cytomegalovirus colitis.56 In the current scenario of the SARS-CoV-2 pandemic, no evidence has yet been published about using rituximab and the development of a severe illness, if the rheumatology patient receiving this biologic agent has COVID-19. The panel considered that it is not prudent to continue biologic rituximab therapy during active infection, including COVID-19; therefore, the recommendation is that patients with rheumatic diseases diagnosed with COVID-19 should discontinue rituximab therapy.
TocilizumabThe panel recommends that patients with rheumatic diseases without COVID-19 should continue tocilizumab biologic therapy. The clinical trials with tocilizumab for rheumatoid arthritis, have not documented an increased incidence of opportunistic infections.57 In the current SARS-CoV-2 pandemic setting, no evidence has yet been published showing that the use of tocilizumab may result in a severe illness if the rheumatic patient receiving this agent has COVID-19 infection.
In addition to the approvals for rheumatoid arthritis and juvenile idiopathic arthritis, tocilizumab has been FDA58 and EMA59 approved for the treatment of CART – chimeric antigen receptor T-cell syndrome. However, this new indication has not been the result of a specific clinical development, but emerged from the empirical use of tocilizumab in the context of clinical trials associated with T-CAR drugs.60 The hypothesis of the potential benefit of tocilizumab use in COVID-19 patients is closely linked to such indication.61,62 In the trial by Xu et al.,63 without a control group, in addition to other methodological weaknesses, 21 patients with COVID-19 and severe pneumonia received tocilizumab, and the majority experienced improvement of various parameters (oxygen requirement, lung X-rays, lymphocyte count and C-reactive protein levels). Another case series in China showed similar results with the use of tocilizumab in 15 patients with COVID-19.64 Some studies are currently being conducted to assess the effectiveness of tocilizumab in COVID-19: a prospective cohort trial led by the University of L’Aquila in Italia (TOSCA trial), to assess the efficacy of tocilizumab in patients with ARDS and cytokines release syndrome secondary to COVID-19 (NCT042332913),65 as well as a randomized, multicenter clinical trial, led by Roche laboratories (COVACTA trial), to assess the efficacy of tocilizumab in patients with COVID-19 and severe pneumonia (NCT04320615).66
The panel considered that in view of the current evidence, it is not sensible to continue biologic therapy with tocilizumab during a viral infection processes, including COVID-19; hence, the recommendation is that patients with rheumatic diseases and diagnosed with COVID-19 should discontinue tocilizumab biologic therapy. It must be highlighted that this recommendation is addressed to the management of patients with rheumatic conditions; suggesting therapeutic options for the management of the COVID-19-induced cytokine release syndrome is beyond the scope of this document.
BelimumabThe panel recommends that patients with rheumatic diseases without COVID-19 should continue biologic therapy with belimumab. The joint results of the BLISS-52 and 76 trials showed a numerical increase in the infectious processes, at the expense of respiratory infections, although the differences were not statistically significant, and no differences were found in the development of herpetic or opportunistic infections or sepsis.67 Based on the recommendations published at the international level, belimumab is only mentioned as part of the indications by the United Kingdom National Health Service,15 under the classification of other biotechnological agents with high and very high risk. No mention is made about the specific approach for patients undergoing belimumab therapy. In the current SARS-CoV-2 pandemic scenario, no evidence has been yet published on the use of belimumab leading to the development of a severe disease if the rheumatic patient receiving this biologic agent has COVID-19. The panel considered that it is not sensible to continue biologic therapy with belimumab during active infectious processes, including COVID-19; hence the recommendation is that patients with rheumatic diseases, diagnosed with COVID-19 should discontinue belimumab treatment.
IL-1 inhibitorsThe panel recommends that patients with rheumatic diseases without COVID-19 should continue biologic therapy with IL-1 inhibitors (anakinra and canakinumab). In the current SARS-CoV-2 pandemic setting, no evidence has yet been published on the use of IL-1 inhibitors and the development of a severe illness, if the rheumatic patient receiving this biologic agent has COVID-19. The panel considered that it is not sensible to continue biologic therapy with IL-1 inhibitors during active infection, including COVID-19; therefore, they recommend that patients with rheumatic diseases diagnosed with COVID-19 should discontinue treatment with IL-1 inhibitors (anakinra and canakinumab).
IL-12/23 inhibitorsThe panel recommends that patients with rheumatic diseases without COVID-19 should continue biologic therapy with IL-12/23inhibitors (ustekinumab). Ustekinumab has shown a similar safety profile with regards to infections, as other biologic therapies used for the treatment of psoriatic arthritis and psoriasis.68 In the current SARS-CoV-2 pandemic scenario, no evidence has yet been published about the use of IL-12/23 inhibitors and the development of a severe illness, if the rheumatic patient receiving this biologic agent has COVID-19. The panel considered that it is not sensible to continue IL-12/23 inhibition therapy during active infectious processes, including COVID-19; therefore, the recommendation is that patients with rheumatic diseases and a diagnosis of COVID-19 should discontinue therapy with IL-12/23inhibitors (ustekinumab).
IL-17 inhibitorsThe panel recommends that patients with rheumatic diseases without COVID-19 should continue biologic therapy with IL-17 inhibitors (ixekizumab and secukinumab). The long-term studies in patients treated for psoriasis, psoriatic arthritis and anchylosing spondylitis with secukinumab, cases of opportunistic infection by Candida at the skin level or in the mucosae, have been reported; all of these cases have been of mild to moderate severity and no cases have yet been reported of patients with systemic candidiasis.69 Similar results have been reported with ixekizumab.70 Some cases of herpes zoster have also been reported with the use of IL-17; however, this increase has not been statistically significant, as compared against placebo.71 In the current SARS-CoV-2 pandemic setting, no evidence has yet been published on the use of IL-17 inhibitors and the development of a severe illness if the rheumatic patient receiving this biologic agent has COVID-19. The panel considered that it is nor sensible to continue biologic therapy with IL-17 inhibitors during active infectious processes, including COVID-19; therefore, the recommendation is that patients with rheumatic diseases diagnosed with COVID-19 should discontinue therapy with IL-17 inhibitors (ixekizumab and secukinumab).
JAK inhibitorsThe panel recommends that patients with rheumatic diseases without COVID-19 should continue treatment with JAK inhibitors (baricitinib and tofacitinib). A meta-analysis assessing the safety of using tofacitinib in patients with rheumatoid arthritis, showed that the risk of severe infections with tofacitinib is comparable to the rates reported in the studies on biologic therapies for rheumatoid arthritis.72 In a recent review of the trials with baricitinib, similar results were reported in terms of safety with regards to infections.73 In the current SARS-CoV-2 pandemic scenario, no evidence has yet been published on the use of JAK inhibitors and the development of a severe disease if the rheumatic patient receiving this agent has COVID-19. The panel considered that it is not prudent to continue JAK inhibition therapy during an active infectious processes, including COVID-19; therefore, the recommendation is that patients with rheumatic diseases with a diagnosis of COVID-19 should discontinue therapy with JAK inhibitors (baricitinib and tofacitinib).
Janus kinase 1 and 2 are associated with inflammation and the AP-2-associated protein kinase 1 (AAK1) plays a key role in the virus entry to the cell. Based on the information generated by biotechnological analyses, baricitinib may help to inhibit the SARS-CoV-2 infection, by inhibiting AAK1, and also inhibiting the inflammation due to the block of the JAK ½ pathway74; hence it has been suggested as a treatment option for COVID-19.75 An open, non-randomized trial is currently underway (BARI-COVID trial) to assess the efficacy of baricitinib in patients with mild to moderate COVID-19, as well as radiological findings of pneumonia (NCT04320277).76 While conducting the literature review for this congress, two additional trials on baricitinib registered in ClinicalTrials.org were identified: “Safety and efficacy for baricitinib for COVID-19” (NCT04340232)77 and “Treatment of moderate to severe coronavirus disease (COVID-19) in hospitalized patients” (NCT04321993).78 However, none of them were yet in the patient enrolment phase. Another trial registered with tofacitinib, the “TOFAcitinib in SARS-CoV2 pneumonia” trial (NCT04332042) was also identified,79 which starts recruiting patients late in April 2020.
ConclusionThis article discusses the recommendations addressed to healthcare practitioners caring for patients with rheumatic diseases, who receive immunomodulators and immunosuppressors (conventional drugs, biologics and small molecules), in the context of the COVID-19 pandemic. These recommendations were designed by a panel of members of the Colombian Association of Rheumatology.
Patients with rheumatic diseases, receiving treatment with immunomodulators or immunosuppressive medications, are therefore considered at risk for the development of SARS-CoV-2 infection. However, the use of some of these agents, such anti-malaria agents, JAK inhibitors, tocilizumab and colchicine, is being included in the protocols of clinical trials in various research centers, as part of the management of patients with COVID-19.
The recommendation for most of the treatments used in rheumatology, is to continue with immunomodulation or immunosuppression therapy in rheumatic patients who do not have the infection, and to discontinue therapy in those patients with a COVID-19 diagnosis. Preparing and publishing these recommendations is a timely and necessary response to the therapeutic approach of the infection caused by this new virus, in light of the WHO declaration of the pandemic, and the initiatives of the Ministry of Health to cope with this new situation in Colombia.
The information in the available scientific evidence is exclusively related to adult patients with rheumatological conditions; no information is available on the pediatric population, and therefore the scope of this recommendations should be limited to adult patients with rheumatic diseases only. The literature so far generated on COVID-19 in the context of the rheumatic patient is sparse, so this consensus is a dynamic document that will be updated over the next 6 months, in order to respond to the need to generate new information to establish recommendations consistent with the available evidence.
Please cite this article as: Saldarriaga Rivera LM, Fernández Ávila D, Bautista Molano W, Jaramillo Arroyave D, Bautista Ramírez AJ, Díaz Maldonado A, et al. Recomendaciones sobre el manejo de pacientes adultos con enfermedades reumáticas en el contexto de la infección por SARS-CoV-2/COVID-19. Asociación Colombiana de Reumatología. Rev Colomb Reumatol. 2020;27:230–241.
This article is published simultaneously in Reumatología Clínica (https://doi.org/10.1016/j.reumae.2020.06.006) and in Revista Colombiana de Reumatología (https://doi.org/10.1016/j.rcreue.2020.10.001), with the consent of the authors and editors.